Species Diversity and Community Structure of Macrobenthos in the Ulleung Basin, East Sea, Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Processing
2.3. Comparison with Previous Studies
2.4. Data Processing
3. Results
3.1. Environmental Variability
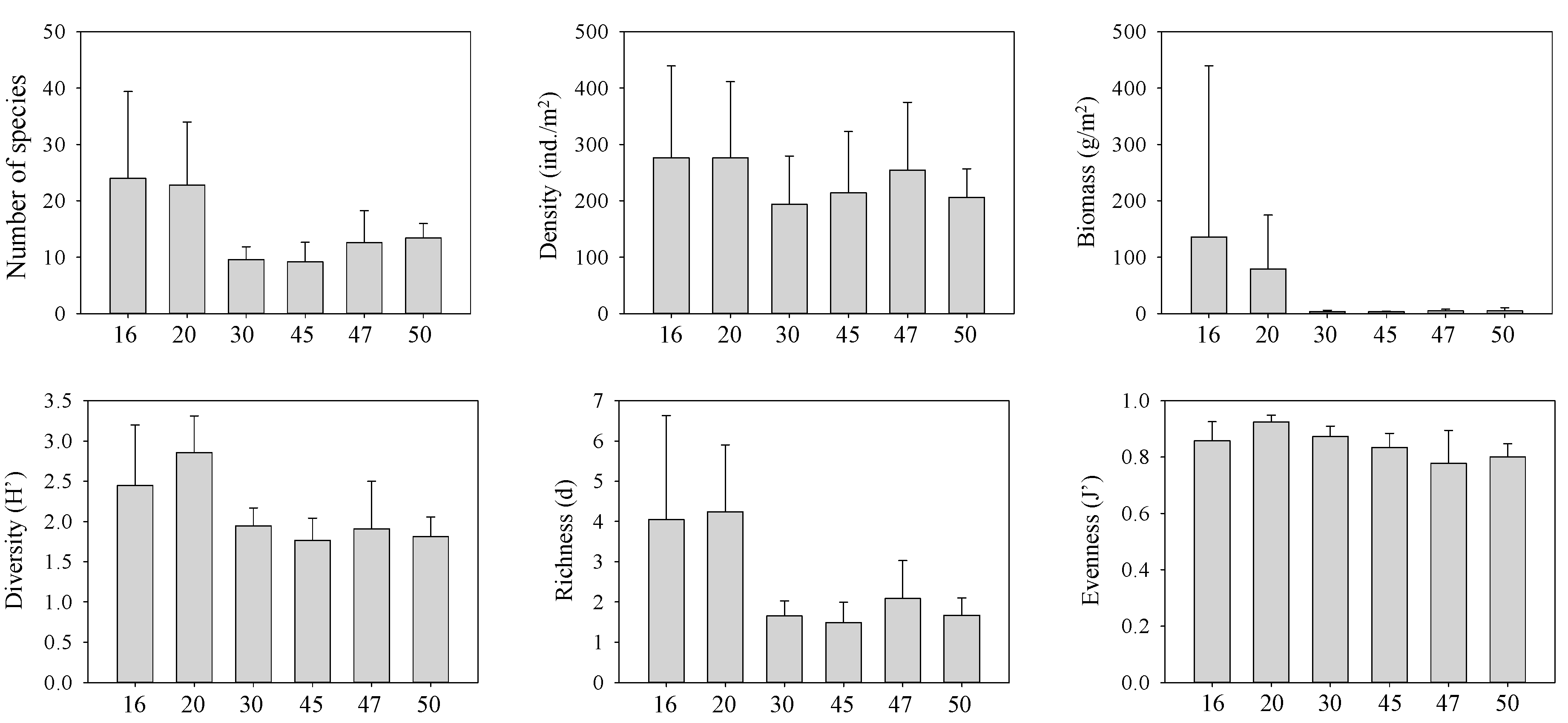
3.2. Macrobenthos
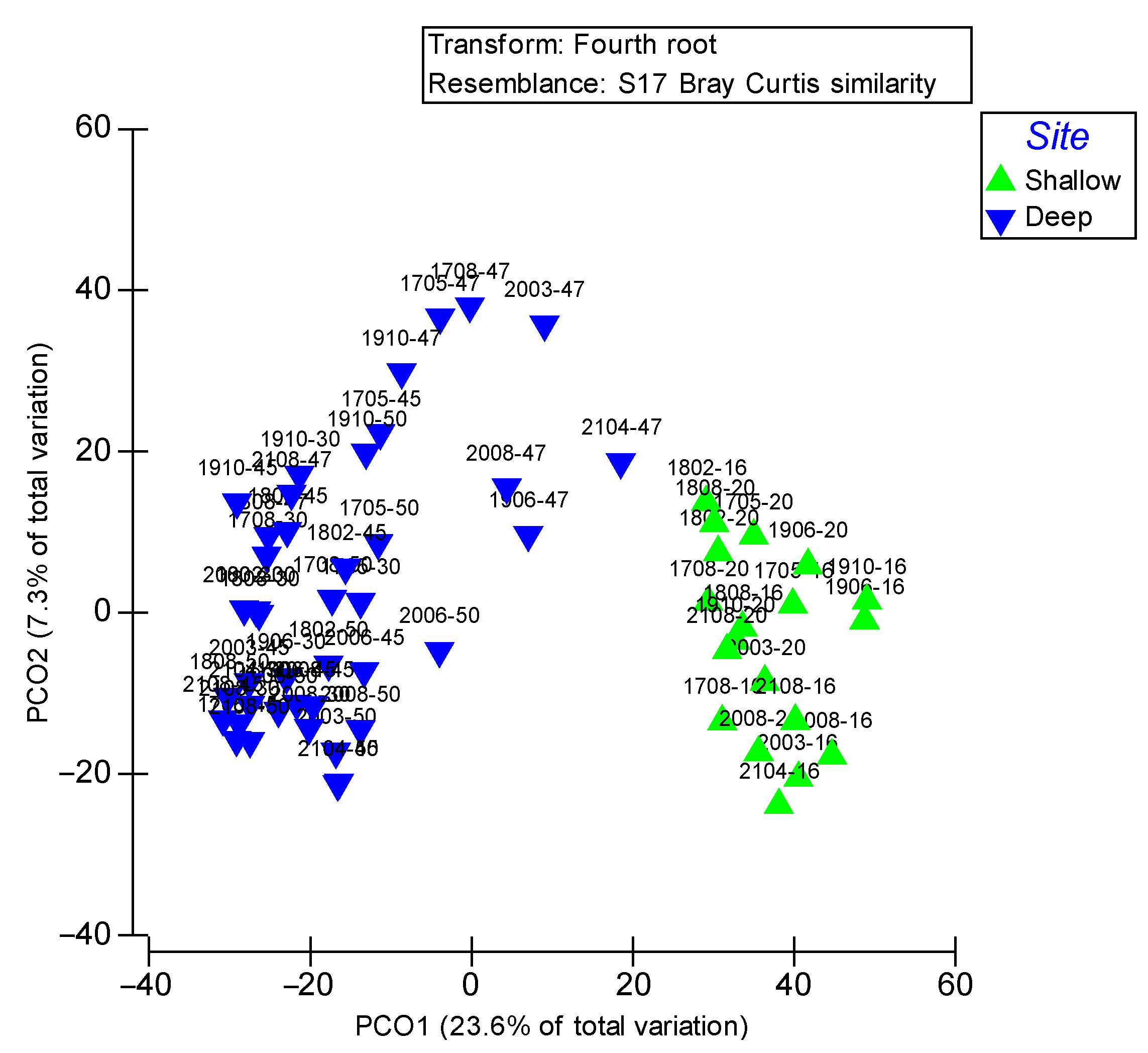
3.3. Community Structure
3.4. Patterns of Macrobenthos Assemblages
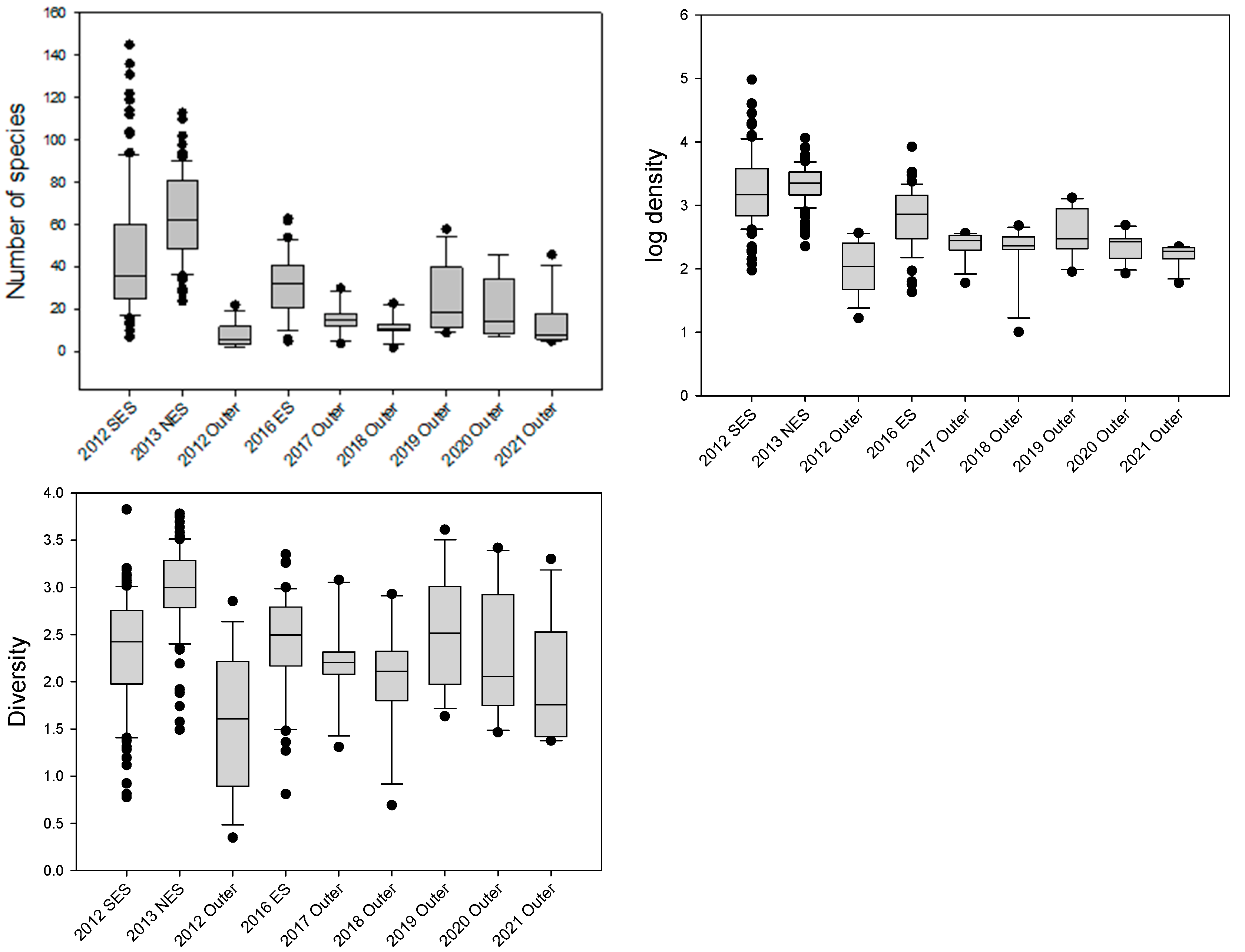
3.5. Comparison with Previous Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.K.; Niiler, P.P. The energetic surface circulation patterns of the Japan/East Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2005, 52, 1547–1563. [Google Scholar] [CrossRef]
- Onishi, M.; Ohtani, K. Volume Transport of the Tsushima Warm Current, West of Tsugaru Strait Bifurcation Area. J. Ocean. 1997, 53, 27–34. [Google Scholar] [CrossRef]
- Kawabe, M. Study of Kuroshio and Tsushima Current. J. Oceanogr. 1986, 42, 319–331. [Google Scholar] [CrossRef]
- Jenkins, W.J. The biogeochemical consequences of changing ventilation in the Japan/East Sea. Mar. Chem. 2008, 108, 137–147. [Google Scholar] [CrossRef]
- Senjyu, T. The Japan sea intermediate water, its characteristics and circulation. J. Oceanogr. 1999, 55, 111–122. [Google Scholar] [CrossRef]
- Morimoto, A.; Yanagi, T. Variability of sea surface circulation in the Japan sea. J. Oceanogr. 2001, 57, 1–13. [Google Scholar] [CrossRef]
- Lee, T.-S.; Kim, I.-N. Chemical Imprints of the Upwelled Waters off the Coast of the Southern East Sea of Korea. Ocean. Sci. J. 2003, 38, 101–110. [Google Scholar]
- Hyun, J.H.; Kim, D.; Shin, C.W.; Noh, J.H.; Yang, E.J.; Mok, J.S.; Kim, S.H.; Kim, H.C.; Yoo, S. Enhanced phytoplankton and bacterioplankton production coupled to coastal upwelling and an anticyclonic eddy in the Ulleung basin, East Sea. Aquat. Microb. Ecol. 2009, 54, 45–54. [Google Scholar] [CrossRef]
- Shin, H.R.; Shin, C.W.; Kim, C.; Byun, S.K.; Hwang, S.C. Movement and structural variation of warm eddy WE92 for three years in the Western East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2005, 52, 1742–1762. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.R.; Min, D.H.; Volkov, Y.; Yoon, J.H.; Takematsu, M. Warming and structural changes in the east (Japan) sea: A clue to future changes in global oceans? Geophys. Res. Lett. 2001, 28, 3293–3296. [Google Scholar] [CrossRef]
- Postlethwaite, C.F.; Rohling, E.J.; Jenkins, W.J.; Walker, C.F. A tracer study of ventilation in the Japan/East Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2005, 52, 1684–1704. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Watts, D.R.; Wimbush, M.; Teague, W.J.; Tracey, K.L.; Book, J.W.; Chang, K.I.; Suk, M.S.; Yoon, J.H. Upper circulation patterns in the Ulleung Basin. Deep. Res. Part II Top. Stud. Oceanogr. 2005, 52, 1617–1638. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hwang, J.S.; Choy, E.J.; Park, H.J.; Kang, D.J.; Lee, T.S.; Chang, K.I.; Kim, K.R.; Kang, C.K. High primary productivity and f-ratio in summer in the Ulleung basin of the East/Japan Sea. Deep. Res. Part I Oceanogr. Res. Pap. 2013, 79, 74–785. [Google Scholar] [CrossRef]
- Chang, K.I.; Teague, W.J.; Lyu, S.J.; Perkins, H.T.; Lee, D.K.; Watts, D.R.; Kim, Y.B.; Mitchell, D.A.; Lee, C.M.; Kim, K. Circulation and currents in the southwestern East/Japan Sea: Overview and review. Prog. Oceanogr. 2004, 61, 105–156. [Google Scholar] [CrossRef]
- Yoo, S.J.; Park, J.S. Why is the southwest the most productive region of the East Sea/Sea of Japan? J. Mar. Syst. 2009, 78, 301–315. [Google Scholar] [CrossRef]
- Cha, J.; Kim, M. A preliminary study for the distribution of rocky intertidal fauna in the Korean coastal areas of the East Sea including Dokdo and Ulleungdo. Korean J. Environ. Biol 2013, 31, 225–231. [Google Scholar] [CrossRef]
- Song, S.J.; Park, J.; Ryu, J.; Rho, H.S.; Kim, W.; Khim, J.S. Biodiversity hotspot for marine invertebrates around the Dokdo, East Sea, Korea: Ecological checklist revisited. Mar. Pollut. Bull. 2017, 119, 162–170. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, H.G.; Kim, S.L.; Choi, J.W.; Park, C.H.; Yu, O.H. Species composition and community structure of macrobenthos during fall on the Dokdo coast, Korea. Ocean Polar Res. 2019, 41, 47–61. [Google Scholar] [CrossRef]
- Kim, S.T.; Hwang, K.S.; Park, G.J.; Choi, C.G. Benthic algal community of Ulleungdo, East coast of Korea. J. Fish. Mar. Sci. Educ. 2016, 28, 83–90. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, K.T. Studies on the Seaweeds in the Islands of Ullungdo and Dokdo: I. Decrease of Algal Species Compositions and Changes of Marine Algal Flora. Korean Soc. Phycol. 2000, 15, 199. [Google Scholar]
- Snelgrove, P.; Blackburn, T.; Hutchings, P. The importance of marine sediment biodiversity in ecosystem processes. Ambio 1997, 26, 578–583. [Google Scholar]
- Dauer, D.M. Biological criteria, environmental health and estuarine macrobenthic community structure. Mar. Pollut. Bull. 1993, 26, 249–257. [Google Scholar] [CrossRef]
- Hu, C.; Dong, J.; Gao, L.; Yang, X.; Wang, Z.; Zhang, X. Macrobenthos functional trait responses to heavy metal pollution gradients in a temperate lagoon. Environ. Pollut. 2019, 253, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Patrício, J.; Neto, J.M.; Teixeira, H.; Salas, F.; Marques, J.C. The robustness of ecological indicators to detect long-term changes in the macrobenthos of estuarine systems. Mar. Environ. Res. 2009, 68, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauvin, J.C.; Ruellet, T. The estuarine quality paradox: Is it possible to define an ecological quality status for specific modified and naturally stressed estuarine ecosystems? Mar. Pollut. Bull. 2009, 59, 38–47. [Google Scholar] [CrossRef]
- Pearson, T.; Rosenberg, H. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–311. [Google Scholar]
- Gray, J.S. Species richness of marine soft sediments. Mar. Ecol. Prog. Ser. 2002, 244, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Rodil, I.F.; Lohrer, A.M.; Chiaroni, L.D.; Hewitt, J.E.; Thrush, S.F. Disturbance of sandflats by thin terrigenous sediment deposits: Consequences for primary production and nutrient cycling. Ecol. Appl. 2011, 21, 416–426. [Google Scholar] [CrossRef]
- Kim, S.L.; Yu, O.H. Benthic polychaete community structure in the Yellow Sea Bottom Cold Water zone (YSBCW): Species diversity, temporal-spatial distribution, and feeding guilds. Mar. Pollut. Bull. 2022, 183, 114071. [Google Scholar] [CrossRef]
- Yu, O.H.; Lee, H.G.; Lee, J.H.; Kim, K.T.; Myung, C.S.; Moon, H.T.; Byun, J.Y. Spatial variation in macrobenthic communities affected by the thermal discharge volumes of a nuclear power plant on the East Coast of Korea. Ocean Polar Res. 2013, 35, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.L.; Lee, H.G.; Yu, O.H. Correlation between rocky reefs and surrounding benthic habitats: Distribution and diversity patterns of polychaetes in the macrobenthic community in the East Sea of South Korea. J. Sea Res. 2021, 174, 102083. [Google Scholar] [CrossRef]
- Choi, J.W.; Hyun, S.M.; Kim, D.S.; Kim, W.S. Macrobenthic faunal assemblages on the soft-bottoms around Dokdo in the East Sea, Korea. Ocean Polar Res. 2002, 24, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Folk, R.; Ward, W. Brazos River bar: A study in the significance of grain-size parameters. J. Sediment Pet. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- KIOST. The Report on a Sustainable Research and Development of Dokdo; Report; Ministry Land, Transport and Maritime Affairs: Seoul, Republic of Korea, 2012; p. 1033. [Google Scholar]
- KOEM. General Investigation of Marine Ecosystem in the Southeastern Sea of Korea; BSPN11-119; Ministry of Oceans and Fisheries: Seoul, Republic of Korea, 2013; p. 600. [Google Scholar]
- KOEM. General Investigation of Marine Ecosystem in the Northeastern Sea of Korea; BSPN11-119; Ministry of Oceans and Fisheries: Seoul, Republic of Korea, 2014; p. 665. [Google Scholar]
- Gotelli, N.; Chao, A. Measuring and estimating species richness, species diversity, and biotic similarity from sampling data. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Colwell, R.K. (Ed.) EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, version 9; User’s Guide and Application; 2013. Available online: http://purl.oclc.org/estimates (accessed on 10 September 2022).
- Dejong, T.M. A Comparison of Three Diversity Indices Based on Their Components of Richness and Evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Chapman, M.G. Relationships between spatial patterns of benthic assemblages in a mangrove forest using different levels of taxonomic resolution. Mar. Ecol. Prog. Ser. 1998, 162, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Hyun, S.; Kim, C.H.; Jou, H.; Kim, J.; Park, C.H. Characteristics of surface sediments distribution and submarine topography around Dokdo Island, Korea. J. Geol. Soc. Korea 2010, 46, 647–660. [Google Scholar]
- Manasrah, R.; Raheed, M.; Badran, M.I. Relationships between water temperature, nutrients and dissolved oxygen in the northern Gulf of Aqaba, Red Sea. Oceanologia 2006, 48, 237–253. [Google Scholar]
- Silva, A.C.F.; Tavares, P.; Shapouri, M.; Stigter, T.Y.; Monteiro, J.P.; Machado, M.; Cancela da Fonseca, L.; Ribeiro, L. Estuarine biodiversity as an indicator of groundwater discharge. Estuar. Coast. Shelf Sci. 2012, 97, 38–43. [Google Scholar] [CrossRef]
- Musale, A.S.; Desai, D.V.; Sawant, S.S.; Venkat, K.; Anil, A.C. Distribution and abundance of benthic macroorganisms in and around Visakhapatnam Harbour on the east coast of India. J. Mar. Biol. Assoc. United Kingd. 2015, 95, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.G.; Kang, R.S.; Jeon, J.O.; Lee, J.-H.H.; Yun, S.G. Distribution patterns of Sandy Bottom Macobenthic Community on the Hupo Coastal Area, in the East Sea of Korea. Ocean Polar Res. 2007, 29, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Joydas, T.V.; Manokaran, S.; Borja, A.; Rabaoui, L.; Manikandan, K.P.; Ashraf, T.T.M.; Aarif, K.M.; Hussain, S.A.; Baig, M.H.; Shoeb, M.; et al. A baseline quantitative assessment of deep-sea benthic fauna of the Gulf of Aqaba (Northern Saudi Arabia, Red Sea). Mar. Pollut. Bull. 2021, 164, 112028. [Google Scholar] [CrossRef] [PubMed]
- Lambshead, P.J.D.; Brown, C.J.; Ferrero, T.J.; Mitchell, N.J.; Smith, C.R.; Hawkins, L.E.; Tietjen, J. Latitudinal diversity patterns of deep-sea marine nematodes and organic fluxes: A test from the central equatorial pacific. Mar. Ecol. Prog. Ser. 2002, 236, 129–135. [Google Scholar] [CrossRef]
- Smith, C.R.; De Leo, F.C.; Bernardino, A.F.; Sweetman, A.K.; Arbizu, P.M. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 2008, 23, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Boorman, B.; Jones, D. Deep-Sea Benthic Sampling. In Methods for the Study of Marine Benthos, 4th ed.; Eleftheriou, A., Ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Hayashi, I.; Sugino, N. Some observations on the sampling efficiency of the Smith-McIntyre grab. Bull. Jpn. Soc. Sci. Fish 1993, 59, 1201–1207. [Google Scholar] [CrossRef]
- Vetriani, C.; Jannasch, H.W.; Macgregor, B.J.; Stahl, D.A.; Reysenbach, A.L. Population structure and phylogenetic characterization of marine benthic Archaea in deep-sea sediments. Appl. Environ. Microbiol. 1999, 65, 4375–4384. [Google Scholar] [CrossRef] [Green Version]
- Gage, J.; Tyler, P. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Hunter, W.R.; Levin, L.A.; Kitazato, H.; Witte, U. Macrobenthic assemblage structure and organismal stoichiometry control faunal processing of particulate organic carbon and nitrogen in oxygen minimum zone sediments. Biogeosciences 2012, 9, 993–1006. [Google Scholar] [CrossRef]
- Domínguez Castanedo, N.; Hernández Alcántara, P.; Solís-Weiss, V.; Granados Barba, A. Distribution of polychaete feeding guilds in sedimentary environments of the Campeche Bank, Southern Gulf of Mexico. Helgol. Mar. Res. 2012, 66, 469–478. [Google Scholar] [CrossRef]
- Pagliosa, P.R. Another diet of worms: The applicability of polychaete feeding guilds as a useful conceptual framework and biological variable. Mar. Ecol. 2005, 26, 246–254. [Google Scholar] [CrossRef]
- Dolbeth, M.; Teixeira, H.; Marques, J.C.; Pardal, M.Â. Feeding guild composition of a macrobenthic subtidal community along a depth gradient. Sci. Mar. 2009, 73, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Maurer, D.; Leathem, W.; Menzie, C. The impact of drilling fluid and well cuttings on polychaete feeding guilds from the US northeastern continental shelf. Mar. Pollut. Bull. 1981, 12, 342–347. [Google Scholar] [CrossRef]
- De Juan, S.; Thrush, S.F.; Demestre, M. Functional changes as indicators of trawling disturbance on a benthic community located in a fishing ground (NW Mediterranean Sea). Mar. Ecol. Prog. Ser. 2007, 334, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.; Moura, A.; Gaspar, M.B.; Pereira, P.; Cancela Da Fonseca, L.; Falcão, M.; Drago, T.; Leitão, F.; Regala, J. Spatial and inter-annual variability of the macrobenthic communities within a coastal lagoon (Óbidos lagoon) and its relationship with environmental parameters. Acta Oecologica 2005, 27, 143–159. [Google Scholar] [CrossRef]
- Carvalho, S.; Pereira, P.; Pereira, F.; de Pablo, H.; Vale, C.; Gaspar, M.B. Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Mar. Environ. Res. 2011, 71, 97–110. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.J.; Heck, K.L. Positive interactions between suspension-feeding bivalves and seagrass—A facultative mutualism. Mar. Ecol. Prog. Ser. 2001, 213, 143–155. [Google Scholar] [CrossRef]
- Kamenev, G.M. Species composition and distribution of bivalves in bathyal and abyssal depths of the Sea of Japan. Deep. Res. Part II Top. Stud. Oceanogr. 2013, 86–87, 124–139. [Google Scholar] [CrossRef]
- Ellingsen, K.E. Soft-sediment benthic biodiversity on the continental shelf in relation to environmental variability. Mar. Ecol. Prog. Ser. 2002, 232, 15–27. [Google Scholar] [CrossRef]
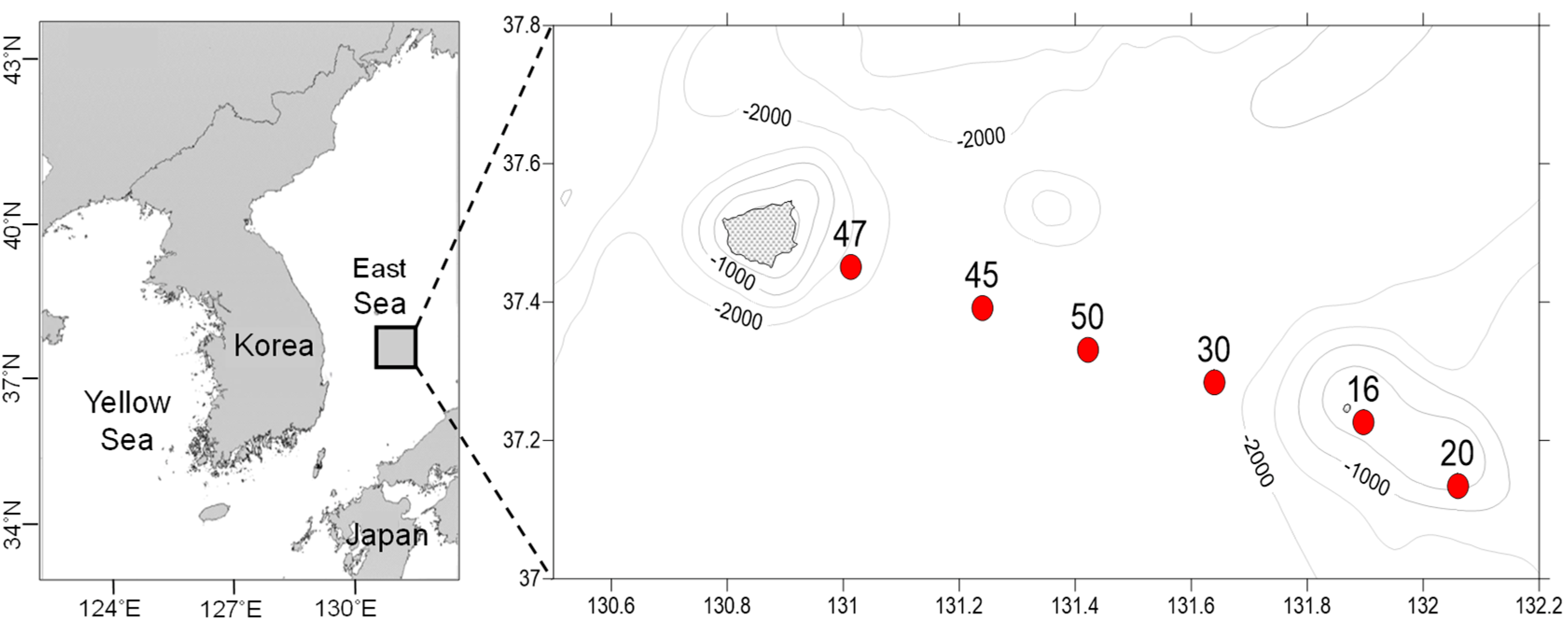


| Variables | st.16 | st.20 | st.30 | st.45 | st.47 | st.50 | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|---|---|---|---|---|---|
| Depth (m) | 192 | 173 | 2200 | 2233 | 1799 | 2344 | ||||
| Temperature (°C) | 5.4 ± 2.6 | 1.7 ± 0.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.8 ± 0.8 | 1.3 ± 0.8 | 1.2 ± 0.4 | 1.2 | 1.4 ± 0.4 |
| Salinity (psu) | 34.1 ± 0.1 | 34.0 ± 0.1 | 34.1 ± 0.1 | 34.1 ± 0.1 | 34.1 ± 0.1 | 34.1 ± 0.1 | 34.1 ± 0.1 | 34.1 ± 0.1 | 34.1 | 34.1 ± 0.1 |
| DO (mg/l) | 5.5 ± 0.8 | 5.5 ± 0.2 | 4.4 ± 0.6 | 4.5 ± 0.7 | 4.4 ± 0.4 | 4.8 ± 0.8 | 4.6 ± 0.3 | 4.9 ± 0.5 | 4.8 | 4.8 ± 0.1 |
| Gravel (%) | 8.9 ± 26.6 | 7.4 ± 6.8 | 0.9 ± 2.7 | 1.6 ± 0.1 | 7.5 ± 7.7 | 0.1 ± 0.1 | 4.8 ± 8.0 | 2.8 ± 1.3 | 4 | 3.9 ± 1.6 |
| Sand (%) | 80.6 ± 38.5 | 62.5 ± 21.7 | 12.4 ± 5.4 | 3.9 ± 31.3 | 16.5 ± 11.8 | 20.5 ± 31.3 | 30.1 ± 19.0 | 26.5 ± 13.8 | 29.8 | 30.5 ± 2.4 |
| Silt (%) | 1.5 ± 4.1 | 10.0 ± 6.6 | 21.6 ± 12.1 | 28.2 ± 14.7 | 21.6 ± 5.7 | 19.2 ± 14.5 | 21.7 ± 16.8 | 19.5 ± 6.2 | 18.4 | 18.7 ± 7.0 |
| Clay (%) | 9.0 ± 27.2 | 20.1 ± 22.6 | 65.1 ± 12.4 | 66.3 ± 24.9 | 54.4 ± 18.1 | 60.2 ± 26.0 | 43.3 ± 10.9 | 51.3 ± 17.5 | 47.8 | 46.9 ± 11.0 |
| Mean grain size (phi) | 2.0 ± 3.0 | 3.6 ± 2.6 | 8.4 ± 0.8 | 8.9 ± 2.6 | 7.2 ± 1.9 | 8.0 ± 2.6 | 6.3 ± 1.9 | 7 ± 1.4 | 6.7 | 6.5 ± 0.8 |
| TOC | 0.4 ± 0.5 | 0.9 ± 0.6 | 1.7 ± 0.9 | 2.0 ± 0.7 | 1.9 ± 0.6 | 1.8 ± 0.8 | 1.9 ± 0.6 | 1.4 ± 0.1 | 1.5 | 1.5 ± 0.1 |
| Taxon | st.16 | st.20 | st.30 | st.45 | st.47 | st.50 | Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Polychaete | 11.8 ± 7.0 | 14 ± 6.0 | 5.2 ± 0.8 | 5.4 ± 1.1 | 5.7 ± 2.1 | 5.5 ± 1.1 | 48.2 | 8.5 | 13.2 | 8 |
| Arthropoda | 4.6 ± 3.7 | 4.4 ± 2.6 | 1.3 ± 1.7 | 0.4 ± 0.8 | 2.9 ± 2.2 | 1.5 ± 1.8 | 5.3 | 2.5 | 2.6 | 2.1 | |
| Mollusca | 3.5 ± 3.5 | 3.3 ± 2.9 | 2.4 ± 1.3 | 2 ± 1.8 | 2.6 ± 2.1 | 2.3 ± 1.7 | 1.4 | 2.9 | 4.0 | 2.9 | |
| Echinoderms | 1.4 ± 1.2 | 1.7 ± 1.1 | 0.5 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.7 | 3 | 0.8 | 1.2 | 1 | |
| Others | 2.6 ± 2.5 | 1.3 ± 1.3 | 0.4 ± 0.7 | 0.6 ± 0.8 | 1.6 ± 1.8 | 0.6 ± 0.7 | 0.9 | 1.25 | 2.1 | 1.4 | |
| total | 23.9 ± 15.5 | 24.7 ± 11.2 | 9.8 ± 2.7 | 8.7 ± 2.8 | 13.1 ± 6.0 | 10.2 ± 3.1 | 8.8 | 16.1 | 16.9 | 15.4 | |
| Density (ind./m2) | Polychaete | 120 ± 70 | 154 ± 66 | 139 ± 57 | 154 ± 53 | 116 ± 64 | 138 ± 40 | 97 | 131.8 | 152.4 | 132.9 |
| Arthropoda | 50.3 ± 60.0 | 37.5 ± 28.0 | 8.2 ± 10.8 | 3.2 ± 7.7 | 20.5 ± 21.1 | 10.8 ± 14.9 | 31.6 | 19.6 | 21.2 | 11.4 | |
| Mollusca | 51.1 ± 56.1 | 26.8 ± 25.6 | 41.2 ± 35.9 | 30.9 ± 26.5 | 91 ± 75.9 | 63.6 ± 40.8 | 35.25 | 54.7 | 77.8 | 51.9 | |
| Echinoderms | 15.8 ± 21.3 | 21.2 ± 21.4 | 10.3 ± 14.9 | 2.5 ± 4.4 | 2.9 ± 5.2 | 2.4 ± 5.4 | 44.8 | 11.6 | 17.9 | 7.9 | |
| Others | 40.3 ± 37.8 | 13.7 ± 16.7 | 2 ± 3.4 | 7.6 ± 11.9 | 40.9 ± 59.6 | 3.4 ± 4.4 | 6.7 | 25.4 | 24.4 | 17.0 | |
| total | 278 ± 163. | 253 ± 120. | 201 ± 95 | 198 ± 81 | 272 ± 124 | 219 ± 72 | 160.6 | 243.3 | 287.1 | 221.3 | |
| Biomass (g/m2) | Polychaete | 10.8 ± 13.2 | 2.7 ± 2.1 | 2.1 ± 1.7 | 2.4 ± 1.4 | 3.5 ± 3.1 | 2.3 ± 2.5 | 7.1 | 4.2 | 6.4 | 1.7 |
| Arthropoda | 1.7 ± 3.0 | 0.2 ± 0.2 | 0.3 ± 0.8 | 0.1 ± 0.3 | 1.4 ± 3.9 | 0.1 ± 0.2 | 56.8 | 0.1 | 7.5 | 2.4 | |
| Mollusca | 7.4 ± 7.3 | 2.2 ± 5.2 | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.5 ± 0.5 | 1.6 ± 3.6 | 1.9 | 1.9 | 1.4 | 1.1 | |
| Echinoderms | 28.4 ± 75.2 | 50.7 ± 88.3 | 0.7 ± 0.7 | 0.2 ± 0.5 | 0.1 ± 0.1 | 0.24 ± 0.41 | 18.2 | 5.1 | 4.0 | 10.2 | |
| Others | 109 ± 298 | 10.6 ± 17.6 | 0.3 ± 0.7 | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.07 ± 0.19 | 52.9 | 2.2 | 12.3 | 14.3 | |
| total | 157 ± 296 | 66.3 ± 92.7 | 3.7 ± 2.5 | 3.5 ± 1.7 | 5.6 ± 4.2 | 4.3 ± 6.0 | 75.9 | 13.7 | 24.1 | 30.0 |
| Taxa | Species | Density (Individuals/m2) | % of Total Density | Frequency (%) |
|---|---|---|---|---|
| Polychaete | Chaetozone setosa | 1604.4 | 11.1 | 100.0 |
| Polychaete | Terebellides horikoshii | 1469.3 | 10.2 | 13.3 |
| Bivalvia | Axinopsida subquadrata | 982.3 | 6.8 | 86.7 |
| Polychaete | Aglaophamus sp. | 773.5 | 5.4 | 43.3 |
| Polychaete | Scalibregma inflatum | 740.1 | 5.1 | 80.0 |
| Polychaete | Sigambra bassi | 514.3 | 3.6 | 46.7 |
| Polychaete | Petaloproctus dentatus | 479.1 | 3.3 | 86.7 |
| Bivalvia | Yoldiella philippiana | 393.2 | 2.7 | 73.3 |
| Others | Phascolosoma sp. | 375.0 | 2.6 | 73.3 |
| Gastropoda | Decorifer matusimanus | 347.2 | 2.4 | 20.0 |
| Bivalvia | Thyasira tokunagai | 291.2 | 2.0 | 53.3 |
| Species | Average Dissimilarity | Contribution (%) | Cumulative (%) |
|---|---|---|---|
| Terebellides horikoshii | 4.13 | 4.49 | 4.49 |
| Chaetozone setosa | 3.01 | 3.27 | 7.76 |
| Scalibregma inflatum | 3 | 3.27 | 11.03 |
| Sigambra bassi | 2.68 | 2.92 | 13.95 |
| Axinopsida subquadrata | 2.56 | 2.79 | 16.74 |
| Glycera nicobarica | 2.25 | 2.45 | 19.19 |
| Aglaophamus sp. | 2.06 | 2.24 | 21.43 |
| Aricidea sp. | 1.32 | 1.43 | 22.86 |
| Aphelochaeta sp. | 1.26 | 1.37 | 24.23 |
| Notomastus latericeus | 1.18 | 1.28 | 25.51 |
| Petaloproctus dentatus | 1.17 | 1.27 | 26.78 |
| Decorifer matusimanus | 1.14 | 1.24 | 28.02 |
| Scoletoma nipponica | 1.12 | 1.21 | 29.24 |
| Yoldiella philippiana | 1.03 | 1.12 | 30.36 |
| Ophiuroglypha kinbergi | 1.01 | 1.1 | 31.46 |
| Synchelidium trioostegitum | 0.99 | 1.08 | 32.54 |
| Laqueus rubellus | 0.99 | 1.08 | 33.62 |
| Scoletoma fragilis | 0.98 | 1.06 | 34.68 |
| Anonyx simplex | 0.95 | 1.03 | 35.71 |
| Phascolosoma sp. | 0.92 | 1 | 36.71 |
| Number of Variables | Correlation (%) | Best Variables |
|---|---|---|
| 4 | 0.809 | Temperature, Salinity, Sand, Depth |
| 4 | 0.808 | Temperature, Salinity, Mz, Depth |
| 5 | 0.807 | Temperature, Salinity, Gravel, Sand, Depth |
| 4 | 0.807 | Temperature, Salinity, Gravel, Depth |
| 3 | 0.806 | Temperature, Salinity, Depth |
| 5 | 0.803 | Temperature, Salinity, Gravel, Mz, Depth |
| 1 | 0.799 | Temperature |
| 5 | 0.798 | Temperature, Salinity, Sand, Mz, Depth |
| 3 | 0.797 | Temperature, Salinity, Mz |
| 4 | 0.797 | Salinity, Gravel, Sand, Depth |
| Year | Area | Sampling Site | Sampling Number | Number of Species | Density (ind./m2) | Dominant Species | Reference |
|---|---|---|---|---|---|---|---|
| 2012 | Southern East Sea | Yeongdeok-Gijang | 27 | 458 | 4859 | (Apo) Spiophanes bombyx (Apo) Lumbrineris longifolia (Apo) Tharyx spp. (Apo) Polydora spp. | KOEM (2013) [35] |
| 2012 | East Sea | Ullungdo-Dokdo | 10 | 135 | 413 | (Cam) Byblis japonicus (Cam) Abludomelita denticulata (Apo) Syllidae sp. (Apo) Terebellides stroemii | KIOST (2012) [34] |
| 2013 | Northern East Sea | Goseong-Yeongdeok | 27 | 487 | 2603 | (Apo) Axinopsida subquadrata (Apo) Spiophanes bombyx (Apo) Magelona sp. | KOEM (2014) [36] |
| 2016 | East Sea | Dokdo | 15 | 177 | 1566 | (Cam) Abludomelita denticulata (Cam) Melita shimizui (Apo) Salvatoria clavata (MBi) Glycymeris aspersa | Kang et al., (2019) [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.L.; Kang, S.M.; Lee, H.G.; Han, G.H.; Yu, O.H. Species Diversity and Community Structure of Macrobenthos in the Ulleung Basin, East Sea, Republic of Korea. J. Mar. Sci. Eng. 2023, 11, 92. https://doi.org/10.3390/jmse11010092
Kim SL, Kang SM, Lee HG, Han GH, Yu OH. Species Diversity and Community Structure of Macrobenthos in the Ulleung Basin, East Sea, Republic of Korea. Journal of Marine Science and Engineering. 2023; 11(1):92. https://doi.org/10.3390/jmse11010092
Chicago/Turabian StyleKim, Sang Lyeol, Su Min Kang, Hyung Gon Lee, Gwan Hee Han, and Ok Hwan Yu. 2023. "Species Diversity and Community Structure of Macrobenthos in the Ulleung Basin, East Sea, Republic of Korea" Journal of Marine Science and Engineering 11, no. 1: 92. https://doi.org/10.3390/jmse11010092





