Molecular Characterization and Expression Analysis of Novel Interleukin-1 Family Member (nIL-1Fm) Gene in Nile Tilapia (Oreochromis niloticus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Fish
2.2. Identification and Cloning of nIL-1Fm
2.3. Bioinformatics Analysis of nIL-1Fm
2.4. Tissue Distribution of nIL-1Fm by Quantitative Real-Time PCR (qRT-PCR)
2.5. Modulation of the Expression of Nile Tilapia nIL-1Fm In Vivo by Bacterial S. agalactiae Infection
2.6. Modulation of nIL-1Fm Expression in PBL by PAMPs
2.7. Statistical Analysis
3. Results
3.1. Sequence Analysis of the nIL-1Fm Gene
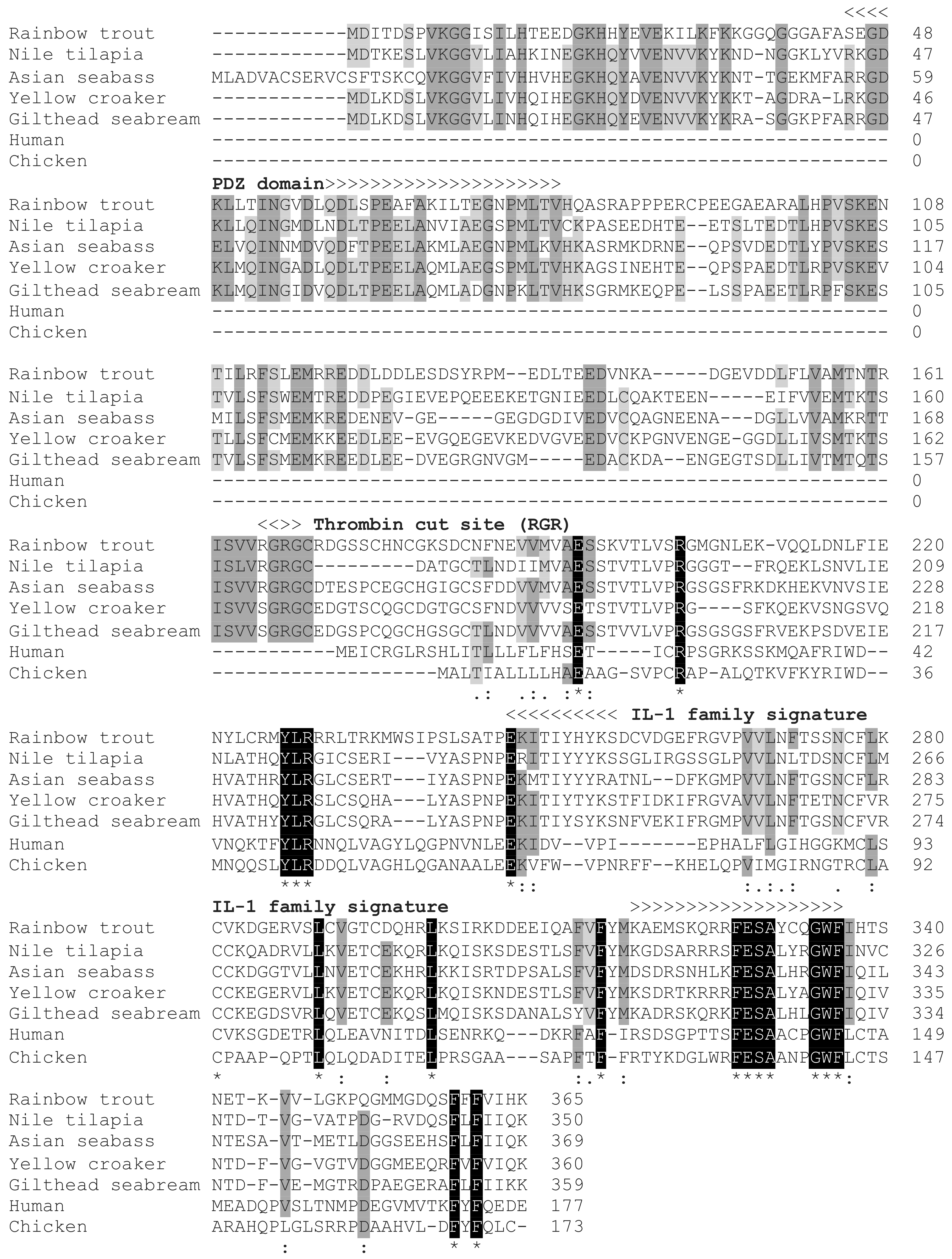
3.2. Multiple Sequence Alignment and Synteny Analysis
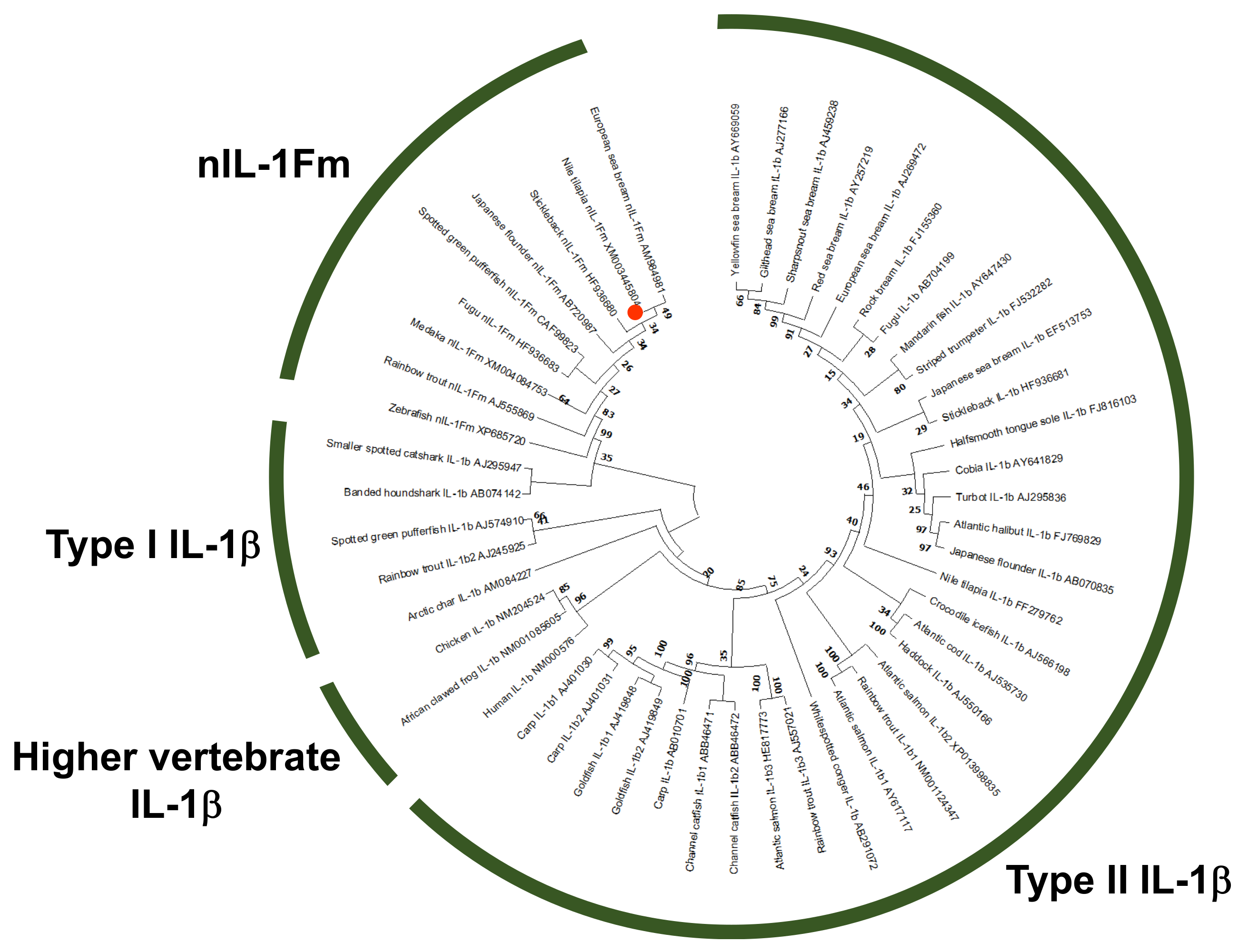
3.3. Phylogenetic Analysis of nIL-1Fm
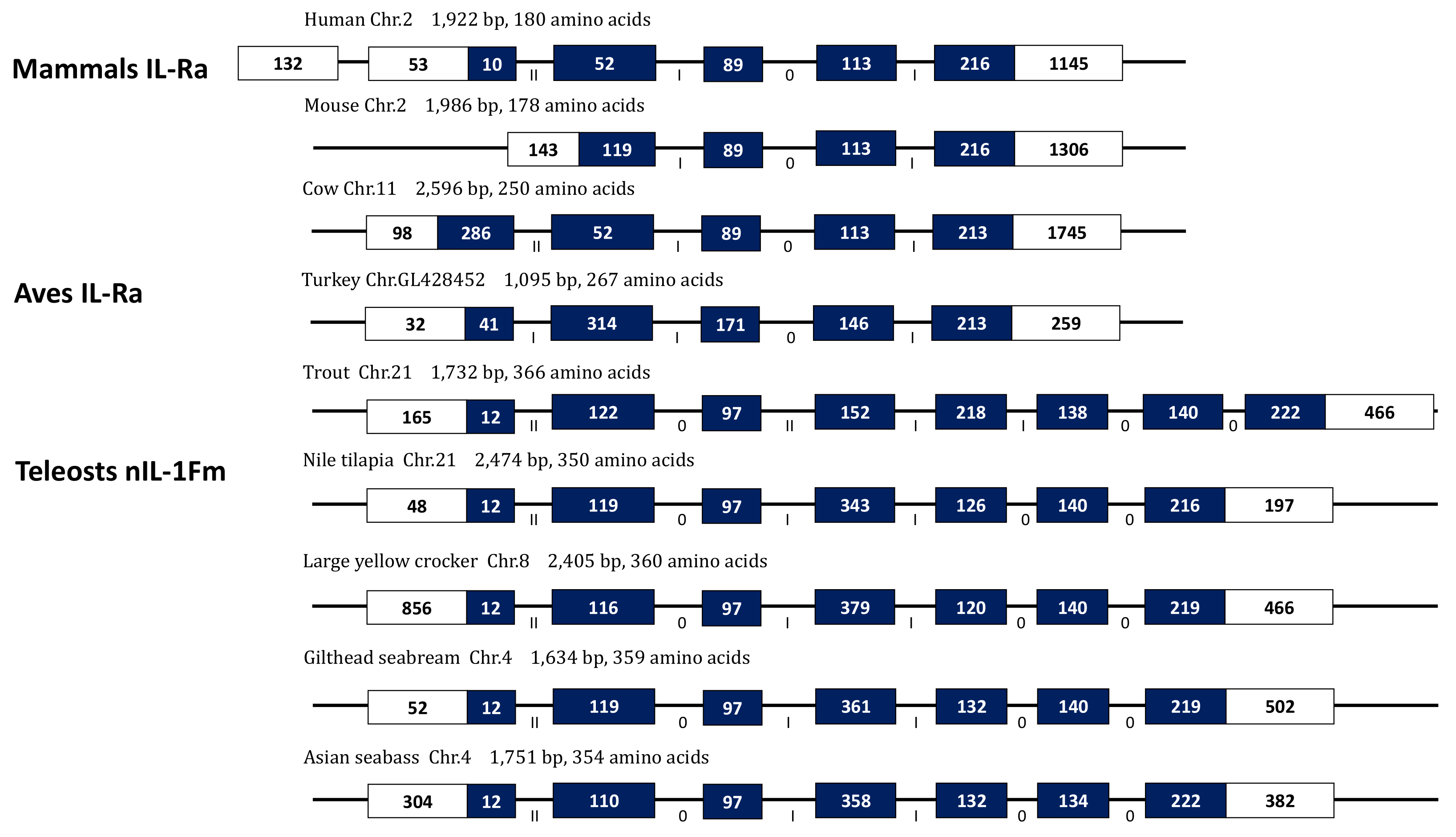
3.4. Gene Organization of Nile Tilapia nIL-1Fm
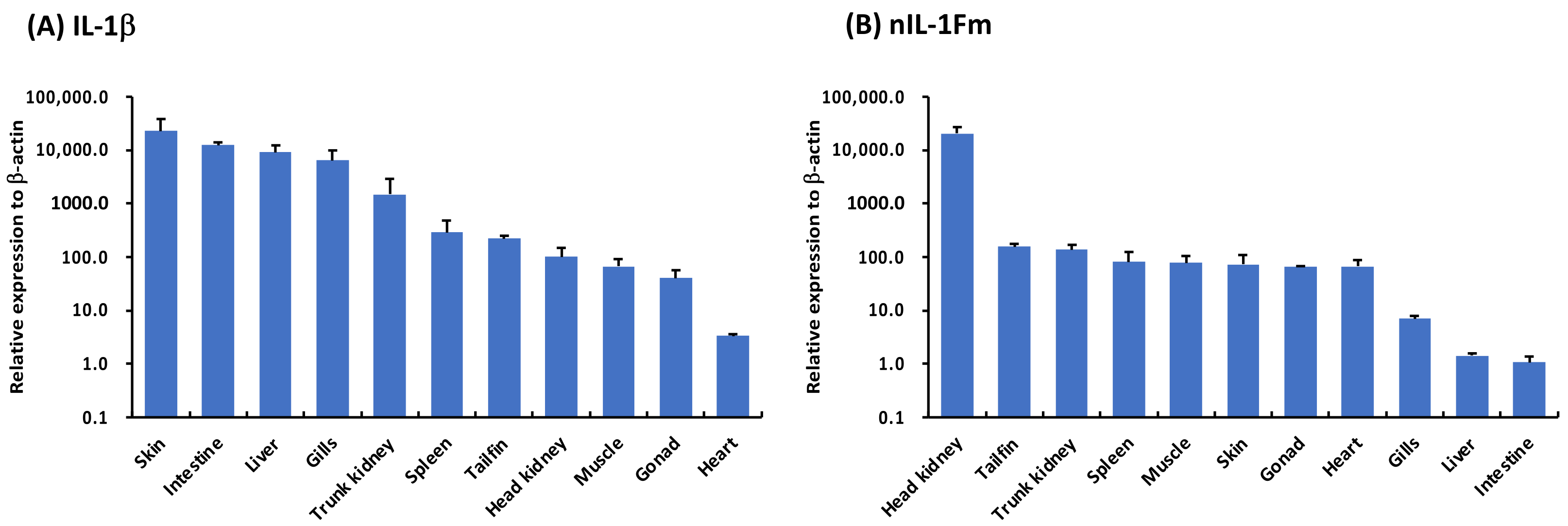
3.5. Tissue Distribution
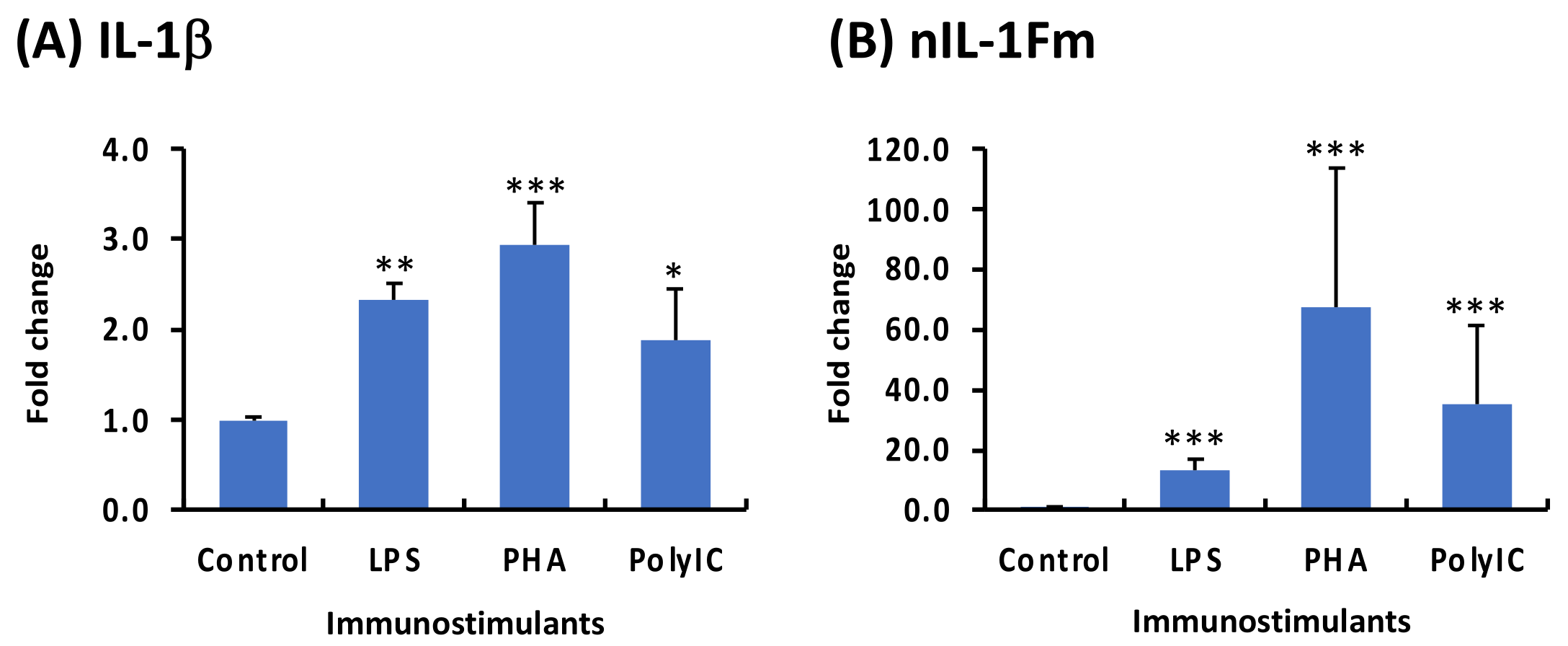
3.6. Modulation of IL-1β and nIL-1Fm Expression to PAMPs in PBLs
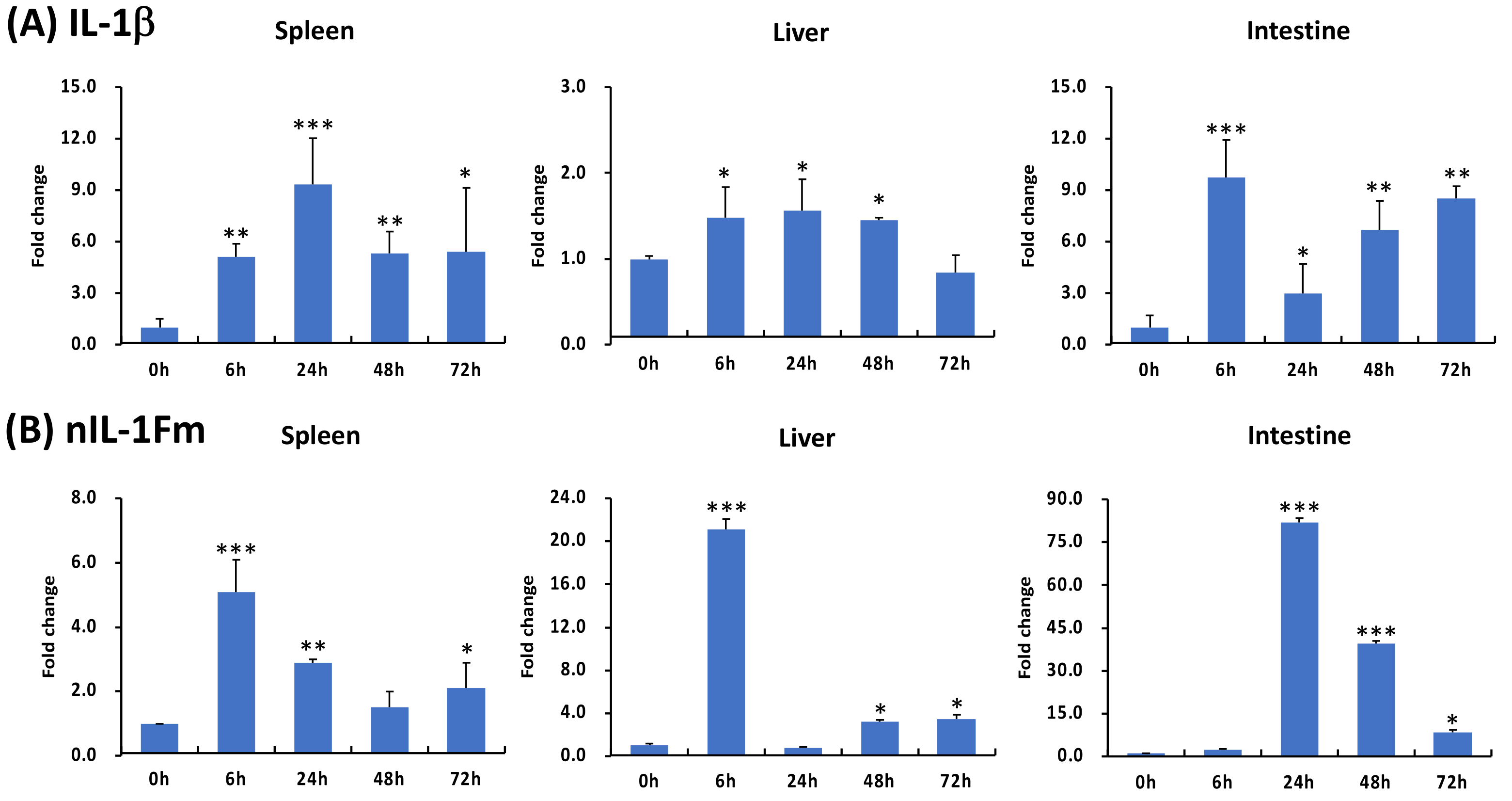
3.7. Expression Profiles of IL-1β and nIL-1Fm in Response to LPS
3.8. Expression of IL-1β and nIL-1Fm under S. agalactiae Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boersma, B.; Jiskoot, W.; Lowe, P.; Bourquin, C. The interleukin-1 cytokine family members: Role in cancer pathogenesis and potential therapeutic applications in cancer immunotherapy. Cytokine Growth Factor Rev. 2021, 62, 1–14. [Google Scholar] [PubMed]
- Dinarello, C.A. The history of fever, leukocytic pyrogen and interleukin-1. Temperature 2015, 2, 8–16. [Google Scholar]
- March, C.J.; Mosley, B.; Larsen, A.; Cerretti, D.P.; Braedt, G.; Price, V.; Gillis, S.; Henney, C.S.; Kronheim, S.R.; Grabstein, K.; et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature 1985, 315, 641–647. [Google Scholar] [PubMed]
- Lin, H.; Ho, A.S.; Haley-Vicente, D.; Zhang, J.; Bernal-Fussell, J.; Pace, A.M.; Hansen, D.; Schweighofer, K.; Mize, N.K.; Ford, J.E. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J. Biol. Chem. 2001, 276, 20597–20602. [Google Scholar]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar]
- Secombes, C.J.; Wang, T.; Bird, S. The interleukins of fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of interleukin-1b. Cytokine Growth Factor Rev. 2002, 13, 483–502. [Google Scholar]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar]
- Secombes, C.; Zou, J.; Daniels, G.; Cunningham, C.; Koussounadis, A.; Kemp, G. Rainbow trout cytokine and cytokine receptor genes. Immunol. Rev. 1998, 166, 333–340. [Google Scholar]
- Fujiki, K.; Shin, D.H.; Nakao, M.; Yano, T. Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1β, high affinity immunoglobulin E Fc receptor γ subunit and serum amyloid A. Fish Shellfish Immunol. 2000, 10, 229–242. [Google Scholar]
- Pelegrín, P.; García-Castillo, J.; Mulero, V.; Meseguer, J. Interleukin-1β isolated from a marine fish reveals up-regulated expression in macrophages following activation with lipopolysaccharide and lymphokines. Cytokine 2001, 16, 67–72. [Google Scholar] [CrossRef]
- Emmadi, D.; Iwahori, A.; Hirono, I.; Aoki, T. cDNA microarray analysis of interleukin-1β-induced Japanese flounder Paralichthys olivaceus kidney cells. Fish Sci. 2005, 71, 519–530. [Google Scholar]
- Wang, Y.; Wang, Q.; Baoprasertkul, P.; Peatman, E.; Liu, Z. Genomic organization, gene duplication, and expression analysis of interleukin-1β in channel catfish (Ictalurus punctatus). Mol. Immunol. 2006, 43, 1653–1664. [Google Scholar] [PubMed]
- Lee, D.S.; Hong, S.H.; Lee, H.J.; Jun, L.J.; Chung, J.K.; Kim, K.H.; Do Jeong, H. Molecular cDNA cloning and analysis of the organization and expression of the IL-1β gene in the Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. Part A Mol. Integr. 2006, 143, 307–314. [Google Scholar]
- Corripio-Miyar, Y.; Bird, S.; Tsamopoulos, K.; Secombes, C.J. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus). Mol. Immunol. 2007, 44, 1361–1373. [Google Scholar] [PubMed]
- Lu, D.Q.; Bei, J.X.; Feng, L.N.; Zhang, Y.; Liu, X.C.; Wang, L.; Chen, J.L.; Lin, H.R. Interleukin-1β gene in orange-spotted grouper, Epinephelus coioides: Molecular cloning, expression, biological activities and signal transduction. Mol. Immunol. 2008, 45, 857–867. [Google Scholar]
- Seppola, M.; Larsen, A.N.; Steiro, K.; Robertsen, B.; Jensen, I. Characterisation and expression analysis of the interleukin genes, IL-1β, IL-8 and IL-10, in Atlantic cod (Gadus morhua L.). Mol. Immunol. 2008, 45, 887–897. [Google Scholar]
- Polinski, M.; Bridle, A.; Nowak, B. Temperature-induced transcription of inflammatory mediators and the influence of Hsp70 following LPS stimulation of southern bluefin tuna peripheral blood leukocytes and kidney homogenates. Fish Shellfish Immunol. 2013, 34, 1147–1157. [Google Scholar]
- Yao, F.; Yang, X.; Wang, X.; Wei, H.; Zhang, A.; Zhou, H. Molecular and functional characterization of an IL-1β receptor antagonist in grass carp (Ctenopharyngodon idella). Dev. Comp. Immunol. 2015, 49, 207–216. [Google Scholar]
- Bird, S.; Wang, T.; Zou, J.; Cunningham, C.; Secombes, C.J. The first cytokine sequence within cartilaginous fish: IL-1b in the small spotted catshark (Scyliorhinus canicula). J. Immunol. 2002, 168, 3329–3340. [Google Scholar] [PubMed]
- Wang, T.; Bird, S.; Koussounadis, A.; Holland, J.W.; Carrington, A.; Zou, J.; Secombes, C.J. Identification of a novel IL-1 cytokine family member in teleost fish. J. Immunol. 2009, 183, 962–974. [Google Scholar] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G. New insights in the immunobiology of IL-1 family members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Angosto, D.; Montero, J.; Lopez-Munoz, A.; Alcaraz-Perez, F.; Bird, S.; Sarropoulou, E.; Abellán, E.; Meseguer, J.; Sepulcre, M.P.; Mulero, V. Identification and functional characterization of a new IL-1 family member, IL-1Fm2, in most evolutionarily advanced fish. Innate Immun. 2014, 20, 487–500. [Google Scholar] [PubMed]
- Taechavasonyoo, A.; Kondo, H.; Nozaki, R.; Suzuki, Y.; Hirono, I. Identification of novel interleukin 1 beta family genes in Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2013, 34, 393–396. [Google Scholar] [PubMed]
- Pleguezuelos, O.; Zou, J.; Cunningham, C.; Secombes, C.J. Cloning, sequencing, and analysis of expression of a second IL-1 β gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics 2000, 51, 1002–1011. [Google Scholar]
- Scapigliati, G.; Buonocore, F.; Bird, S.; Zou, J.; Pelegrin, P.; Falasca, C.; Prugnoli, D.; Secombes, C.J. Phylogeny of cytokines: Molecular cloning and expression analysis of sea bass Dicentrarchus labrax interleukin-1β. Fish Shellfish Immunol. 2001, 11, 711–726. [Google Scholar]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune response and protective efficacy of two new adjuvants, Montanide™ ISA 763B VG and Montanide™ GEL02, administered with a Streptococcus agalactiae ghost vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 116, 19–29. [Google Scholar]
- Wangkahart, E.; Bruneel, B.; Wisetsri, T.; Nontasan, S.; Martin, S.A.; Chantiratikul, A. Interactive effects of dietary lipid and nutritional emulsifier supplementation on growth, chemical composition, immune response and lipid metabolism of juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737341. [Google Scholar]
- Wangkahart, E.; Wachiraamonloed, S.; Lee, P.T.; Subramani, P.A.; Qi, Z.; Wang, B. Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 120, 402–410. [Google Scholar]
- Wang, B.; Wangkahart, E.; Secombes, C.J.; Wang, T. Insights into the evolution of the suppressors of cytokine signaling (SOCS) gene family in vertebrates. Mol. Biol. Evol. 2019, 36, 393–411. [Google Scholar]
- Louis, A.; Muffato, M.; Roest Crollius, H. Genomicus: Five genome browsers for comparative genomics in eukaryota. Nucleic Acids Res. 2012, 41, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, Y.; Wangkahart, E.; Zou, J.; Chang, M.; Yang, D.; Secombes, C.J.; Nie, P.; Wang, T. Sequence and expression analysis of interferon regulatory factor 10 (IRF10) in three diverse teleost fish reveals its role in antiviral defense. PLoS ONE 2016, 11, e0147181. [Google Scholar] [CrossRef]
- Wangkahart, E.; Scott, C.; Secombes, C.J.; Wang, T. Re-examination of the rainbow trout (Oncorhynchus mykiss) immune response to flagellin: Yersinia ruckeri flagellin is a potent activator of acute phase proteins, anti-microbial peptides and pro-inflammatory cytokines in vitro. Dev. Comp. Immunol. 2016, 57, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Benedicenti, O.; Wang, T.; Wangkahart, E.; Milne, D.J.; Holland, J.W.; Collins, C.; Secombes, C.J. Characterisation of arginase paralogues in salmonids and their modulation by immune stimulation/infection. Fish Shellfish Immunol. 2017, 61, 138–151. [Google Scholar] [CrossRef]
- Hu, Y.; Maisey, K.; Subramani, P.A.; Liu, F.; Flores-Kossack, C.; Imarai, M.; Secombes, C.J.; Wang, T. Characterisation of rainbow trout peripheral blood leucocytes prepared by hypotonic lysis of erythrocytes, and analysis of their phagocytic activity, proliferation and response to PAMPs and proinflammatory cytokines. Dev. Comp. Immunol. 2018, 88, 104–113. [Google Scholar] [CrossRef]
- Subramaniam, S.; Stansberg, C.; Cunningham, C. The interleukin 1 receptor family. Dev. Comp. Immunol. 2004, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Tagliabue, A. The interleukin-1 receptor family. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2013; Volume 25, pp. 394–407. [Google Scholar]
- Dinarello, C.A.; Thompson, R.C. Blocking IL-1: Interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today 1991, 12, 404–410. [Google Scholar] [CrossRef]
- Fanning, A.S.; Anderson, J.M. Protein–protein interactions: PDZ domain networks. Curr. Biol. 1996, 6, 1385–1388. [Google Scholar] [CrossRef]
- Eisenberg, S.P.; Brewer, M.T.; Verderber, E.; Heimdal, P.; Brandhuber, B.J.; Thompson, R.C. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: Evolution of a cytokine control mechanism. Proc. Natl. Acad. Sci. USA 1991, 88, 5232–5236. [Google Scholar] [CrossRef] [Green Version]
- Rivers-Auty, J.; Daniels, M.J.; Colliver, I.; Robertson, D.L.; Brough, D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat. Commun. 2018, 9, 1156. [Google Scholar] [CrossRef]
- Hurme, M.; Santtila, S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. Eur. J. Immunol. 1998, 28, 2598–2602. [Google Scholar] [CrossRef]
- Martin, S.A.; Dehler, C.E.; Król, E. Transcriptomic responses in the fish intestine. Dev. Comp. Immunol. 2016, 64, 103–117. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chang, C.J.; Karger, A.; Keller, M.; Pfaff, F.; Wangkahart, E.; Wang, T.; Secombes, C.J.; Kimoto, A.; Furihata, M.; et al. Ancient cytokine interleukin 15-like (IL-15L) induces a type 2 immune response. Front. Immunol. 2020, 5, 2384. [Google Scholar] [CrossRef]
- Wangkahart, E.; Secombes, C.J.; Wang, T. Dissecting the immune pathways stimulated following injection vaccination of rainbow trout (Oncorhynchus mykiss) against enteric redmouth disease (ERM). Fish Shellfish Immunol. 2019, 85, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Wangkahart, E.; Secombes, C.J.; Wang, T. Studies on the use of flagellin as an immunostimulant and vaccine adjuvant in fish aquaculture. Front. Immunol. 2019, 9, 3054. [Google Scholar] [CrossRef]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14. [Google Scholar] [CrossRef]



| Gene Name | Primer Name | Primer Sequence (5′–3′) | Application |
|---|---|---|---|
| nIL-1Fm | Full IL-1X F | ATGGACACGAAGGAGTCTCTGGT | Cloning |
| Full IL-1X R | TCACTTTTGAATGATGAAGAGGAA | Cloning | |
| IL-1X F | GGAGGATGACCCGGAGGGAA | qRT-PCR | |
| IL-1X R | ATCACAGCCCCTGCCTCTCA | qRT-PCR | |
| IL-1β | IL-1B F | AAGATGAATTGTGGAGCTGTGTT | qRT-PCR |
| IL-1B R | AAAAGCATCGACAGTATGTGAAAT | qRT-PCR | |
| β-actin | β-actin F | ACAGGATGCAGAAGGAGATCACAG | qRT-PCR |
| β-actin R | GTACTCCTGCTTGCTGATCCACAT | qRT-PCR |
| Species Name | GenBank ID | Length (bp) | ORF (AA) | Mw (kDa)/pI | Similarity (%) | Identity (%) |
|---|---|---|---|---|---|---|
| Mammalian IL-1Ra | ||||||
| Human | CAA36262 | 1740 | 177 | 20.05/5.82 | 17.7 | 11.7 |
| Rhesus monkey | XP_001091833 | 1723 | 177 | 19.89/6.89 | 19.6 | 12.9 |
| Bird IL-1Ra | ||||||
| Chicken | CCD83119 | 554 | 173 | 19.37/8.68 | 19.7 | 9.3 |
| Duck | XP_032060067 | 515 | 162 | 18.17/7.74 | 22.1 | 11.6 |
| Fish nIL-1Fm | ||||||
| Nile tilapia | ON568309 | 2474 | 350 | 39.12/4.97 | - | - |
| Common carp | XM_042734003 | 1766 | 353 | 40.40/5.22 | 47.8 | 32.6 |
| Fathead minnow | XP_039529760 | 2016 | 347 | 39.07/5.13 | 49.1 | 33.4 |
| Grass carp | KM066966 | 1260 | 347 | 39.48/4.96 | 49.1 | 33.4 |
| Zebrafish | NP_001277347 | 1071 | 356 | 39.55/5.17 | 49.6 | 32.6 |
| Channel catfish | XP_017314635 | 2034 | 354 | 39.35/5.45 | 51.7 | 35.7 |
| Rainbow trout | AJ555869 | 1736 | 365 | 41.18/5.67 | 60.0 | 45.4 |
| Medaka | XM004084753 | 1380 | 348 | 39.57/7.53 | 65.1 | 47.4 |
| Fugu | HF936683 | 1122 | 373 | 42.27/5.21 | 67.8 | 50.7 |
| Flounder | AB720987 | 1083 | 354 | 39.83/5.15 | 68.8 | 50.4 |
| Asian seabass | XP_018539191 | 2161 | 354 | 39.77/5.59 | 69.8 | 55.4 |
| Gilthead seabream | XP_030271237 | 1879 | 359 | 39.82/5.63 | 73.1 | 57.3 |
| Yellow croaker | XP_010730145 | 1785 | 360 | 39.97/5.30 | 76.1 | 61.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangkahart, E.; Jumpalueang, S.; Ardprachan, S.; Phudkliang, J.; Sunthamala, P.; Pholchamat, S.; Qi, Z. Molecular Characterization and Expression Analysis of Novel Interleukin-1 Family Member (nIL-1Fm) Gene in Nile Tilapia (Oreochromis niloticus). J. Mar. Sci. Eng. 2022, 10, 1272. https://doi.org/10.3390/jmse10091272
Wangkahart E, Jumpalueang S, Ardprachan S, Phudkliang J, Sunthamala P, Pholchamat S, Qi Z. Molecular Characterization and Expression Analysis of Novel Interleukin-1 Family Member (nIL-1Fm) Gene in Nile Tilapia (Oreochromis niloticus). Journal of Marine Science and Engineering. 2022; 10(9):1272. https://doi.org/10.3390/jmse10091272
Chicago/Turabian StyleWangkahart, Eakapol, Sukanda Jumpalueang, Supanniga Ardprachan, Janjira Phudkliang, Phitcharat Sunthamala, Sirinya Pholchamat, and Zhitao Qi. 2022. "Molecular Characterization and Expression Analysis of Novel Interleukin-1 Family Member (nIL-1Fm) Gene in Nile Tilapia (Oreochromis niloticus)" Journal of Marine Science and Engineering 10, no. 9: 1272. https://doi.org/10.3390/jmse10091272





