Spatial Impact of Recreational-Grade Echosounders and the Implications for Killer Whales
Abstract
:1. Introduction
2. Materials and Methods
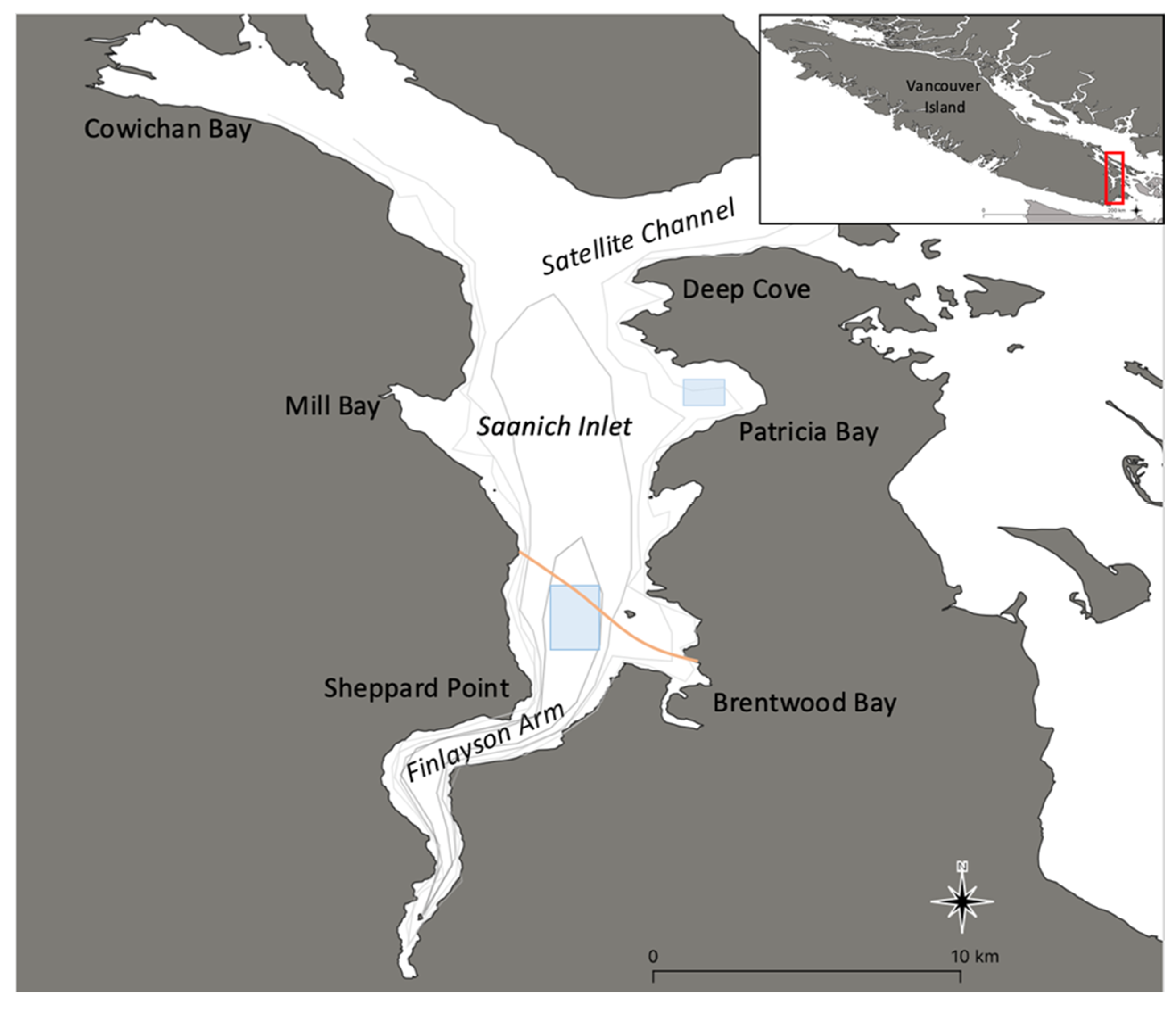
2.1. Study Site
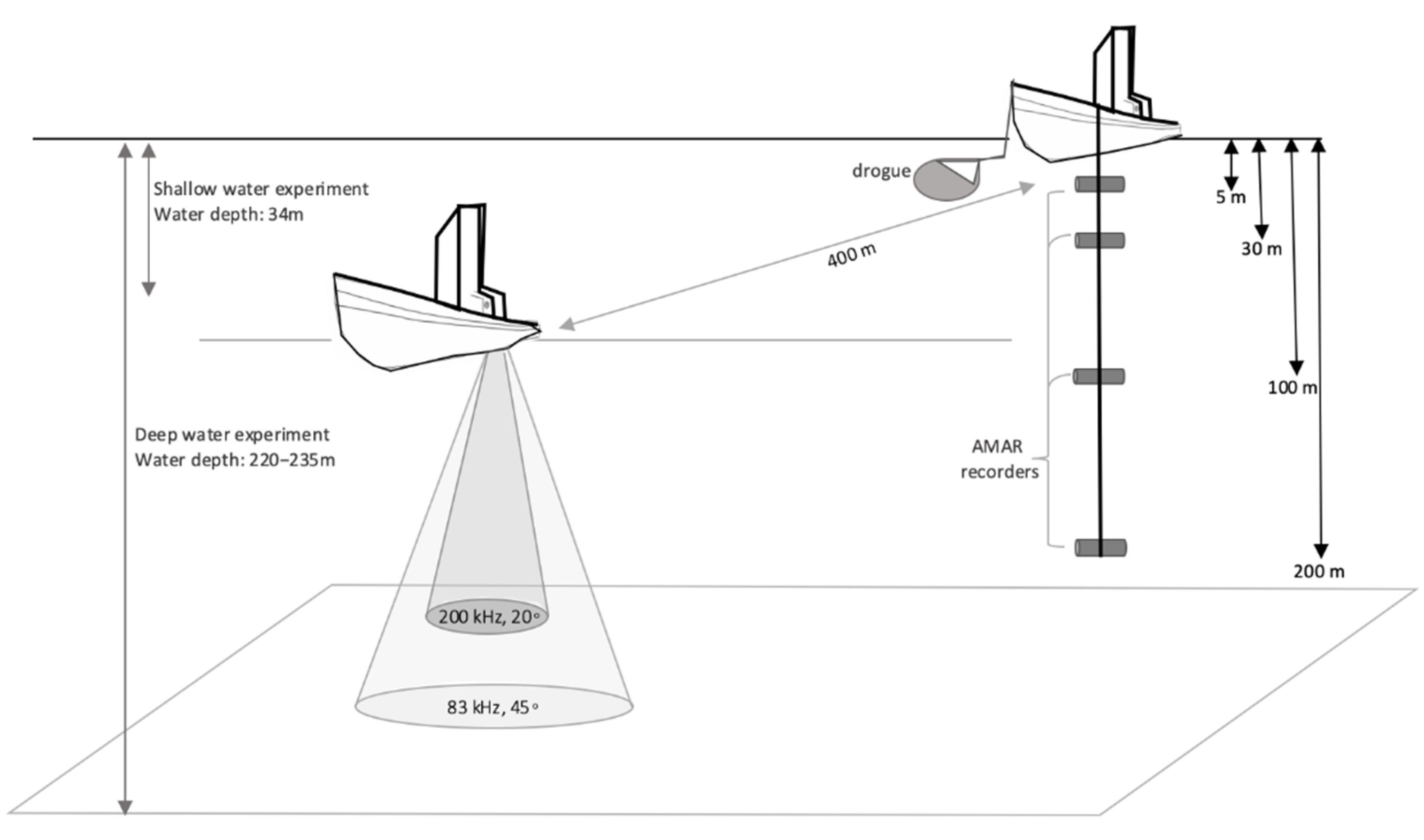
2.2. Active Acoustic Sound Source and Passive Acoustic Recordings
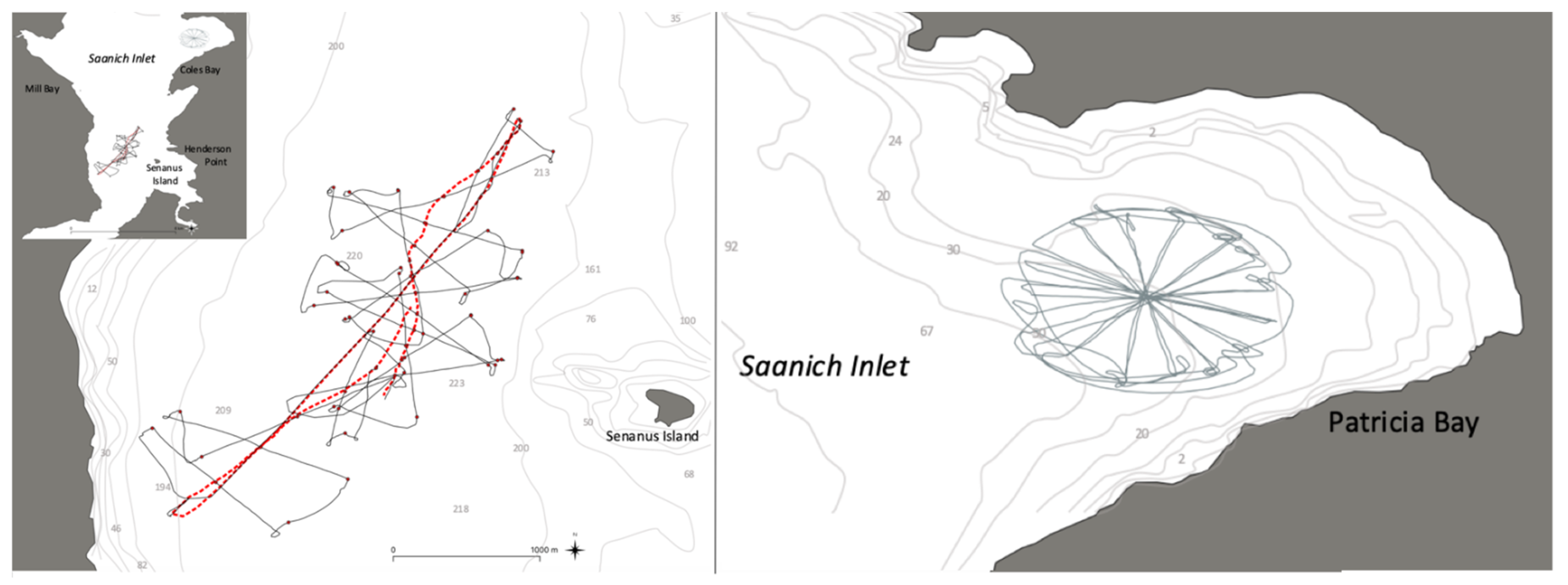
2.3. Experimental Design and Implementation
2.4. Acoustic Analysis
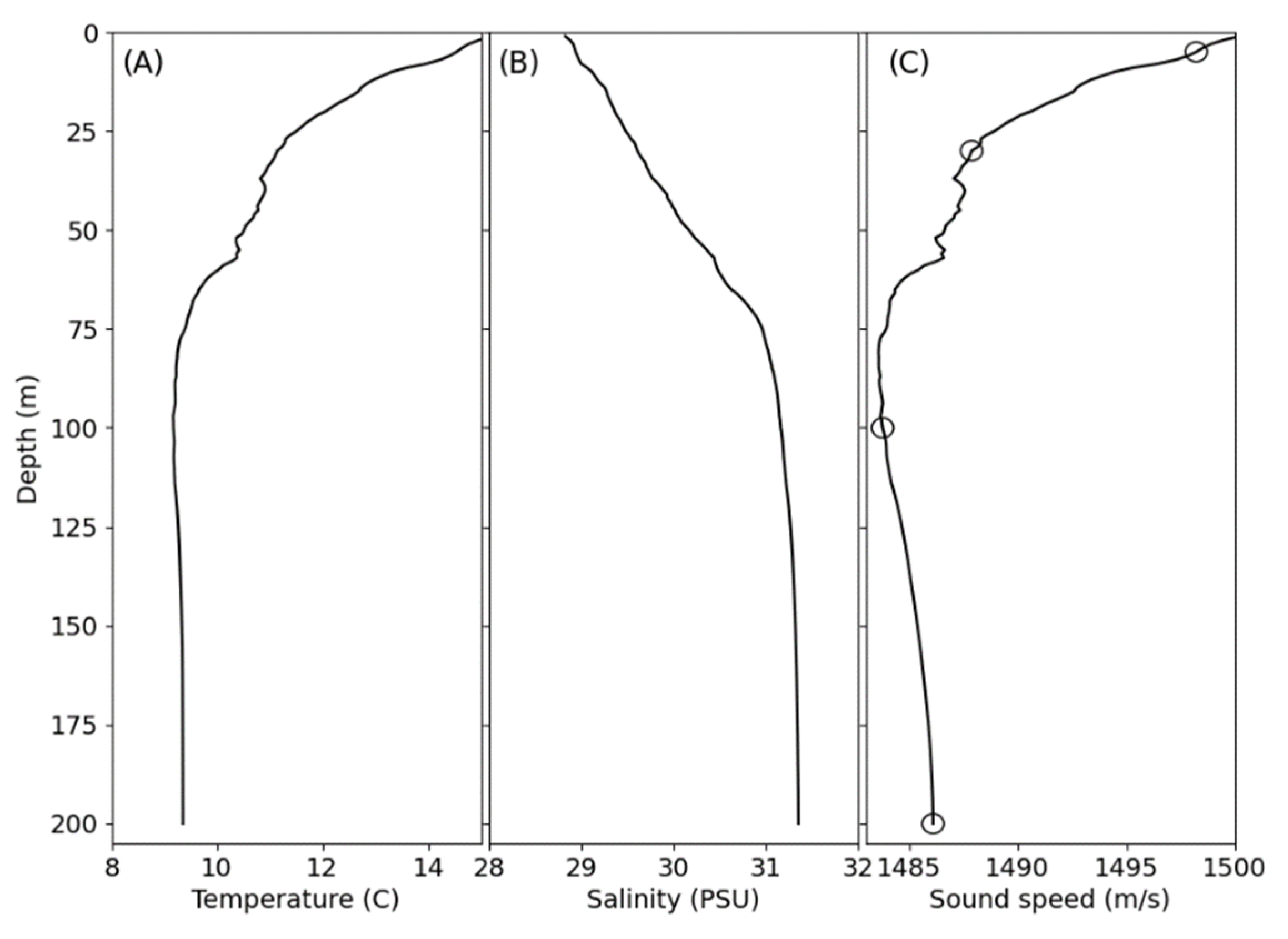
2.5. Water Properties and Environmental Conditions
3. Results
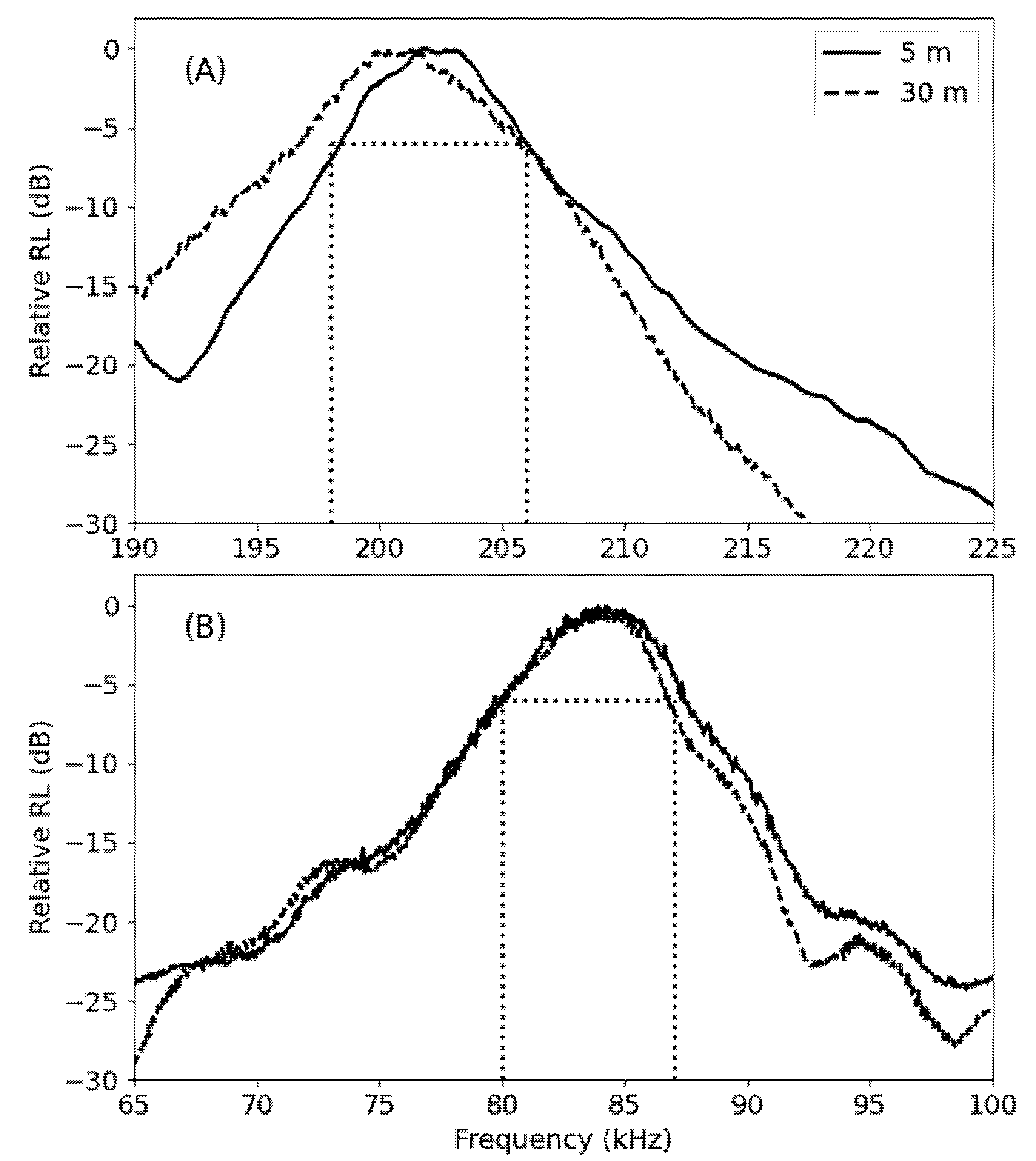
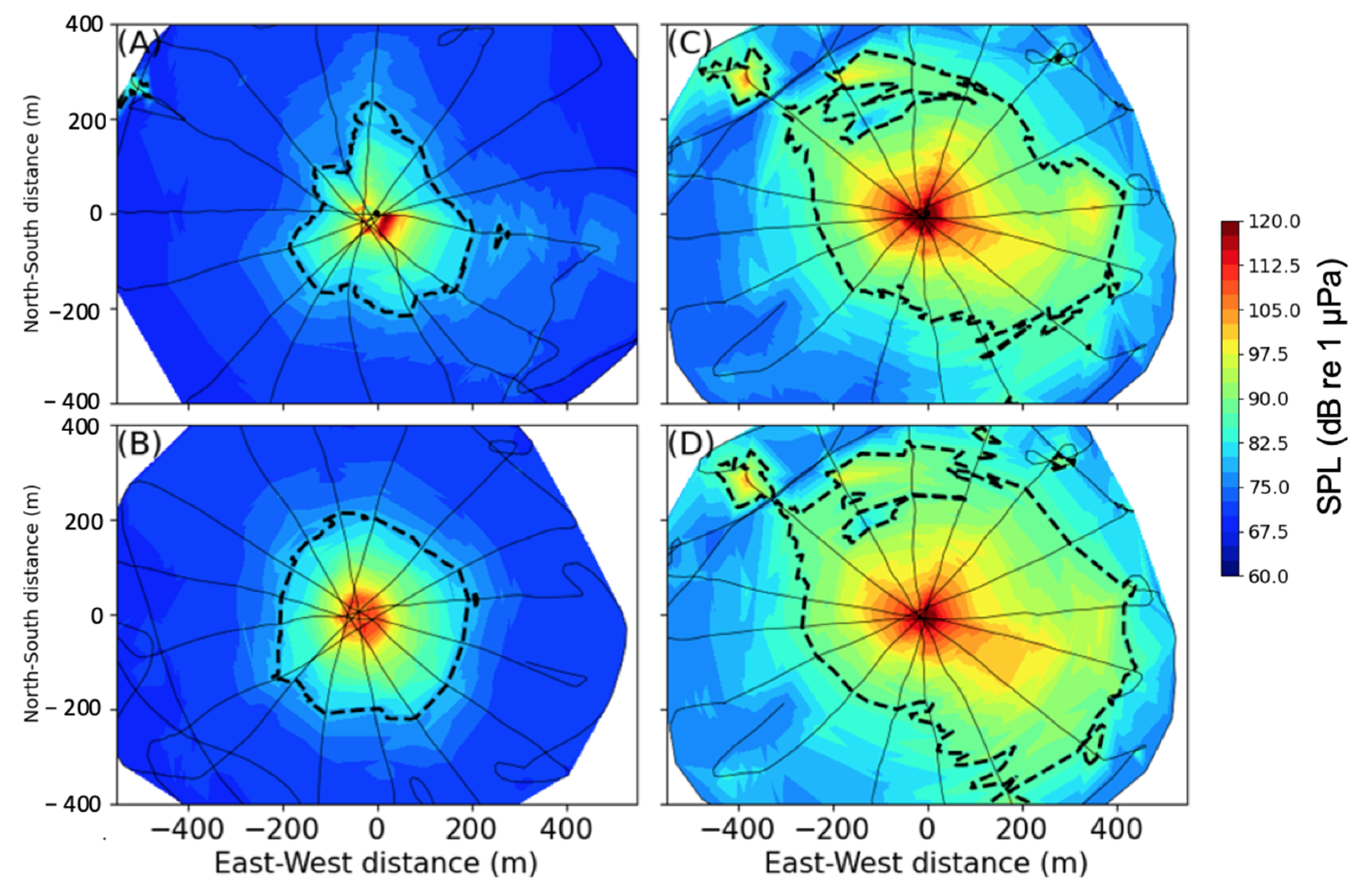
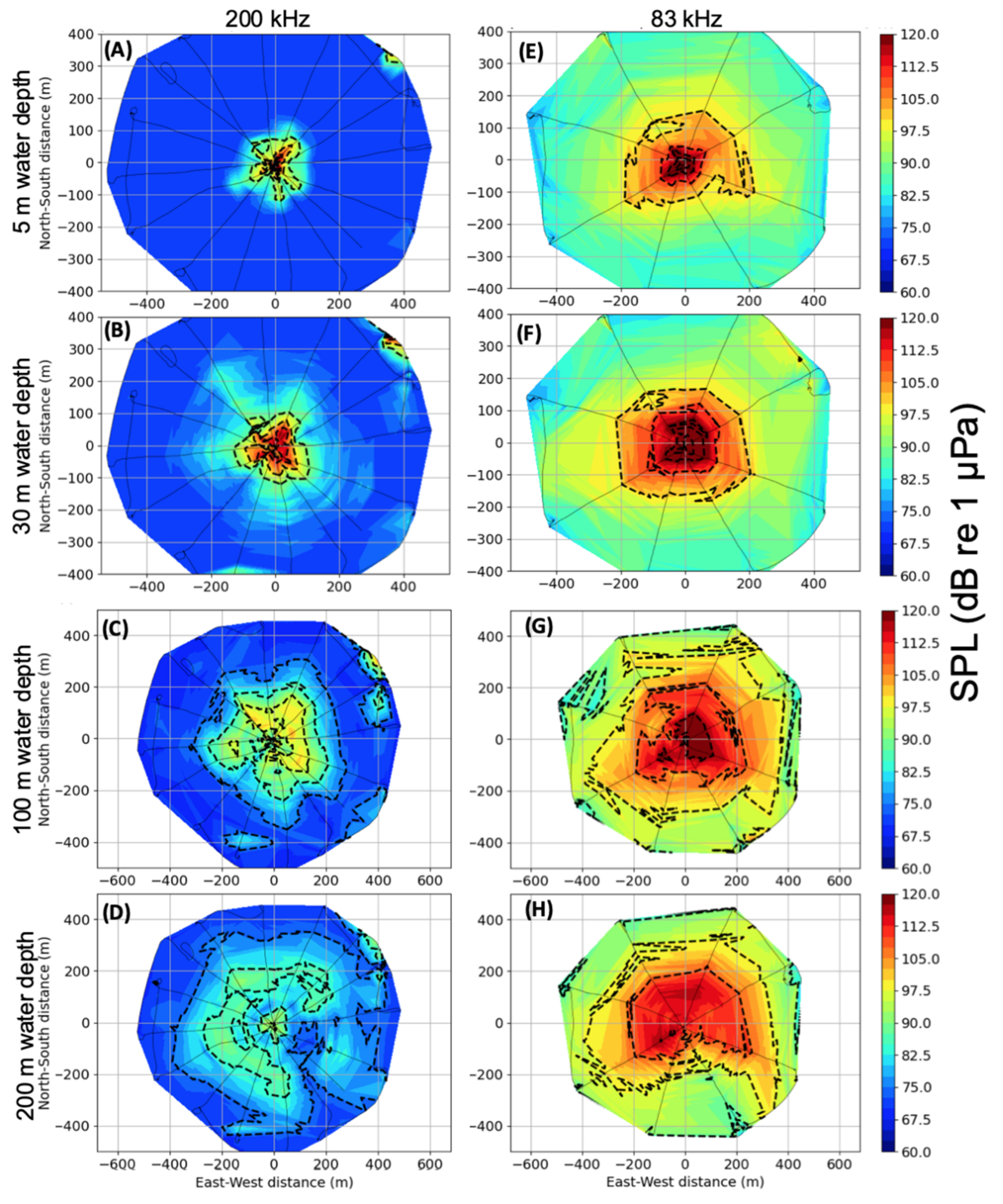
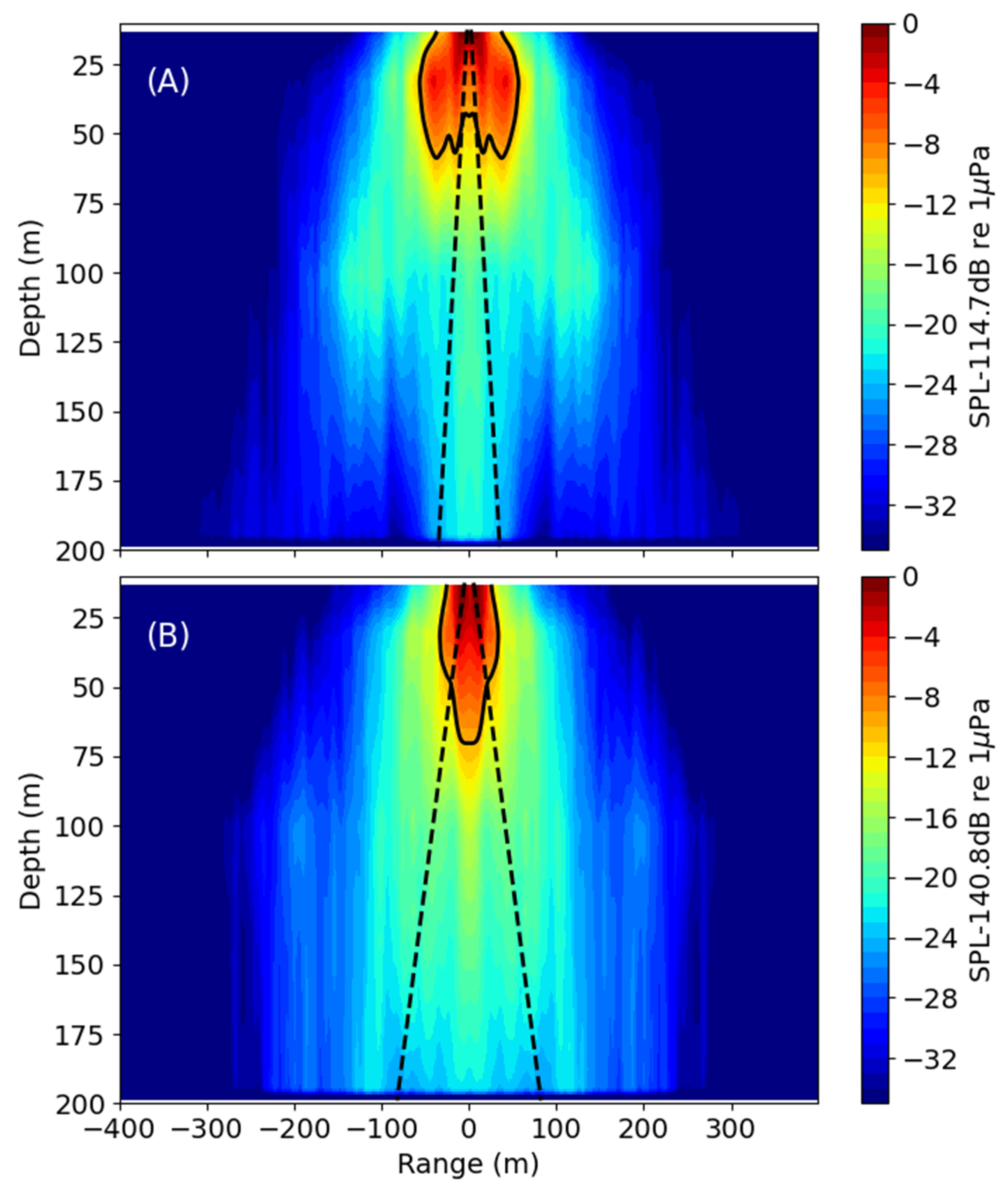
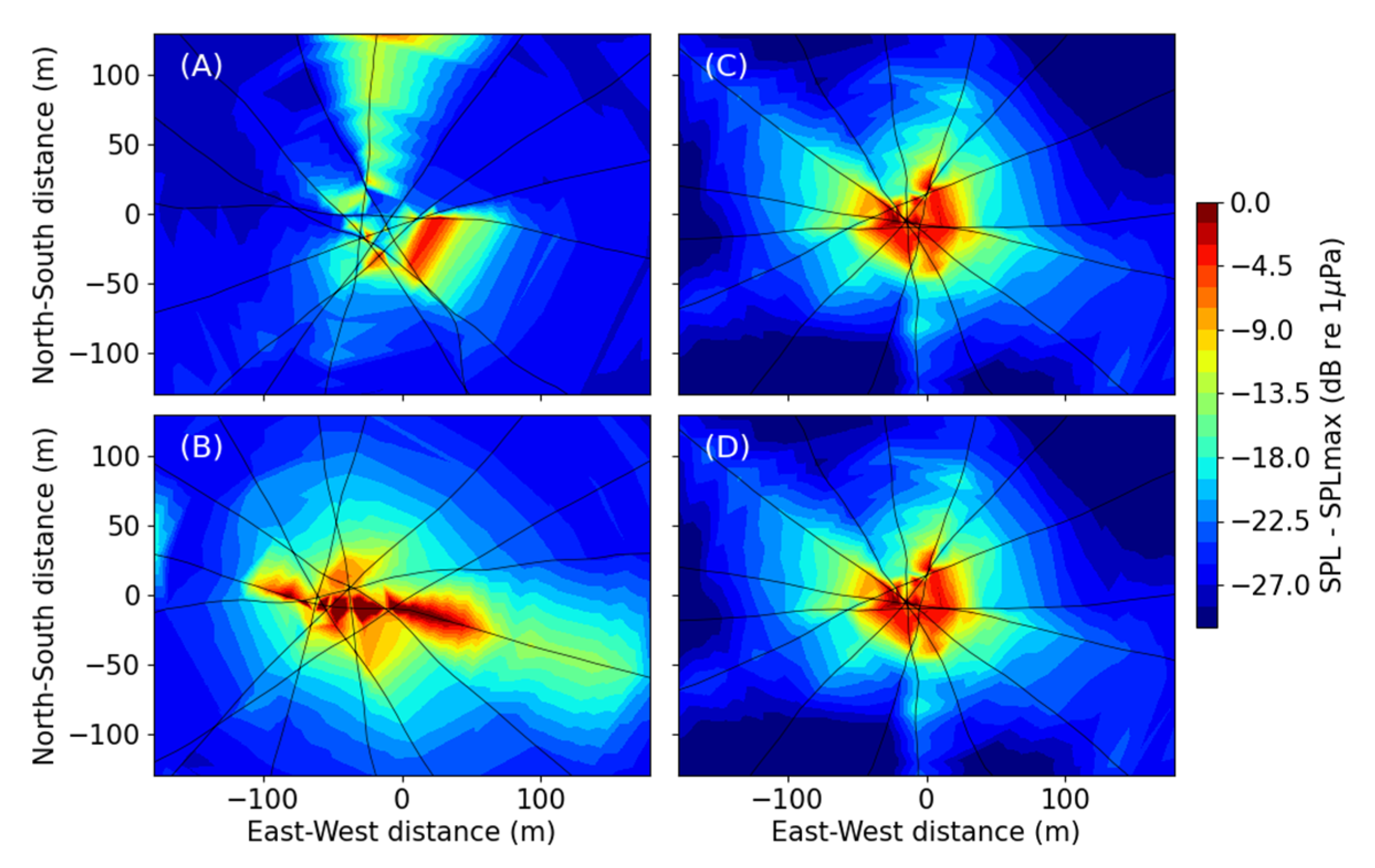
3.1. Acoustic Analysis
3.1.1. Shallow Water (34 m)
3.1.2. Deep Water (220–235 m)
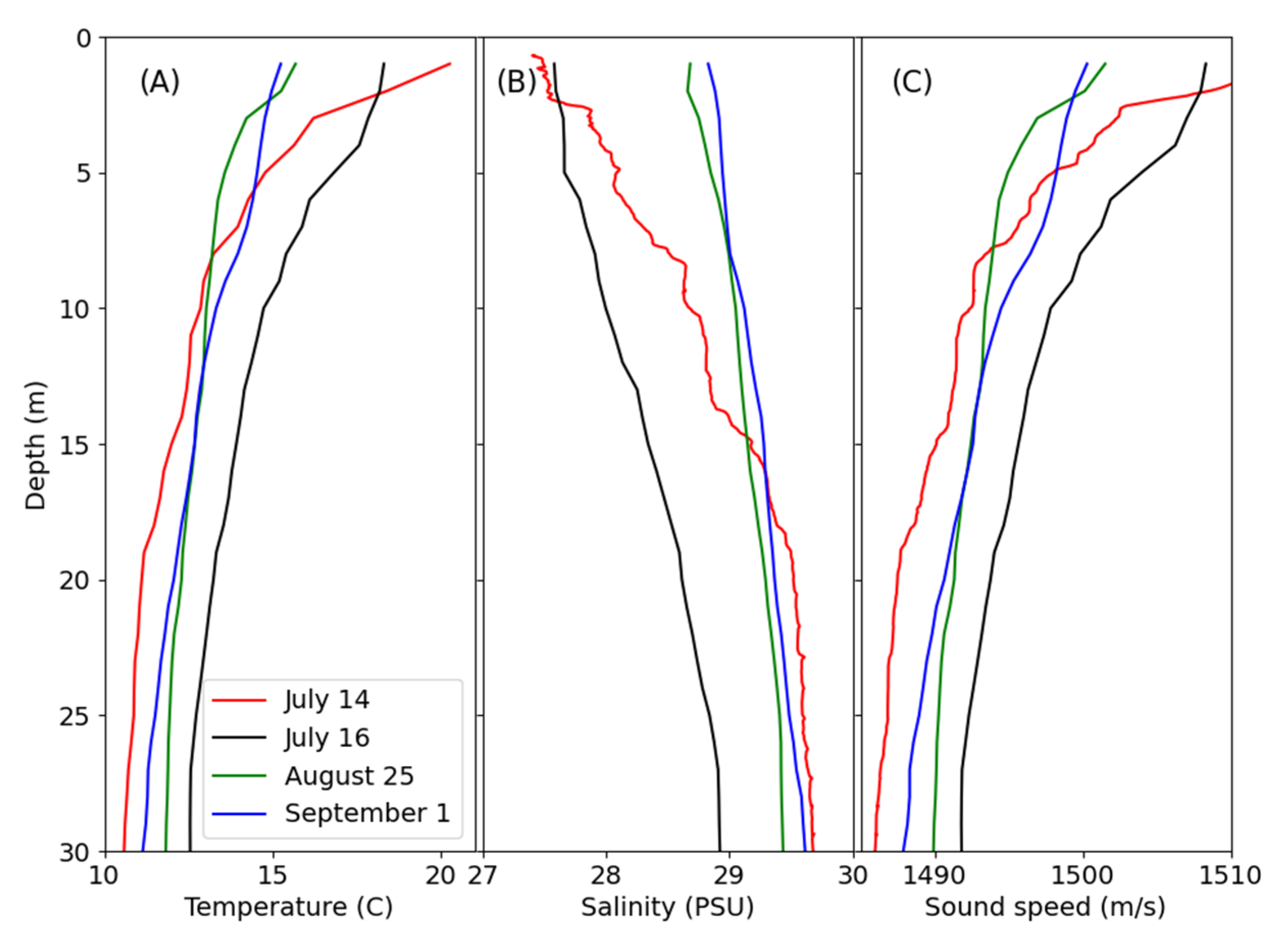
3.2. Water Properties and Environmental Conditions
3.3. Impact on SRKWs
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurton, X. An Introduction to Underwater Acoustics: Principles and Applications; Springer: London, UK, 2002; pp. 172–180. [Google Scholar]
- Anderson, J.T.; Van Holliday, D.; Kloser, R.; Reid, D.G.; Simard, Y. Acoustic seabed classification: Current practice and future directions. ICES J. Mar. Sci. 2008, 65, 1004–1011. [Google Scholar] [CrossRef]
- Lurton, X.; DeRuiter, S. Sound radiation of seafloor-mapping echosounders in the water column, in relation to the risks posed to marine mammals. Int. Hydrogr. Rev. 2011, 6, 7–17. [Google Scholar]
- Calvert, J.; Strong, J.A.; McGonigle, C.; Quinn, R. An evaluation of supervised and unsupervised classification techniques for marine benthic habitat mapping using multibeam echosounder data. ICES J. Mar. Sci. 2014, 72, 1498–1513. [Google Scholar]
- Howe, J.A.; Anderton, R.; Arosio, R.; Dove, D.; Bradwell, T.; Crump, P.; Cooper, R.; Cocuccio, A. The seabed geomorphology and geological structure of the Firth of Lorn, western Scotland, UK, as revealed by multibeam echo-sounder survey. Earth Environ. Sci. Trans. R. Soc. Edinb. 2015, 105, 273–284. [Google Scholar] [CrossRef]
- Lurton, X. Modelling of the sound field radiated by multibeam echosounders for acoustical impact assessment. Appl. Acoust. 2016, 101, 201–221. [Google Scholar] [CrossRef]
- Deng, Z.D.; Southall, B.L.; Carlson, T.J.; Xu, J.; Martinez, J.J.; Weiland, M.A.; Ingraham, J.M. 200 kHz Commercial Sonar Systems Generate Lower Frequency Side Lobes Audible to Some Marine Mammals. PLoS ONE 2014, 9, e95315. [Google Scholar] [CrossRef]
- Finneran, J.J. Noise-induced hearing loss in marine mammals: A review of temporary threshold shift studies from 1996 to 2015. J. Acoust. Soc. Am. 2015, 138, 1702–1726. [Google Scholar] [CrossRef]
- Finneran, J.J. Auditory Weighting Functions and TTS/PTS Exposure Functions for Marine Mammals Exposed to Underwater Noise; SSC Pacific: San Diego, CA, USA, 2016. [Google Scholar]
- Southall, B.L.; Finneran, J.J.; Reichmuth, C.; Nachtigall, P.E.; Ketten, D.R.; Bowles, A.E.; Ellison, W.T.; Nowacek, D.P.; Tyack, P.L. Marine Mammal Noise Exposure Criteria: Updated Scientific Recommendations for Residual Hearing Effects. Aquat. Mamm. 2019, 45, 125–232. [Google Scholar] [CrossRef]
- De Robertis, A.; Higginbottom, I. A post-processing technique to estimate the signal-to-noise ratio and remover echosounder background noise. ICES J. Mar. Sci. 2007, 64, 1282–1291. [Google Scholar] [CrossRef]
- Knudsen, H. Long-term evaluation of scientific-echosounder performance. ICES J. Mar. Sci. 2009, 66, 1335–1340. [Google Scholar]
- Greenaway, S.F. Linearity Tests if a Multibeam Echosounder. Masters Thesis, University of New Hampshire, Durham, NH, USA, 2010; p. 71. [Google Scholar]
- McCarthy, E.; Moretti, D.; Thomas, L.; DiMarzio, N.; Morrissey, R.; Jarvis, S.; Ward, J.; Izzi, A.; Dilley, A. Changes in spatial and temporal distribution and vocal behavior of Blainville’s beaked whales (Mesoplodon densirostris) during multiship exercises with mid-frequency sonar. Mar. Mammal. Sci. 2011, 27, E206–E226. [Google Scholar] [CrossRef]
- DeRuiter, S.L.; Southall, B.L.; Calambokidis, J.; Zimmer, W.M.X.; Sadykova, D.; Falcone, E.A.; Friedlaender, A.S.; Joseph, J.E.; Moretti, D.; Schorr, G.S.; et al. First direct measurements of behavioural responses by Cuvier’s beaked whales to mid-frequency active sonar. Biol. Lett. 2013, 9, 20130223. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.M.; Morrisey, R.P.; Moretti, D.J.; DiMarzio, N.A.; Shaffer, J.A. Marine mammal monitoring on navy ranges (M3R): A toolset for automated detection, localization, and monitoring of marine mammals in open ocean environments. Mar. Technol. Soc. J. 2014, 48, 5–20. [Google Scholar] [CrossRef]
- Manzano-Roth, R.; Henderson, E.; Martin, S.; Martin, C.; Matsuyama, B. Impacts of U.S. navy training events on Blainville’s beaked whale (Mesoplodon densirostris) foraging dives in Hawaiian waters. Aquat. Mamm. 2016, 42, 507–518. [Google Scholar] [CrossRef]
- Falcone, E.A.; Schorr, G.S.; Watwood, S.L.; DeRuiter, S.; Zerbini, A.; Andrews, R.D.; Morrissey, R.P.; Moretti, D.J. Diving behaviour of Cuvier’s beaked whales exposed to two types of military sonar. R. Soc. Open Sci. 2017, 4, 170629. [Google Scholar] [CrossRef] [PubMed]
- DiMarzio, N.; Watwood, S.; Fetherston, T.; Moretti, D. Marine Mammal Monitoring on Navy Ranges (M3R) on the Southern California Anti- Submarine Warfare Range (SOAR) and the Pacific Missile Range Facility (PMRF) 2018; Naval Undersea Warfare Center: Newport, RI, USA, 2019. [Google Scholar]
- Frantzis, A. Does acoustic testing strand whales? Nature 1998, 392, 29. [Google Scholar] [CrossRef]
- Evans, D.L.; England, G.R. Joint Interim Report Bahamas Marine Mammal Stranding Event of 15–16 March 2000, U.S.; Department of Commerce and Secretary of the Navy: Washington, DC, USA, 2001. [Google Scholar]
- D’Amico, A.; Gisiner, R.C.; Ketten, D.R.; Hammock, J.A.; Johnson, C.; Tyack, P.L.; Mead, J. Beaked whale strandings and naval exercises. Aquat. Mamm. 2009, 35, 452–472. [Google Scholar] [CrossRef]
- Fernandez, A.; Sierra, E.; Martin, V.; Mendez, M.; Sacchinnin, S.; Bernaldo de Quiros, Y.; Andrada Borzollino, M.A.; Bolaños Rivero, M.; Quesada Canales, I.Ó.; Tejedor, M.; et al. Last “atypical” beaked whales mass stranding in the Canary Islands (July, 2004). J. Mar. Sci. Res. Dev. 2012, 2, 107. [Google Scholar] [CrossRef]
- Vires, G. Echosounder Effects on Beaked Whales in the Tongue of the Ocean, Bahamas. Masters Thesis, Duke University, Durham, NH, USA, 2011; p. 40. [Google Scholar]
- Cholewiak, D.; DeAngelis, A.I.; Palka, D.; Corkeron, P.J.; Van Parijs, S.M. Beaked whales demonstrate a marked acoustic response to the use of shipboard echosounders. R. Soc. Open Sci. 2017, 4, 170940. [Google Scholar] [CrossRef]
- Quick, N.; Scott-Hayward, L.; Sadykova, D.; Nowacek, D.; Read, A. Effects of a scientific echo sounder on the behavior of short finned pilot whales (Globicephala macrorhynchus). Can. J. Fish. Aquat. Sci. 2017, 74, 716–726. [Google Scholar] [CrossRef]
- Varghese, H.K.; Miksis-Olds, J.; DiMarzio, N.; Lowell, K.; Linder, E.; Mayer, L.; Moretti, D. The Effect of two 12 kHz Multibeam mapping surveys on the foraging behavior of Cuvier’s beaked whales off of Southern California. J. Acoust. Soc. Am. 2020, 147, 3849–3858. [Google Scholar] [CrossRef]
- Ketten, D.R. Sonars and strandings: Are beaked whales the aquatic acoustic canary? Acoust. Today 2014, 10, 45–55. [Google Scholar]
- Olesiuk, P.F.; Lawson, J.W.; Trippel, E.A. Pathways of effects of noise associated with aquaculture on natural marine ecosystems in Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2012, 6, 64. [Google Scholar]
- Be Whale Wise. Marine Wildlife Guidelines. Available online: http://www.bewhalewise.org/marine-wildlife-guidelines/ (accessed on 27 February 2021).
- Au, W.W.L.; Ford, J.K.B.; Horne, J.K.; Allman, K.A.N. Echolocation signals of free-ranging killer whales (Orcinus orca) and modeling of foraging for chinook salmon (Oncorhynchus tshawytscha). J. Acoust. Soc. Am. 2004, 115, 901–909. [Google Scholar] [CrossRef]
- Heise, K.A.; Barrett-Lennard, L.G.; Chapman, N.R.; Dakin, D.T.; Erbe, C.; Hannay, D.E.; Merchant, N.D.; Pilkington, J.S.; Thornton, S.J.; Tollit, D.J.; et al. Proposed Metrics for the Management of Underwater Noise for Southern Resident Killer Whales; Coastal Ocean Report Series (2); Ocean Wise: Vancouver, BC, Canada, 2017; p. 30. [Google Scholar]
- Soldevilla, M.S.; Henderson, E.E.; Campbell, G.S.; Wiggins, S.M.; Hildebrand, J.A.; Roch, M.A. Classification of Risso’s and Pacific white-sided dolphins using spectral properties of echolocation clicks. J. Acoust. Soc. Am. 2008, 127, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Basset, H.R.; Baumann, S.; Campbell, G.S.; Wiggins, S.M.; Hildebrand, J.A. Dall’s porpoise (Phocoenoides dalli) echolocation click spectral structure. J. Acoust. Soc. Am. 2009, 125, 2677. [Google Scholar] [CrossRef]
- Miller, L.A.; Wahlber, M. Echolocation by the harbour porpoise: Life in coastal waters. Front. Physiol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Farcas, A.; Thompson, P.M.; Merchant, N.D. Underwater noise modelling for environmental impact assessment. Environ. Impact Assess. Rev. 2016, 57, 114–122. [Google Scholar] [CrossRef]
- Olson, J.K.; Wood, J.; Osborne, R.W.; Barrett-Lennard, L.; Larson, S. Sightings of southern resident killer whales in the Salish Sea 1976−2014: The importance of a long-term opportunistic dataset. Endang. Species Res. 2018, 37, 37,105–118. [Google Scholar] [CrossRef]
- Cominelli, S.; Devillers, R.; Yurk, H.; MacGillivray, A.; McWhinnie, L.; Canessa, R. Noise exposure from commercial shipping for the southern resident killer whale population. Mar. Poll. Bull. 2018, 136, 177–200. [Google Scholar] [CrossRef]
- Department of Fisheries and Oceans Canada, DFO. Identification of areas for mitigation of vessel-related threats to survival and recovery for Southern Resident Killer Whales. DFO Can. Advis. Sec. Sci. Advis. Re. 2021, 2021, 25. [Google Scholar]
- Holt, M.M.; Tennessen, J.B.; Ward, E.J.; Hanson, M.B.; Emmons, C.K.; Giles, D.A.; Hogan, J.T. Effects of vessel distance and sex on the behavior of endangered killer whales. Front. Mar. Sci. 2021, 7, 1211. [Google Scholar] [CrossRef]
- Herlinveaux, R.H. Oceanography of Saanich Inlet in Vancouver Island, British Columbia. J. Fish. Res. Board Canada 1962, 19, 1–37. [Google Scholar] [CrossRef]
- Gargett, A.E.; Stucchi, D.; Whitney, F. Physical processes associated with high primary production in Saanich Inlet, British Columbia. Estuarine Coast. Shelf Sci. 2003, 56, 1141–1156. [Google Scholar] [CrossRef]
- Transport Canada. Government of Canada Announces Second Year of Enhanced Measures to Protect Southern Resident Killer Whales. Available online: https://www.canada.ca/en/transport-canada/news/2020/05/government-of-canada-announces-second-year-of-enhanced-measures-to-protect-southern-resident-killer-whales.html (accessed on 27 February 2020).
- Baird, R.W.; Hanson, M.B.; Ashe, E.E.; Heithaus, M.R.; Marshall, G.J. Studies of foraging in ‘southern resident’ killer whales during July 2002: Dive depths, bursts in speed, and the use of a ‘crittercam’ system for examining sub-surface behaviour. In Report Prepared under Order Number AB 133F-02-SE-1744 for the National Marine Fisheries Service; National Marine Fisheries Service, National Marine Mammal Laboratory: Seattle, WA, USA, 2003. [Google Scholar]
- Baird, R.W.; Hanson, M.B.; Dill, L.M. Factors influencing the diving behaviour of fish eating killer whales: Sex differences and diel and interannual variation in diving rates. Can. J. Zool. 2005, 83, 257–267. [Google Scholar] [CrossRef]
- Wright, B.M.; Ford, J.K.B.; Ellis, G.M.; Deecke, V.B.; Shapiro, A.D.; Battaile, B.C.; Trites, A.W. Fine-scale foraging movements by fish-eating killer whales (Orcinus orca) relate to the vertical distributions and escape responses of salmonid prey (Oncorhynchus spp.). Move Ecol. 2017, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Tennessen, J.B.; Holt, M.M.; Hansen, M.B.; Emmons, C.K.; Giles, D.A.; Hogan, J.T. Kinematic signatures of prey capture from archival tags reveal sex differences in killer whale foraging activity. J. Exp. Bio. 2019, 222, jeb191874. [Google Scholar] [CrossRef]
- Tennessen, J.B.; Holt, M.M.; Ward, E.J.; Hansen, M.B.; Emmons, C.K.; Giles, D.A.; Hogan, J.T. Hidden Markov models reveal temporal patterns and sex difference in killer whale behaviour. Nature Sci. Rep. 2019, 9, 14951. [Google Scholar]
- Merchant, N.D.; Fristrup, K.M.; Johnson, M.P.; Tyack, P.L.; Witt, M.J.; Blondel, P.; Parks, S.E. Measuring acoustic habitats. Methods Ecol. Evol. 2015, 6, 257–265. [Google Scholar] [CrossRef]
- Wladichuk, J.L.; Hannay, D.E.; MacGillivray, A.O.; Li, Z.; Thornton, S. Systematic Source Level Measurements of Whale Watching and other small boats. J. Ocean Tech. 2018, 14, 108–126. [Google Scholar]
- Burnham, R.E.; Vagle, S.; O’Neill, C. Spatiotemporal patterns in the natural and anthropogenic additions to the soundscape in parts of the Salish Sea, British Columbia, 2018–2020. Mar. Poll. Bull. 2021, 170, 112647. [Google Scholar] [CrossRef]
- Branstetter, B.; St Leger, J.; Acton, D.; Stewart, J.; Houser, D.; Finneran, J.J.; Jenkins, K. Killer whale (Orcinus orca) behavioral audiograms. J. Acoust. Soc. Am. 2017, 141, 2387. [Google Scholar] [CrossRef]
- Nachtigall, P.E.; Pawloski, J.L.; Au, W.W.L. Temporary threshold shifts and recovery following noise exposure in the Atlantic bottlenosed dolphin (Tursiops truncatus). J. Acoust. Soc. Am. 2003, 113, 3425–3429. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Nieuwstraten, S.H.; Staal, C.; van Ligtenberg, C.L.; Versteegh, D. Low- frequency aerial hearing of a harbour porpoise (Phocoena phocoena). In The Biology of the Harbour Porpoise; Read, A.J., Wiepkema, P.R., Nachtigall, P.E., Eds.; De Spil: Woerden, The Netherlands, 1997; pp. 295–312. [Google Scholar]
- Verboom, W.C.; Kastelein, R.A. Structure of harbour porpoise (Phocoena phocoena) acoustic signals with high repetition rates. In Echolocation in Bats and Dolphins; Thomas, J.A., Moss, C., Vater, M., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 40–43. [Google Scholar]
- Szymanski, M.D.; Bain, D.E.; Kiehl, K.; Pennington, S.; Wong, S.; Henry, K.R. Killer whale (Orcinus orca) hearing: Auditory brainstem response and behavioral audiograms. J. Acoust. Soc. Am. 1999, 106, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.C.; Robinson, S.P.; Goldsmith, M.J. A new equation for the accurate calculation of sound speed in all oceans. J. Acoust. Soc. Am. 2008, 124, 2774–2782. [Google Scholar] [CrossRef]
- Vagle, S.; Burnham, R.E.; O’Neill, C.; Yurk, H. Variability in anthropogenic underwater noise due to bathymetry and sound speed characteristics. J. Mar. Sci. Engin. 2021, 9, 1047. [Google Scholar] [CrossRef]
- Childerhouse, S.; Douglas, L. Review of multibeam echosounder surveys and marine mammals. In Report Prepared by Blue Planet Marine; BPM: Canberra, Australia, 2016; Document Reference Number: BPM-16-MDC. [Google Scholar]
- Stimpert, A.; DeRuiter, S.; Southall, B.; Moretti, D.; Falcone, E.; Goldbogen, J.; Friedlaender, A.; Schorr, G.; Calambokidis, J. Acoustic and foraging behavior of a Baird’s beaked whale, Berardius bairdii, exposed to simulated sonar. Sci. Rep. 2014, 4, 7031. [Google Scholar] [CrossRef]
- Miller, P.J.; Kvadsheim, P.H.; Lam, F.-P.A.; Wensveen, P.J.; Antunes, R.; Alves, A.C.; Visser, F.; Kleivane, L.; Tyack, P.L.; Silve, L.D. The severity of behavioural changes observed during experimental exposures of killer (Orcinus orca), Long-Finned Pilot (Globicephala melas) and Sperm (Physeter macrocephalus) Whles to Naval Sonar. Aquat. Mamm. 2012, 38, 362–401. [Google Scholar] [CrossRef]
- Rendell, L.; Gordon, J. Vocal response of long-finned pilot whales (Globicephala melas) to military sonar in the Ligurian Sea. Mar. Mamm. Sci. 1999, 15, 198–204. [Google Scholar] [CrossRef]
- Alves, A.; Antunes, R.; Bird, A.; Tyack, P.; Miller, P.; Lam, F.; Kvadsheim, P. Vocal matching of naval sonar signals by long-finned pilot whales (Globicephala melas). Mar. Mamm. Sci. 2014, 30, 1248–1257. [Google Scholar] [CrossRef]
- Ridgway, S.H.; Carder, D.; Finneran, J.; Keough, M.; Kamolnick, T.; Todd, M.; Goldblatt, A. Dolphin continuous auditory vigilance for five days. J. Exp. Biol. 2006, 209, 3621–3628. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.M.; Carder, D.A.; Ridgway, S.H. Vigilance in female bottlenose dolphins (Tursiops sp.) before and after calving. J. Comp. Psychol. 2008, 21, 35–57. [Google Scholar]
- Williams, R.; Erbe, C.; Ashe, E.; Beerman, A.; Smith, J. Severity of killer whale behavioral responses to ship noise: A dose-response study. Mar. Poll. Bull. 2014, 79, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Joy, R.; Tollit, D.; Wood, J.; MacGillivray, A.; Li, Z.; Trounce, K.; Robinson, O. Potential benefits of vessel slowdowns on endangered southern resident killer whales. Front. Mar. Sci. 2019, 6, 344. [Google Scholar] [CrossRef]
- Lusseau, D.; Bain, D.E.; Williams, R.; Smith, J.C. Vessel traffic disrupts the foraging behavior of southern resident killer whales Orcinus orca. Endang. Species Res. 2009, 6, 211–221. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Hazen, E.L.; Golbogen, J.A.; Stimpert, A.K.; Calambokidis, J.; Southall, B. Prey-mediated behavioral responses of feeding blue whales in controlled sound exposure experiments. Ecol. App. 2016, 26, 1075–1085. [Google Scholar] [CrossRef]
- Isojunno, S.; Curé, C.; Kvadsheim, P.H.; Lam, F.-P.A.; Tyack, P.; Wensveen, P.J.; Miller, P.J.O. Sperm whales reduce foraging effort during exposure to 1–2 kHz sonar and killer whale sounds. Ecol. Appl. 2016, 26, 77–93. [Google Scholar] [CrossRef]
- Wisniewska, D.M.; Johnson, M.; Teilmann JSiebert, U.; Galatius, A.; Dietz, R.; Madsen, P.T. High rates of vessel noise disrupt foraging in wild harbour porpoises (Phocoena phocoena). Proc. R. Soc. B 2018, 285, 20172314. [Google Scholar] [CrossRef]
- Koski, K.; Osborne, R.; Tallmon, R. Soundwatch Public Outreach/Boater Education Project 2004–2005 Final Program Report, NMFS Contract No. AB133F-04-SE-0835 (Report No. NFFP 5000-4-00026); The Whale Museum: Friday Harbor, WA, USA, 2006; p. 25. [Google Scholar]
- Holt, M.M.; Noren, D.P.; Veirs, V.; Emmons, C.K.; Veirs, S. Speaking up: Killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoust. Soc. Am. 2009, 125, EL27–EL32. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnham, R.; Vagle, S.; Van Buren, P.; Morrison, C. Spatial Impact of Recreational-Grade Echosounders and the Implications for Killer Whales. J. Mar. Sci. Eng. 2022, 10, 1267. https://doi.org/10.3390/jmse10091267
Burnham R, Vagle S, Van Buren P, Morrison C. Spatial Impact of Recreational-Grade Echosounders and the Implications for Killer Whales. Journal of Marine Science and Engineering. 2022; 10(9):1267. https://doi.org/10.3390/jmse10091267
Chicago/Turabian StyleBurnham, Rianna, Svein Vagle, Peter Van Buren, and Christie Morrison. 2022. "Spatial Impact of Recreational-Grade Echosounders and the Implications for Killer Whales" Journal of Marine Science and Engineering 10, no. 9: 1267. https://doi.org/10.3390/jmse10091267
APA StyleBurnham, R., Vagle, S., Van Buren, P., & Morrison, C. (2022). Spatial Impact of Recreational-Grade Echosounders and the Implications for Killer Whales. Journal of Marine Science and Engineering, 10(9), 1267. https://doi.org/10.3390/jmse10091267







