A Comprehensive Review on Material Compatibility and Safety Standards for Liquid Hydrogen Cargo and Fuel Containment Systems in Marine Applications
Abstract
:1. Introduction
2. Issues with Metallic Materials Applied to Liquid Hydrogen Storage
2.1. Effect of Low Temperature
2.2. Effect of Hydrogen Embrittlement
2.2.1. Hydrogen Environment for Testing
2.2.2. Maximum Hydrogen Embrittlement Temperature
- -
- Nickel base alloys: Room temperature [73];
- -
- -
- Aluminum alloys: Room temperature;
- -
- Austenitic stainless steel (300–series Cr–Ni stainless steel): 200–220 K [72].
2.2.3. Partial Pressure of Hydrogen
2.3. Effect of Material Characteristics
2.4. Effect of Testing Method
3. Comparison of LNG and LH2 System for Ships
3.1. System Configuration
| Target | Category | Pressure (bar) | Temp. Range (K) | Ref. |
|---|---|---|---|---|
| GODU–LH2 project | Design pressure of the inner tank | 6.55 | Abt. 20 | [105] |
| LH2 terminal | Design pressure of the inner tank | 2.2 | Abt. 20 | [104] |
| CGA Standard | MAWP of the inner tank | 10.3 | Abt. 20 | [103] |
| LH2 tank | MAWP of the inner tank | 9.0 | Abt. 20 | [106] |
| PEMFC | MAWP of the inlet | ≤3.5 | Ambient | [106] |
| CGH tank | Maximum fueling pressure | ≤875 | 233 to 358 | SAE J2579 |
3.2. System Minimum Requirements
- Risk factors related to LNG
- -
- Cryogenic burns;
- -
- Low-temperature embrittlement;
- -
- Risk of suffocation;
- -
- Expansion and pressure;
- -
- Fire;
- -
- Rapid phase separation (evaporation).
- Measures to reduce risk factors
- -
- Protection from external environments (cofferdams, airlocks, etc.);
- -
- Pressure relief and ventilation;
- -
- Secondary sealing (e.g., dual tubes and secondary barriers);
- -
- Application of welded connections rather than flange connections;
- -
- Drip tray capacity and liquid detection;
- -
- Protection of the hull structure from released cryogenic, high-pressure steam and gas.
- Risk factors related to hydrogen
- -
- Combustion-related factors: low ignition energy, a wide range of combustion conditions, less visible flame, and fast flame propagation;
- -
- Low temperature-related factors: low-temperature burns, oxygen enrichment by liquefaction/condensation, solidification of other fluids;
- -
- High permeability;
- -
- Low viscosity;
- -
- Hydrogen embrittlement in parent materials and weld metals.
- Measures to reduce risk factors (focused on hydrogen embrittlement)
- -
- Reduction in working stress (to the level of 30–50% of yield strength) [107];
- -
- Minimization of low-temperature plastic deformation during cold working (estimated while considering all generated stress);
- -
- Solution heat treatment recommended for stainless steel;
- -
- Carbon steel pipes heat-treated by normalizing;
- -
- Alloy steel with a tensile strength of 950 MPa or less treated by quenching and tempering;
- -
- Seamless steel pipes used in a high-pressure hydrogen environment with a pressure level of 200 bar or more;
- -
- Flange connection methods avoided when connecting pipes if weld joints can be applied;
- -
- Full penetration butt weld.
- -
- Cargo/fuel tanks and pressure vessels for processes;
- -
- Piping system for cargo/fuel and processes;
- -
- Drip trays;
- -
- Outer shells.
3.3. Applicable Materials
- -
- 9% nickel steel;
- -
- Austenitic stainless steel: AISI 304, 304 L, 316, 316 L, 321, 347;
- -
- Aluminum alloys: Al 5083;
- -
- Austenitic Fe-Ni alloy steel: 36% nickel steel;
- -
- Cryogenic high-manganese steel.
- Category 1: Materials that are almost not affected by hydrogen embrittlement under limited conditions;
- Category 2: Materials that undergo hydrogen embrittlement and thus can only be used under limited conditions;
- Category 3: Materials that are significantly affected by hydrogen embrittlement, for example, ending up failing or breaking, in an elastic range in which the stress is lower than the yield strength of the material.
4. Analysis of Safety Regulations for Material Selection
4.1. General Considerations in Material Selection
4.1.1. ISO/TR 15916
- Design and structural aspects
- -
- Reduction in operating stress levels;
- -
- Minimization of low-temperature plastic deformation during cold working;
- -
- Avoidance of repetitive loading to prevent fatigue cracks.
- Material aspects
- -
- Constraints on the hardiness and strength of metallic materials;
- -
- Minimization of residual stress;
- -
- Application of materials with excellent resistance to hydrogen, e.g., austenitic stainless steel;
- -
- Assessments based on ISO 11114-4 in material selection.
4.1.2. KGS Code
- -
- KGS AC111 2021 (Code for Facilities, Technology and Inspection for Manufacturing of High-Pressure Gas Storage Tanks and Pressure Vessels);
- -
- KGS AC213 2021 (Code for Facilities, Technology and Inspection for Manufacturing of Cryogenic Cylinders);
- -
- KGS FU671 2021 (Facility/Technical/Inspection Code for Use of Hydrogen Gases).
- (1)
- Major Details of KGS AC111: 2021
- (2)
- Major Details of KGS AC213: 2021
- -
- Tensile tests for welded seams: in terms of tensile strength/yield strength;
- -
- Bending tests for the inside of the welded joint: in terms of the amount of cracking;
- -
- Bending tests for the side of the welded joint: in terms of the amount of cracking;
- -
- Bending tests for the backside of the welded joint: in terms of the amount of cracking;
- -
- Tensile tests for the used weld metal: in terms of tensile strength/yield strength, with an elongation of 22% or more;
- -
- Impact tests for welded joints (applied only to stainless steel): in terms of impact absorption energy, measured by Charpy impact tests at −150 °C or less (with an impact absorption energy of at least 20 J/cm2 and 30 J/cm2 or more on average.
- (3)
- Major Details of KGS FU671: 2021
4.1.3. CGA Standard
- -
- CGA H-3 (2019) Standard for Cryogenic Hydrogen Storage;
- -
- CGA H-5 (2020) Standard for Bulk Hydrogen Supply Systems;
- -
- CGA G-5.4 (2019) Standard for Hydrogen Piping System at User Locations;
- -
- CGA G-5.6 (2005) Hydrogen Pipeline Systems.
- (1)
- Major Details of CGA H-3 (2019)
- -
- All annular-space pipelines must be composed of seamless tubes or pipes made of austenitic stainless steel.
- -
- Welding on tube or pipe connections should be avoided as much as possible, and if unavoidable, butt welding should be used.
- -
- For 304 stainless steel, it must be ensured that any stress that exceeds 20% of its tensile strength is not generated. Here, any stress generated during cold working should also be counted in the estimation process.
- -
- The 316 stainless steel is a material with excellent resistance to hydrogen embrittlement.
- (2)
- Major Details of CGA H-5 (2020)
- (3)
- Major Details of CGA G-5.4 (2019)
- (4)
- Major Details of CGA G-5.6 (2005)
- -
- Ensure that the austenite stability factor has a positive value (typically 300-series stainless steel alloys with a Ni content of 10.5% or more).
- -
- Metallic materials with a high austenite stability factor (or with a high nickel equivalent) should be selected as much as possible.
- -
- The use of stainless steel grades with a metastable austenite phase, such as 201, 301, 302, 304, 304 L, and 321, must be avoided in a high-pressure hydrogen atmosphere.
- -
- Ferritic stainless steel, martensitic stainless steel, duplex stainless steel, and precipitation-hardened stainless steel may only be used for parts with low operating stress.
4.1.4. ISO 19881
- Material characteristics
- -
- Steel: Vessels must be made of aluminum-killed steel with fine grains that is resistant to corrosion, deformation, and degradation when exposed to high-pressure hydrogen.
- -
- The content of additives must be specified, such as carbon, manganese, aluminum, silicon, nickel, chromium, molybdenum, boron, and vanadium.
- Impact tests
- -
- Applicable to steel structures.
- -
- Tests are performed in accordance with ISO 148-1 (Metallic materials—Charpy pendulum impact test-Part 1: Test method) or ASTM E23 (Standard Test Methods for Notched Bar Impact Testing of Metallic Materials).
- -
- The notch is made in the C-L direction (perpendicular to the circumference and along the length direction).
- -
- All impact tests are performed at the lowest test temperature (−40 °C).
- Tensile tests
- -
- Applicable to all metallic materials.
- -
- Subject to ASTM E8 (Standard Test Methods for Tension Testing of Metallic Materials), etc.
- Sustained load cracking tests
- -
- Applicable to aluminum alloys.
- -
- Tests are performed in accordance with Appendix B of ISO 7866:2012 (Gas Cylinders-Refillable Seamless Aluminum Alloy Gas Cylinders-Design, Construction and Testing).
- Corrosion tests
- -
- Applicable to aluminum alloys.
- -
- Tests are performed in accordance with Appendix A of ISO 7866:2012 (Gas Cylinders-Refillable Seamless Aluminum Alloy Gas Cylinders-Design, Construction and Testing).
4.1.5. ASME B31.12
4.2. Material Compatibility Test
4.2.1. CSA/ANSI CHMC-1
- Hydrogen atmosphere: Tests are performed by designing a test chamber that complies with ASTM G142 or using hydrogen-precharged specimens.
- Test temperature: At a temperature within the operating temperature range where the effect of hydrogen embrittlement is the most pronounced
- -
- At 220 K for 300-series stainless steel and at room temperature for other metallic materials.
- Test pressure: Set equivalent or superior to the MAWP.
- SSRT: Tests are performed on notched or smooth tensile specimens either in a hydrogen atmosphere or in a non-hydrogen atmosphere.
- -
- Verified to be compatible if the notch–tensile strength ratio (under ASTM G129) or the relative reduction area (under ASTM E8) is 0.9 or more.
- -
- Notched specimens must comply with ASTM G142, or the notch stress concentration coefficient should be 3 or more.
- -
- For smooth tensile specimens, the upper limit of the strain rate is twice the rate of 10−5 s−1. For notched specimens, the gauge length is set to 25.4 mm, and the effective strain rate is 10−6 s−1.
- Fracture toughness tests: JIC threshold fracture toughness measured according to ASTM E1820.
- Fatigue crack growth rate tests: ASTM E647.
- Fatigue life tests: Evaluated according to ASTM E466 and ASTM E606.
- -
- In load-controlled tests, the stress ratio is 0.1 for notched specimens and −1.0 or 0.1 for smooth specimens.
4.2.2. ASME BPVC Division III Article KD-10
- Applicable materials (in a high-pressure hydrogen atmosphere with a pressure of 1030 bar or less)
- -
- Alloy steel grades, including SA-372 and SA-723.
- -
- Stainless steel grades, including SA-336 Gr. F316.
- -
- Al 6061-T6.
- Required tests for high-pressure hydrogen atmospheres
- -
- Crack growth initiation tests (KIC) according to ASTM E399 or E1820.
- -
- Crack growth stoppage tests (KIH) according to ASTM E1681.
- -
- Crack growth rate (da/dN) tests according to ASTM E647.
4.2.3. SAE J2579
- Applicable to: Compressed hydrogen vessels for automobiles with a pressure of 700 bar
- Test conditions for SSRT
- -
- Test temperature: 228 ± 5 K (room temperature only for aluminum alloys).
- -
- Hydrogen pressure: 1.25 times the pressure level of 700 bar (under the same gas purity condition as specified in CSA CHMC-1).
- -
- Test specimen: Only smooth tensile specimens allowed.
- -
- Strain rate control: 5.0 × 10−5 s−1 or less (2.0 × 10−5 s−1 or less under CSA CHMC-1).
- -
- Evaluation criteria: The yield strength in a hydrogen atmosphere should be at least 0.8 times that measured in the air.
- Test conditions for fatigue life tests
- -
- Test environment: Only at room temperature at the same hydrogen pressure.
- -
- Test specimen: Either notched specimens or round-bar tensile specimens.
- -
- In load-controlled tests, the maximum stress should be at least 1/3 of the tensile strength, and the frequency is set to 1 Hz.
- -
- Stress ratio: 0.1 for notched specimens and −1.0 for round-bar tensile specimens.
- -
- Evaluation criteria: 105 or more for notched specimens and 2.0 × 105 or more for round-bar tensile specimens.
4.2.4. Other Standards
5. Proposal of New Guidelines for the Acceptance of Alternative Metallic Materials for Hydrogen Service in Ships
5.1. Review of MSC.1/Circular.1622 for Use with Liquid Hydrogen
5.2. Test Methodology Based on MSC.1/Circular.1622 for Hydrogen Service
- Hydrogen Atmosphere Identification
- -
- Evaluate exposure to hydrogen environment.
- -
- Assess design temperature and hydrogen pressure.
- Material Specifications Verification
- -
- Examine chemical compositions, microstructure, mechanical performance, and heat treatment.
- -
- Ensure certification and other relevant documentation.
- Review Material Acceptance
- -
- Verify that the material is permissible for the intended environment, considering temperature and hydrogen pressure.
- -
- If the material is not on the approved lists, initiate material compatibility assessments.
- Material Compatibility Assessments for the Target Environment
- -
- Conduct simplified hydrogen sensitivity assessment based on SAE J2579.
- -
- Evaluate fracture toughness, taking into account the concurrent effects of low-temperature embrittlement and gaseous hydrogen-induced embrittlement.
- Slow strain rate tensile test (SSRT)
- -
- Test temperature: Design temperature and the temperature where hydrogen embrittlement is most pronounced (or the temperature close to the range).
- -
- Hydrogen exposure conditions: Exposed to high-purity gaseous hydrogen at the maximum allowable working pressure (or tests performed after injecting an equivalent amount of hydrogen into the specimen).
- -
- Test specimen: Typical round-bar tensile specimen (ASTM E8/E8M).
- -
- Strain rate control: ≤5.0 × 10−5 s−1.
- -
- Acceptance criteria: relative reduction in area ≥ 0.5.
- -
- If notched specimens are used in fatigue life tests, SSRT may be omitted.
- Fatigue life test
- -
- Test temperature: Ambient temperature (fatigue life increased when exposed to low-temperature hydrogen).
- -
- Hydrogen exposure conditions: Same as in SSRT.
- -
- Test specimen: Either of notched specimen (ASTM G142) or typical round-bar tensile specimen (ASTM E8/E8M).
- -
- Load control: Maximum stress set to 1/3 or more of the tensile strength with a frequency of 1 Hz.
- -
- Stress ratio: 0.1 for notched specimens and −1.0 for round-bar specimens.
- -
- Evaluation criteria: Further discussion needed for the criteria for fatigue life (105 or more for notched specimens and 2.0 × 105 or more for round-bar tensile specimens according to the reference standards).
- Fracture toughness test
- -
- Test temperature: Design temperature and the temperature where hydrogen embrittlement is most pronounced (or the temperature close to the range).
- -
- Hydrogen exposure conditions: Same as in SSRT.
- -
- Test specimen and procedure: ASTM E1820 (JIC threshold fracture toughness or CTOD).
- -
- Acceptance criteria for CTOD can be adopted as an industry standard. Further discussion needed for JIC.
- -
- Remarks: Charpy impact tests is not allowed for hydrogen service.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Alloy Type | Ni Contents (%) | Hydrogen Environment | Relative Tensile Strength | Relative Elongation | Relative Reduction in Area | Remarks | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Pressure (Bar) | Temp. (K) | |||||||
| 304 | 8.11 | 10 | 300 | 0.87 | - | 0.41 | [48,51] | |
| 304 | 8.64 | 10 | 300 | 0.96 | - | 0.52 | [48,51] | |
| 321 D | 9.3 | 90 | 300 | 0.92 | 0.47 | 0.57 | [85] | |
| 304 LN | 9.6 | 90 | 300 | 0.95 | 0.79 | 0.57 | [85] | |
| 304 | 9.9 | 90 | 300 | 1.01 | 0.93 | 0.75 | [85] | |
| 304 heat C | 8.15 | 830 | 300 | - | - | 0.37 | [84] | |
| 304 | 8.11 | 345 | 300 | 0.94 | - | 0.42 | Internal H2 | [48,51] |
| 304 | 8.64 | 345 | 300 | 1.03 | - | 0.47 | Internal H2 | [48,51] |
| 316 | 10 | 10 | 300 | 1.01 | - | 0.99 | [48,51] | |
| 316 | 10 | 345 | 300 | 1.10 | - | 0.68 | Internal H2 | [48,51] |
| 316 | 10.04 | 10 | 300 | 0.98 | - | 0.82 | [48,51] | |
| 316 | 10.04 | 345 | 300 | 1.13 | - | 0.55 | Internal H2 | [48,51] |
| 316 heat C | 10.12 | 830 | 300 | - | - | 0.6 | [84] | |
| 316 | 10.18 | 10 | 300 | 1.00 | - | 0.70 | [48,51] | |
| 304 | 10.18 | 345 | 300 | 1.08 | - | 0.71 | Internal H2 | [48,51] |
| TP316NG | 10.4 | 90 | 300 | 1.01 | 0.99 | 0.85 | [85] | |
| 347 B | 10.6 | 90 | 300 | 0.91 | 0.62 | 0.63 | [85] | |
| TP304 L | 11 | 90 | 300 | 0.99 | 0.93 | 0.77 | [85] | |
| 316 | 11.08 | 10 | 300 | 0.98 | - | 0.79 | [48,51] | |
| 316 | 11.08 | 345 | 300 | 1.04 | - | 0.83 | Internal H2 | [48,51] |
| 316 | 11.15 | 10 | 300 | 1.00 | - | 1.01 | [48,51] | |
| 316 | 11.15 | 345 | 300 | 1.09 | - | 0.86 | Internal H2 | [48,51] |
| 304 L | 11.2 | 90 | 300 | 0.99 | 0.90 | 0.84 | [85] | |
| 347 A | 11.3 | 90 | 300 | 0.98 | 0.97 | 0.94 | [85] | |
| 321 C | 11.5 | 90 | 300 | 1.02 | 0.97 | 0.88 | [85] | |
| 316 L | 12.02 | 780 | 300 | - | - | 0.94 | [84] | |
| 316 L | 12.15 | 10 | 300 | 0.97 | - | 0.99 | [48,51] | |
| 316 L | 12.15 | 345 | 300 | 1.04 | - | 0.89 | Internal H2 | [48,51] |
| 316 L | 12.19 | 10 | 300 | 1.00 | - | 0.99 | [48,51] | |
| 316 L | 12.19 | 345 | 300 | 1.07 | - | 0.90 | Internal H2 | [48,51] |
| 316 | 12.56 | 10 | 300 | 1.00 | - | 0.97 | [48,51] | |
| 316 | 12.56 | 345 | 300 | 1.05 | - | 0.91 | Internal H2 | [48,51] |
| 316 | 12.6 | 10 | 300 | 1.00 | - | 1.00 | [48,51] | |
| 316 | 12.6 | 345 | 300 | 1.07 | - | 0.90 | Internal H2 | [48,51] |
| 316 | 12.74 | 10 | 300 | 1.00 | - | 0.93 | [48,51] | |
| 316 | 12.74 | 345 | 300 | 1.04 | - | 0.84 | Internal H2 | [48,51] |
| 316 LN | 13.2 | 90 | 300 | 1.00 | 1.02 | 0.99 | [85] | |
| 316 L | 17.06 | 1150 | 300 | - | - | 0.95 | high-Ni | [84] |
| 304 L forged | 10.44 | 1380 | 293 | 1.12 | - | 0.60 | Internal H2 | [23] |
| 304 L forged | 10.6 | 1380 | 293 | 1.13 | - | 0.82 | Internal H2 | [23] |
| 304 heat C | 8.15 | 1090 | 249 | - | - | 0.2 | [84] | |
| 316 heat C | 10.12 | 1020 | 233 | - | - | 0.28 | [84] | |
| 304 L forged | 10.44 | 1380 | 233 | 1.00 | - | 0.30 | Internal H2 | [23] |
| 316 L | 12.02 | 1150 | 233 | - | - | 0.84 | [84] | |
| 316 L | 12.19 | 900 | 233 | 0.96 | 0.80 | 0.63 | [86] | |
| 316 L | 12.19 | 900 | 233 | 0.98 | 1.01 | 0.93 | [88] | |
| 316 L | 12.19 | 900 | 233 | 0.98 | 0.98 | 0.80 | [88] | |
| 316 L | 12.19 | 900 | 233 | 1.04 | 0.94 | 0.73 | [88] | |
| 316 L | 17.06 | 1150 | 233 | - | - | 0.73 | high-Ni | [84] |
| 304 | 8.11 | 400 | 223 | 0.51 | - | 0.17 | [48,51] | |
| 316 | 8.78 | 400 | 223 | 0.57 | - | 0.22 | [48,51] | |
| 304 | 8.11 | 345 | 223 | 0.65 | - | 0.23 | Internal H2 | [48,51] |
| 316 | 8.3 | 345 | 223 | 0.73 | - | 0.21 | Internal H2 | [48,51] |
| 304 | 8.64 | 345 | 223 | 0.66 | - | 0.27 | Internal H2 | [48,51] |
| 316 | 8.78 | 345 | 223 | 0.68 | - | 0.31 | Internal H2 | [48,51] |
| 316 | 10 | 345 | 223 | 0.88 | - | 0.27 | Internal H2 | [48,51] |
| 316 | 10.04 | 400 | 223 | 0.66 | - | 0.16 | [48,51] | |
| 316 | 10.04 | 345 | 223 | 0.89 | - | 0.29 | Internal H2 | [48,51] |
| 316 | 10.1 | 345 | 223 | 0.88 | - | 0.30 | Internal H2 | [48,51] |
| 304 | 10.18 | 400 | 223 | 0.63 | - | 0.21 | [48,51] | |
| 304 | 10.18 | 345 | 223 | 0.82 | - | 0.28 | Internal H2 | [48,51] |
| 316 | 10.5 | 345 | 223 | 0.98 | - | 0.37 | [48,51] | |
| 316 | 11.08 | 400 | 223 | 0.72 | - | 0.30 | [48,51] | |
| 316 | 11.08 | 345 | 223 | 0.93 | - | 0.44 | Internal H2 | [48,51] |
| 316 | 11.15 | 400 | 223 | 0.80 | - | 0.35 | [48,51] | |
| 316 | 11.15 | 345 | 223 | 0.97 | - | 0.44 | Internal H2 | [48,51] |
| 316 | 11.2 | 345 | 223 | 1.04 | - | 0.41 | Internal H2 | [48,51] |
| 316 L | 12.15 | 400 | 223 | 0.90 | - | 0.36 | [48,51] | |
| 316 L | 12.15 | 345 | 223 | 1.08 | - | 0.53 | Internal H2 | [48,51] |
| 316 L | 12.19 | 400 | 223 | 0.90 | - | 0.44 | [48,51] | |
| 316 L | 12.19 | 345 | 223 | 1.07 | - | 0.49 | Internal H2 | [48,51] |
| 316 | 12.56 | 400 | 223 | 0.99 | - | 0.53 | [48,51] | |
| 316 | 12.56 | 345 | 223 | 1.04 | - | 0.81 | Internal H2 | [48,51] |
| 316 | 12.6 | 400 | 223 | 0.99 | - | 0.83 | [48,51] | |
| 316 | 12.6 | 345 | 223 | 1.06 | - | 0.67 | Internal H2 | [48,51] |
| 316 | 12.74 | 400 | 223 | 1.00 | - | 0.68 | [48,51] | |
| 316 | 12.74 | 345 | 223 | 1.05 | - | 0.93 | Internal H2 | [48,51] |
| 304 L | 10.7 | 1380 | 113 | 1.19 | 0.97 | 0.59 | Internal H2 | [87] |
| 304 L | 10.7 | 1380 | 77 | 1.08 | 1.06 | 0.75 | Internal H2 | [87] |
| 304 LN | 9.6 | 1 | 20 | 0.98 | 0.95 | 0.61 | [87] | |
| 304 L | 10.7 | 1380 | 20 | 1.01 | 1.14 | 0.62 | Internal H2 | [87] |
| 316 LN | 13.2 | 1 | 20 | 1.04 | 0.93 | 0.77 | [85] | |
| Alloy Type | Ni Contents (%) | H2 Environment | Fracture Toughness | Relative Fracture Toughness | Remarks | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Pressure (Bar) | Temp. (K) | w/H2 | w/o H2 | |||||
| 316 L | 10.04 | 10 | 293 | 361 | 940 | 0.38 | ASTM E1820 violated. | [24] |
| 317 L | 12.56 | 10 | 293 | 925 | 1051 | 0.88 | ASTM E1820 violated. | [24] |
| 316 L | 13.2 | 340 | 293 | 350 | 480 | 0.73 | Internal H2 (3730 appm) | [101] |
| 304 L | 10.44 | 410 | 293 | 390 | 629 | 0.62 | Internal H2 (66 wppm) | [23,25] |
| 304 L | 10.44 | 1380 | 293 | 256 | 629 | 0.41 | Internal H2 (140 wppm) | [23] |
| 304 L | 10.6 | 1380 | 293 | 323 | 670 | 0.48 | Internal H2 (140 wppm) | [23] |
| 304 L | 10.44 | 410 | 223 | 159 | 716 | 0.22 | Internal H2 (66 wppm) | [23,25] |
| 304 L | 10.44 | 1380 | 223 | 166 | 716 | 0.23 | Internal H2 (140 wppm) | [23] |
| 304 L | 10.6 | 1380 | 223 | 164 | 768 | 0.21 | Internal H2 (140 wppm) | [23] |
| 304 | 8.08 | 0 | 4 | - | 66 | - | - | [26] |
| 304 | 8.2 | 0 | 77 | - | 161 | - | - | [27] |
| 304 | 8.2 | 0 | 4 | - | 110 | - | - | [27] |
| 304 | 8.6 | 0 | 293 | - | 953 | - | - | [28] |
| 304 | 8.6 | 0 | 183 | - | 378 | - | - | [28] |
| 304 | 8.6 | 0 | 183 | - | 430 | - | - | [28] |
| 304 | 8.6 | 0 | 110 | - | 317 | - | - | [28] |
| 304 L | 9.15 | 0 | 77 | - | 496 | - | - | [29] |
| 304 L | 9.15 | 0 | 4 | - | 205 | - | - | [29] |
| 316 L | 9.67 | 0 | 4 | - | 195 | - | - | [26] |
| 304 L | 10 | 0 | 293 | - | 529 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 304 L | 10 | 0 | 273 | - | 418 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 304 L | 10 | 0 | 223 | - | 556 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 304 L | 10 | 0 | 183 | - | 386 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 304 L | 10 | 0 | 110 | - | 395 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 304 L | 10 | 0 | 77 | - | 300 | - | 0.03C-2Mn-18Cr-10Ni | [30] |
| 316 LN | 12.16 | 0 | 4 | - | 500 | - | - | [26] |
| List of Test | Testing Method | Acceptance Criteria | Testing Conditions to Be Considered | Remarks | |
|---|---|---|---|---|---|
| 20 K | H2 Environment | ||||
| Tensile test | Section 6.3.1 of the IGC Code and Recognized standards | √ | √ | ||
| Charpy impact test | Section 6.3.2 of the IGC Code and Recognized standards | 27 J (Transverse test specimen for ferrous alloy plates) | - | - | To be excluded |
| Fatigue test | Paragraph 4.18.2.4.2 of the IGC Code and IIW or DNVGL-RP-C203 or BS 7608 | 105 to 108 cycles | - | √ | |
| CTOD test | Recognized standard (such as ASTM E1820, BS 7448 or ISO 12135) | a minimum CTOD value of 0.2 mm | √ | √ | |
| Ductile fracture toughness test | Recognized standard (such as ASTM E1820, ASTM E2818, ISO 15653 or ISO 12135) | √ | √ | ||
References
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222. [Google Scholar] [CrossRef]
- Berstad, D.; Gardarsdottir, S.; Roussanaly, S.; Voldsund, M.; Ishimoto, Y.; Nekså, P. Liquid hydrogen as prospective energy carrier: A brief review and discussion of underlying assumptions applied in value chain analysis. Renew. Sustain. Energy Rev. 2022, 154, 111772. [Google Scholar] [CrossRef]
- Ye, M.; Sharp, P.; Brandon, N.; Kucernak, A. System-level comparison of ammonia, compressed and liquid hydrogen as fuels for polymer electrolyte fuel cell powered shipping. Int. J. Hydrogen Energy 2022, 47, 8565–8584. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Tong, L.; Wang, L. Research Progress of Cryogenic Materials for Storage and Transportation of Liquid Hydrogen. Metals 2021, 11, 1101. [Google Scholar] [CrossRef]
- Takaoka, Y.; Mizumukai, K.; Kameno, Y. Suiso Frontier, the First LH2 Carrier—Demonstration of Technologies and Operational Phase. In Proceedings of the 33rd International Ocean and Polar Engineering Conference, Ottawa, QC, Canada, 18–23 June 2023. [Google Scholar]
- ISO/TR 15916; Basis Considerations for the Safety of Hydrogen Systems. International Organization for Standardization: Geneva, Switzerland, 2019.
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Oriani, R.A. Hydrogen Embrittlement of Steels. Annu. Rev. Mater. Sci. 1978, 8, 327–357. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Gupta, N.; Zhang, K.; van Doorne, C.; Fesmire, J.; Dindoruk, B.; Balakotaiah, V. Hydrogen supply chain and challenges in large-scale LH2 storage and transportation. Int. J. Hydrogen Energy 2021, 46, 24149–24168. [Google Scholar] [CrossRef]
- Kern, A.; Schriever, U.; Stumpfe, J. Development of 9% Nickel Steel for LNG Applications. Steel Res. Int. 2007, 78, 189–194. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, M. An Investigation of the Mechanical Properties of a Weldment of 7% Nickel Alloy Steels. Metals 2016, 6, 285. [Google Scholar] [CrossRef]
- Oh, D.-J.; Lee, J.-M.; Noh, B.-J.; Kim, W.-S.; Ando, R.; Matsumoto, T.; Kim, M.-H. Investigation of fatigue performance of low temperature alloys for liquefied natural gas storage tanks. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2015, 229, 1300–1314. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, B.K.; Nam, D.G.; Kim, M.H. Effect of Nickel Contents on Fatigue Crack Growth Rate and Fracture Toughness for Nickel Alloy Steels. Metals 2022, 12, 173. [Google Scholar] [CrossRef]
- Takeda, T.; Hiraide, T.; Ueda, K. Safety Evaluation of the High Manganese Steel LNG SPB® Tank; Springer: Berlin/Heidelberg, Germany, 2021; pp. 715–728. [Google Scholar] [CrossRef]
- Han, I.W.; Lee, B.K.; Park, S.H.; Kang, C.Y. Microstructure and Mechanical Properties of Cryogenic High-Manganese Steel Weld Metal. Int. J. Offshore Polar Eng. 2017, 27, 260–265. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Lee, S.-G.; Jang, W.-K.; Choi, J.-K.; Kim, Y.-J.; Kim, S. Cryogenic S–N Fatigue and Fatigue Crack Propagation Behaviors of High Manganese Austenitic Steels. Metall. Mater. Trans. A 2013, 44, 4601–4612. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.-K.; Jang, W.; Lee, J.-H. Strain behavior of very new high manganese steel for 200,000 m3 LNG cryogenic storage tank. Energy 2023, 271, 126889. [Google Scholar] [CrossRef]
- Fekete, J.R.; Sowards, J.W.; Amaro, R.L. Economic impact of applying high strength steels in hydrogen gas pipelines. Int. J. Hydrogen Energy 2015, 40, 10547–10558. [Google Scholar] [CrossRef]
- Korean Register. Research Report of Material Compatibility for Liquid Hydrogen Storage on Marine Application; Korea Institute of Machinery & Materials, Pusan National University: Busan, Republic of Korea, 2023. [Google Scholar]
- Jackson, H.; San Marchi, C.; Balch, D.; Somerday, B.; Michael, J. Effects of Low Temperature on Hydrogen-Assisted Crack Growth in Forged 304L Austenitic Stainless Steel. Metall. Mater. Trans. A 2016, 47, 4334–4350. [Google Scholar] [CrossRef]
- Michler, T.; Lindner, M.; Eberle, U.; Meusinger, J. Assessing Hydrogen Embrittlement in Automotive Hydrogen Tanks. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 94–125. [Google Scholar] [CrossRef]
- Ronevich, J.; San Marchi, C.; Maguire, M.; Balch, D. Fracture Properties of Welded 304L in Hydrogen Environments. In Proceedings of the 2021 International Conference on Hydrogen Safety, Albuquerque, NM, USA, 21–24 September 2021. [Google Scholar] [CrossRef]
- Murase, S.; Kobatake, S.; Tanaka, M.; Tashiro, I.; Horigami, O.; Ogiwara, H.; Shibata, K.; Nagai, K.; Ishikawa, K. Effects of a high magnetic field on fracture toughness at 4.2 K for austenitic stainless steels. Fusion Eng. Des. 1993, 20, 451–454. [Google Scholar] [CrossRef]
- Rana, M.D.; Doty, W.D.; Yukawa, S.; Zawierucha, R. Fracture Toughness Requirements for ASME Section VIII Vessels for Temperatures Colder Than 77K. J. Press. Vessel. Technol. 2001, 123, 332–337. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, C.-S.; Jo, D.-H.; Kim, D.-S.; Yun, I.-S. Low Temperature Effects on the Strength and Fracture Toughness of Membrane for LNG Storage Tank. Trans. Korean Soc. Mech. Eng. A 2000, 24, 710–717. [Google Scholar]
- Lee, H.M.; Shin, J.Y.; Shin, H.S. Fracture Toughness of Structural Materials for LNG Storage Tank at Cryogenic Temperatures. Fracture Toughness of Structural Materials for LNG Storage Tank at Cryogenic Temperatures; The Korean Institute of GAS: Daejeon, Republic of Korea, 1998; pp. 340–349. [Google Scholar]
- Park, J.; Lee, K.; Sung, H.; Kim, Y.J.; Kim, S.K.; Kim, S. J-integral Fracture Toughness of High-Mn Steels at Room and Cryogenic Temperatures. Metall. Mater. Trans. A 2019, 50, 2678–2689. [Google Scholar] [CrossRef]
- Alomari, A.S. Serrated yielding in austenitic stainless steels. Mater. High Temp. 2021, 1–14. [Google Scholar] [CrossRef]
- Rodriguez, P. Serrated plastic flow. Bull. Mater. Sci. 1984, 6, 653–663. [Google Scholar] [CrossRef]
- Robinson, J.M.; Shaw, M.P. Microstructural and mechanical influences on dynamic strain aging phenomena. Int. Mater. Rev. 1994, 39, 113–122. [Google Scholar] [CrossRef]
- Pink, E.; Grinberg, A. Serrated flow in a ferritic stainless steel. Mater. Sci. Eng. 1981, 51, 1–8. [Google Scholar] [CrossRef]
- ASTM E1450-16; Standard Test Method for Tension Testing of Structural Alloys in Liquid Helium. ASTM International: Conshohocken, PA, USA, 2016.
- Ogata, T.; Ishikawa, K.; Hiraga, K.; Nagai, K.; Yuri, T. Temperature rise during the tensile test in superfluid helium. Cryogenics 1985, 25, 444–446. [Google Scholar] [CrossRef]
- Ogata, T. Evaluation of Mechanical Properties of Structural Materials at Cryogenic Temperatures and International Standardization for Those Methods; AIP Publishing: Melville, NY, USA, 2014; pp. 320–326. [Google Scholar] [CrossRef]
- Benzing, J.; Derimow, N.; Lucon, E.; Weeks, D. Fracture Toughness Tests at 77K and 4K on 316L Stainless Steel Welded Plates; The National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022. [CrossRef]
- Zheng, C.; Yu, W. Effect of low-temperature on mechanical behavior for an AISI 304 austenitic stainless steel. Mater. Sci. Eng. A 2018, 710, 359–365. [Google Scholar] [CrossRef]
- Nanstad, R.; Swain, R.; Berggren, R. Influence of Thermal Conditioning Media on Charpy Specimen Test Temperature; Charpy Impact Test: Factors and Variables; ASTM International: West Conshohocken, PA, USA, 1990; pp. 195–210. [Google Scholar] [CrossRef]
- Wolfenden, A.; Tobler, R.; Reed, R.; Hwang, I.; Morra, M.; Ballinger, R.; Nakajima, H.; Shimamoto, S. Charpy Impact Tests Near Absolute Zero. J. Test. Eval. 1991, 19, 34–40. [Google Scholar] [CrossRef]
- Yoshida, H.; Kozuka, T.; Miyata, K.; Kodaka, H. Instrumented Charpy Impact Tests at Low Temperatures for Several Steels. Austenitic Steels at Low Temperatures; Springer: Boston, MA, USA, 1983; pp. 349–354. [Google Scholar] [CrossRef]
- Jin, S.; Horwood, W.A.; Morris, J.W.; Zackay, V.F. A Simple Method for Charpy Impact Testing below 6K. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1995; pp. 373–378. [Google Scholar] [CrossRef]
- Venezuela, J.; Gray, E.; Liu, Q.; Zhou, Q.; Tapia-Bastidas, C.; Zhang, M.; Atrens, A. Equivalent hydrogen fugacity during electrochemical charging of some martensitic advanced high-strength steels. Corros. Sci. 2017, 127, 45–58. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Pressouyre, G.M. Role of Traps in the Microstructural Control of Hydrogen Embrittlement of Steels; Defense Technical Information Center: Fort Belvoir, VA, USA, 1985. [Google Scholar]
- Zhang, T.; Chu, W.Y.; Gao, K.W.; Qiao, L.J. Study of correlation between hydrogen-induced stress and hydrogen embrittlement. Mater. Sci. Eng. A 2003, 347, 291–299. [Google Scholar] [CrossRef]
- An, H.; Lee, J.; Park, H.; Yoo, J.; Chung, S.; Park, J.; Kang, N. Cold Cracks in Fillet Weldments of 600 MPa Tensile Strength Low Carbon Steel and Microstructural Effects on Hydrogen Embrittlement Sensitivity and Hydrogen Diffusion. Korean J. Met. Mater. 2021, 59, 21–32. [Google Scholar] [CrossRef]
- San Marchi, C.; Michler, T.; Nibur, K.A.; Somerday, B.P. On the physical differences between tensile testing of type 304 and 316 austenitic stainless steels with internal hydrogen and in external hydrogen. Int. J. Hydrogen Energy 2010, 35, 9736–9745. [Google Scholar] [CrossRef]
- Michler, T.; Yukhimchuk, A.A.; Naumann, J. Hydrogen environment embrittlement testing at low temperatures and high pressures. Corros. Sci. 2008, 50, 3519–3526. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, M.; Imade, M.; Fukuyama, S.; Yokogawa, K. Effect of nickel equivalent on hydrogen gas embrittlement of austenitic stainless steels based on type 316 at low temperatures. Acta Mater. 2008, 56, 3414–3421. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Hydrogen environment embrittlement of austenitic stainless steels at low temperatures. Int. J. Hydrogen Energy 2008, 33, 2111–2122. [Google Scholar] [CrossRef]
- San Marchi, C.W. Austenitic Stainless Steels in Gaseous Hydrogen Embrittlement of High Performance Metals in Energy Systems; Sandia National Laboratories: Livermore, CA, USA, 2011. [Google Scholar]
- Kobayashi, H.; Yamada, T.; Kobayashi, H.; Matsuoka, S. Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2016; Volume 6B. [Google Scholar] [CrossRef]
- Ogata, T.; Balachandran, U.; Amm, K.; Evans, D.; Gregory, E.; Lee, P.; Osofsky, M.; Pamidi, S.; Park, C.; Wu, J.; et al. Hydrogen Embrittlement Evaluation in Tensile Properties of Stainless Steels at Cryogenic Temperatures. AIP Conf. Proc. 2008, 986, 124–131. [Google Scholar] [CrossRef]
- Sun, D.; Han, G.; Vaodee, S.; Fukuyama, S.; Yokogawa, K. Tensile behaviour of type 304 austenitic stainless steels in hydrogen atmosphere at low temperatures. Mater. Sci. Technol. 2001, 17, 302–308. [Google Scholar] [CrossRef]
- Nelson, H.G. Hydrogen Embrittlement; Elsevier: Amsterdam, The Netherlands, 1983; pp. 275–359. [Google Scholar] [CrossRef]
- Fidelle, J.-P. Closing Commentary—IHE-HEE: Are They the Same? Hydrogen Embrittlement Testing; ASTM International: West Conshohocken, PA, USA, 1974; pp. 267–272. [Google Scholar] [CrossRef]
- Nelson, H. Closing Commentary—IHE-HEE: Are They the Same? Hydrogen Embrittlement Testing; ASTM International: West Conshohocken, PA, USA, 1974; pp. 273–274. [Google Scholar] [CrossRef]
- Fukuyama, S.; Imade, M.; Yokogawa, K. Development of New Material Testing Apparatus in High-Pressure Hydrogen and Evaluation of Hydrogen Gas Embrittlement of Metals; Materials and Fabrication; ASMEDC: Washington, DC, USA, 2007; Volume 6, pp. 527–533. [Google Scholar] [CrossRef]
- Imade, M.; Zhang, L.; Wen, M.; Iijima, T.; Fukuyama, S.; Yokogawa, K. Internal Reversible Hydrogen Embrittlement and Hydrogen Gas Embrittlement of Austenitic Stainless Steels Based on Type 316; Materials and Fabrication, Parts A and B; ASMEDC: Washington, DC, USA, 2009; Volume 6, pp. 205–213. [Google Scholar] [CrossRef]
- Gibbs, P.J.; San Marchi, C.; Nibur, K.A.; Tang, X. Comparison of Internal and External Hydrogen on Fatigue-Life of Austenitic Stainless Steels; Volume 6B: Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Matsuoka, S.; Furuya, Y.; Takeuchi, E.; Hirukawa, H.; Matsunaga, H. Effect of external/internal hydrogen on SSRT properties of austenitic stainless steels and the role of martensitic transformation-induced plasticity. Trans. JSME 2020, 86, 20-00306. (In Japanese) [Google Scholar] [CrossRef]
- Omura, T.; Nakamura, J.; Hirata, H.; Jotoku, K.; Ueyama, M.; Osuki, T.; Terunuma, M. Effect of Surface Hydrogen Concentration on Hydrogen Embrittlement Properties of Stainless Steels and Ni Based Alloys. ISIJ Int. 2016, 56, 405–412. [Google Scholar] [CrossRef]
- ANSI/CSA CHMC 1-2014; Test Methods for Evaluating Material Compatibility in Compressed Hydrogen Applications—Metals (R2018). American National Standards Institute: Washington, DC, USA, 2018.
- San Marchi, C.; Ronevich, J.; Sabisch, J.; Sugar, J.; Medlin, D.; Somerday, B. Effect of microstructural and environmental variables on ductility of austenitic stainless steels. Int. J. Hydrogen Energy 2021, 46, 12338–12347. [Google Scholar] [CrossRef]
- Fukunaga, A. Differences between internal and external hydrogen effects on slow strain rate tensile test of iron-based superalloy A286. Int. J. Hydrogen Energy 2022, 47, 2723–2734. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A.D.; Shi, Z.; Verbeken, K.; Atrens, A. Determination of the hydrogen fugacity during electrolytic charging of steel. Corros. Sci. 2014, 87, 239–258. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Eliezer, D. Hydrogen assisted cracking in type 304L and 316L stainless steel. In Proceedings of the Third International Conference on Effect of Hydrogen on Behavior of Materials, The Metallurgical Society of AIME, Moran, WY, USA, 26–31 August 1981; pp. 565–574. [Google Scholar]
- ISO 16573-2; Steel—Measurement Method for the Evaluation of Hydrogen Embrittlement Resistance of High-Strength Steels—Part 2, Slow Strain Rate Test. ISO: Geneva, Switzerland, 2022.
- Hwang, J.-S.; Kim, J.-H.; Kim, S.-K.; Lee, J.-M. Effect of PTFE coating on enhancing hydrogen embrittlement resistance of stainless steel 304 for liquefied hydrogen storage system application. Int. J. Hydrogen Energy 2020, 45, 9149–9161. [Google Scholar] [CrossRef]
- Fukuyama, S.; Sun, D.; Zhang, L.; Wen, M.; Yokogawa, K. Effect of Temperature on Hydrogen Environment Embrittlement of Type 316 Series Austenitic Stainless Seels at Low Temperatures. J. Jpn. Inst. Met. 2003, 67, 456–459. [Google Scholar] [CrossRef]
- Holbrook, J.H.; West, A.J. The effect of temperature and strain rate on the tensile properties of hydrogen charged 304L. In Proceedings of the Third International Conference on Effect of Hydrogen Behavior of Materials, Moran, WY, USA, 26–31 August 1981; pp. 655–663. [Google Scholar]
- Hofmann, W.; Rauls, W. Ductility of steel under the influence of external high pressure hydrogen (Hydrogen embrittlement of plain steel and Armco iron). Weld. J. 1965, 44, 225–230. [Google Scholar]
- Farrell, K.; Quarrell, A.G. Hydrogen Embrittlement of Ultra-high-tensile Steel. J. Iron Steel Inst. 1964, 102, 1002–1011. [Google Scholar]
- Iijima, T.; Enoki, H.; Yamabe, J.; An, B. Effect of High Pressure Gaseous Hydrogen on Fatigue Properties of SUS304 and SUS316 Austenitic Stainless Steel; Volume 6B: Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Kimura, M.; Yoshikawa, N.; Tamura, H.; Iijima, T.; Ishizuka, A.; Yamabe, J. Test Method to Establish Hydrogen Compatibility of Materials in High Pressure Hydrogen Gas Environments for Fuel Cell Vehicles. ISIJ Int. 2021, 61, 1333–1336. [Google Scholar] [CrossRef]
- Weber, S.; Martin, M.; Theisen, W. Impact of heat treatment on the mechanical properties of AISI 304L austenitic stainless steel in high-pressure hydrogen gas. J. Mater. Sci. 2012, 47, 6095–6107. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Zhou, C.; Hong, Y.; Tao, H.; Zheng, J.; Zhang, L. Improved resistance to hydrogen environment embrittlement of warm-deformed 304 austenitic stainless steel in high-pressure hydrogen atmosphere. Corros. Sci. 2019, 148, 159–170. [Google Scholar] [CrossRef]
- Olden, V.; Saai, A.; Jemblie, L.; Johnsen, R. FE simulation of hydrogen diffusion in duplex stainless steel. Int. J. Hydrogen Energy 2014, 39, 1156–1163. [Google Scholar] [CrossRef]
- Fussik, R.; Egels, G.; Theisen, W.; Weber, S. Stacking Fault Energy in Relation to Hydrogen Environment Embrittlement of Metastable Austenitic Stainless CrNi-Steels. Metals 2021, 11, 1170. [Google Scholar] [CrossRef]
- Martin, F.; Feaugas, X.; Oudriss, A.; Tanguy, D.; Briottet, L.; Kittel, J. State of Hydrogen in Matter: Fundamental Ad/Absorption, Trapping and Transport Mechanisms. In Mechanics—Microstructure—Corrosion Coupling; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–197. [Google Scholar] [CrossRef]
- Han, G.; He, J.; Fukuyama, S.; Yokogawa, K. Effect of strain-induced martensite on hydrogen environment embrittlement of sensitized austenitic stainless steels at low temperatures. Acta Mater. 1998, 46, 4559–4570. [Google Scholar] [CrossRef]
- Matsuoka, S.; Yamabe, J.; Matsunaga, H. Criteria for determining hydrogen compatibility and the mechanisms for hydrogen-assisted, surface crack growth in austenitic stainless steels. Eng. Fract. Mech. 2016, 153, 103–127. [Google Scholar] [CrossRef]
- Deimel, P.; Sattler, E. Austenitic steels of different composition in liquid and gaseous hydrogen. Corros. Sci. 2008, 50, 1598–1607. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.; Nahm, S.H.; Baek, U.B. An experimental study for qualifying hydrogen compatibility of austenitic stainless steel under low temperature. J. Mech. Sci. Technol. 2022, 36, 157–165. [Google Scholar] [CrossRef]
- Merkel, D.R.; Nickerson, E.K.; Seffens, R.J.; Simmons, K.L.; Marchi, C.S.; Kagay, B.J.; Ronevich, J.A. Effect of Hydrogen on Tensile Properties of 304L Stainless Steel at Cryogenic Temperatures; Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2021; Volume 4. [Google Scholar] [CrossRef]
- Current status of round-robin tests for hydrogen material compatibility. In Proceedings of the 6th Meeting of the Informal Working Group on GTR No.13 (Phase 2), Tianjin, China, 18–20 June 2019.
- Koide, K.; Minami, T.; Anraku, T.; Iwase, A.; Inoue, H. Effect of Hydrogen Partial Pressure on the Hydrogen Embrittlement Susceptibility of Type304 Stainless Steel in High-pressure H2/Ar Mixed Gas. ISIJ Int. 2015, 55, 2477–2482. [Google Scholar] [CrossRef]
- Michler, T.; Elsässer, C.; Wackermann, K.; Schweizer, F. Effect of Hydrogen in Mixed Gases on the Mechanical Properties of Steels—Theoretical Background and Review of Test Results. Metals 2021, 11, 1847. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Izawa, C.; Wagner, S.; Pundt, A.; Theisen, W. Influence of machining-induced martensite on hydrogen-assisted fracture of AISI type 304 austenitic stainless steel. Int J Hydrogen Energy 2011, 36, 11195–11206. [Google Scholar] [CrossRef]
- Tsong-Pyng, P.; Altstetter, C.J. Effects of deformation on hydrogen permeation in austenitic stainless steels. Acta Metall. 1986, 34, 1771–1781. [Google Scholar] [CrossRef]
- Louthan, M.R.; Caskey, G.R.; Donovan, J.A.; Rawl, D.E. Hydrogen embrittlement of metals. Mater. Sci. Eng. 1972, 10, 357–368. [Google Scholar] [CrossRef]
- Louthan, M.R.J.; McNitt, R.P.; Sisson, R.D.J. Environmental Degradation of Engineering Materials in Hydrogen; No. CONF-810913-Vol. 2; Virginia Polytechnic Inst. and State Univ., Lab. for the Study of Environmental Degradation of Engineering Materials: Blacksburg, WV, USA, 1981. [Google Scholar]
- Schijve, J. Fatigue of Structures and Materials, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J.; Sattler, E. Influence of high pressure gaseous hydrogen on S–N fatigue in two austenitic stainless steels. Int. J. Fatigue 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Ogata, T. Hydrogen Environment Embrittlement on Austenitic Stainless Steels from Room Temperature to Low Temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2015, 102, 012005. [Google Scholar] [CrossRef]
- San Marchi, C.; Zimmerman, J.A.; Tang, X.; Kernion, S.J.; Thürmer, K.; Nibur, K.A. Fatigue Life of Austenitic Stainless Steel in Hydrogen Environments; Volume 6B: Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen Embrittlement Mechanism in Fatigue of Austenitic Stainless Steels. Metall. Mater. Trans. A 2008, 39, 1327. [Google Scholar] [CrossRef]
- Ronevich, J.A.; San Marchi, C.; Balch, D.K. Temperature Effects on Fracture Thresholds of Hydrogen Precharged Stainless Steel Welds; Volume 6B: Materials and Fabrication; American Society of Mechanical Engineers: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Morgan, M.J. Hydrogen Effects on the Fracture Toughness Properties of Forged Stainless Steels; Materials and Fabrication, Parts A and B; ASMEDC: Washington, DC, USA, 2008; Volume 6, pp. 217–222. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Pratt, J.W.; Madsen, R.T.; Caughlan, S.A.M.; Leach, T.S.; Appelgate, T.B., Jr.; Kelety, S.Z.; Wintervoll, H.-C.; Haugom, G.P.; Teo, A.T. Feasibility of the Zero-V: A Zero-emission Hydrogen Fuel Cell, Coastal Research Vessel; OSTI: Albuquerque, NM, USA; Livermore, CA, USA, 2018. [Google Scholar] [CrossRef]
- Compressed Gas Association. Standard for Cryogenic Hydrogen Storage; Compressed Gas Association: McLean, VA, USA, 2019. [Google Scholar]
- Kim, J.; Park, H.; Jung, W.; Chang, D. Operation scenario-based design methodology for large-scale storage systems of liquid hydrogen import terminal. Int. J. Hydrogen Energy 2021, 46, 40262–40277. [Google Scholar] [CrossRef]
- Swanger, A.M.; Notardonato, W.U.; Jumper, K.M. ASME Section VIII Recertification of a 33,000 Gallon Vacuum-Jacketed LH2 Storage Vessel for Densified Hydrogen Testing at NASA Kennedy Space Center; Design and Analysis; American Society of Mechanical Engineers: New York, NY, USA, 2015; Volume 3. [Google Scholar] [CrossRef]
- MAN Energy Solutions. Cryogenic Solutions for Onshore and Offshore Applications 2021. Available online: https://www.man-es.com/docs/default-source/document-sync/man-cryo-eng.pdf?sfvrsn=f1030ce2_2 (accessed on 15 September 2022).
- Hayden, L.E.; Stalheim, D. ASME B31.12 Hydrogen Piping and Pipeline Code Design Rules and Their Interaction with Pipeline Materials Concerns, Issues and Research; Codes and Standards; ASMEDC: Washington, DC, USA, 2009; Volume 1, pp. 355–361. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen Embrittlement Prevention in High Strength Steels by Application of Various Surface Coatings—A Review; Springer: Singapore, 2021; pp. 673–683. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Laadel, N.-E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation barriers for hydrogen embrittlement prevention in metals—A review on mechanisms, materials suitability and efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Iljima, T.; Abe, T.; Itoga, H. Development of Material Testing Equipment in High Pressure Gaseous Hydrogen and International Collaborative Work of a Testing Method for a Hydrogen Society. Synth. Engl. Ed. 2015, 8, 61–69. [Google Scholar] [CrossRef]


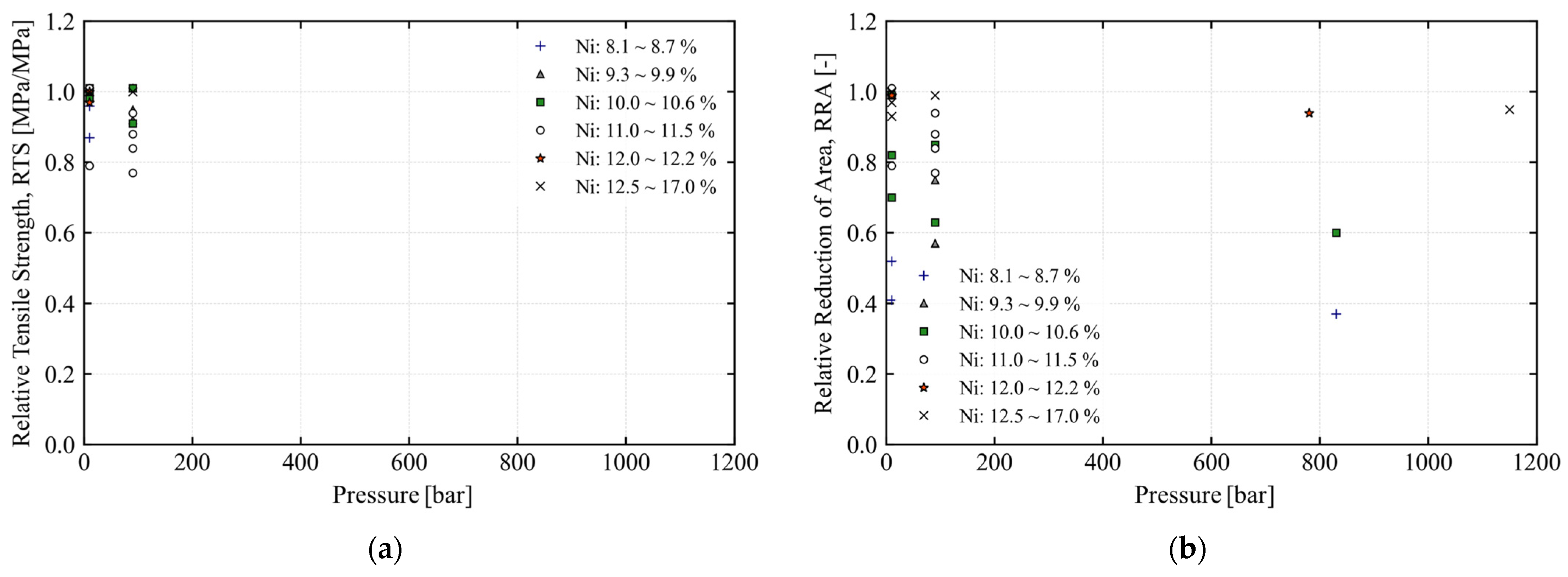
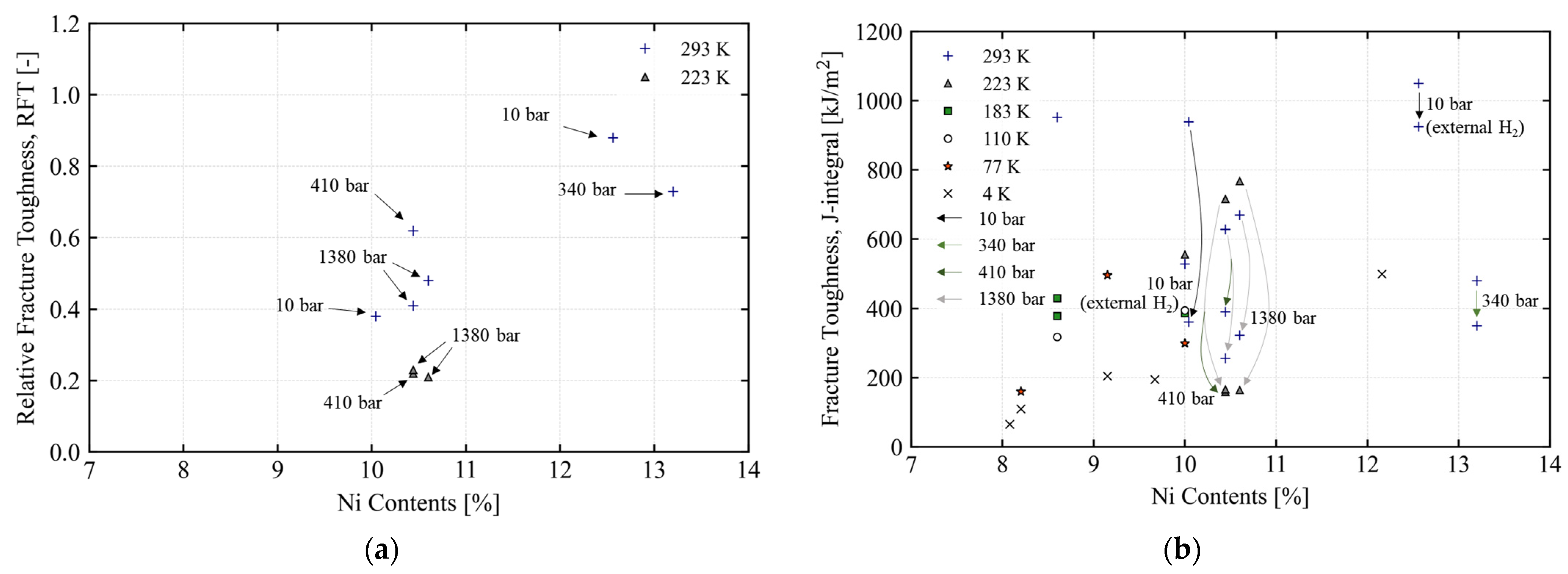
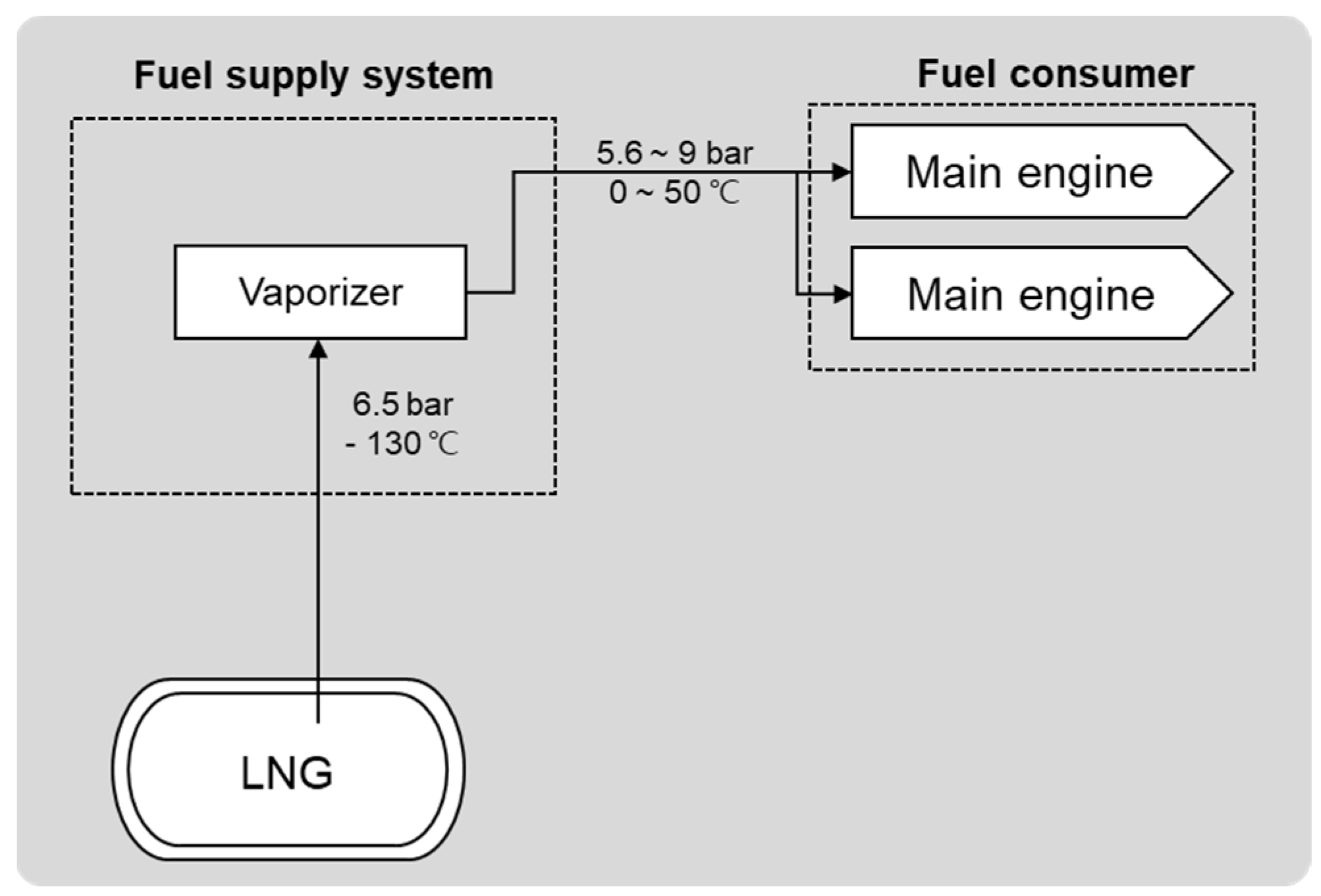
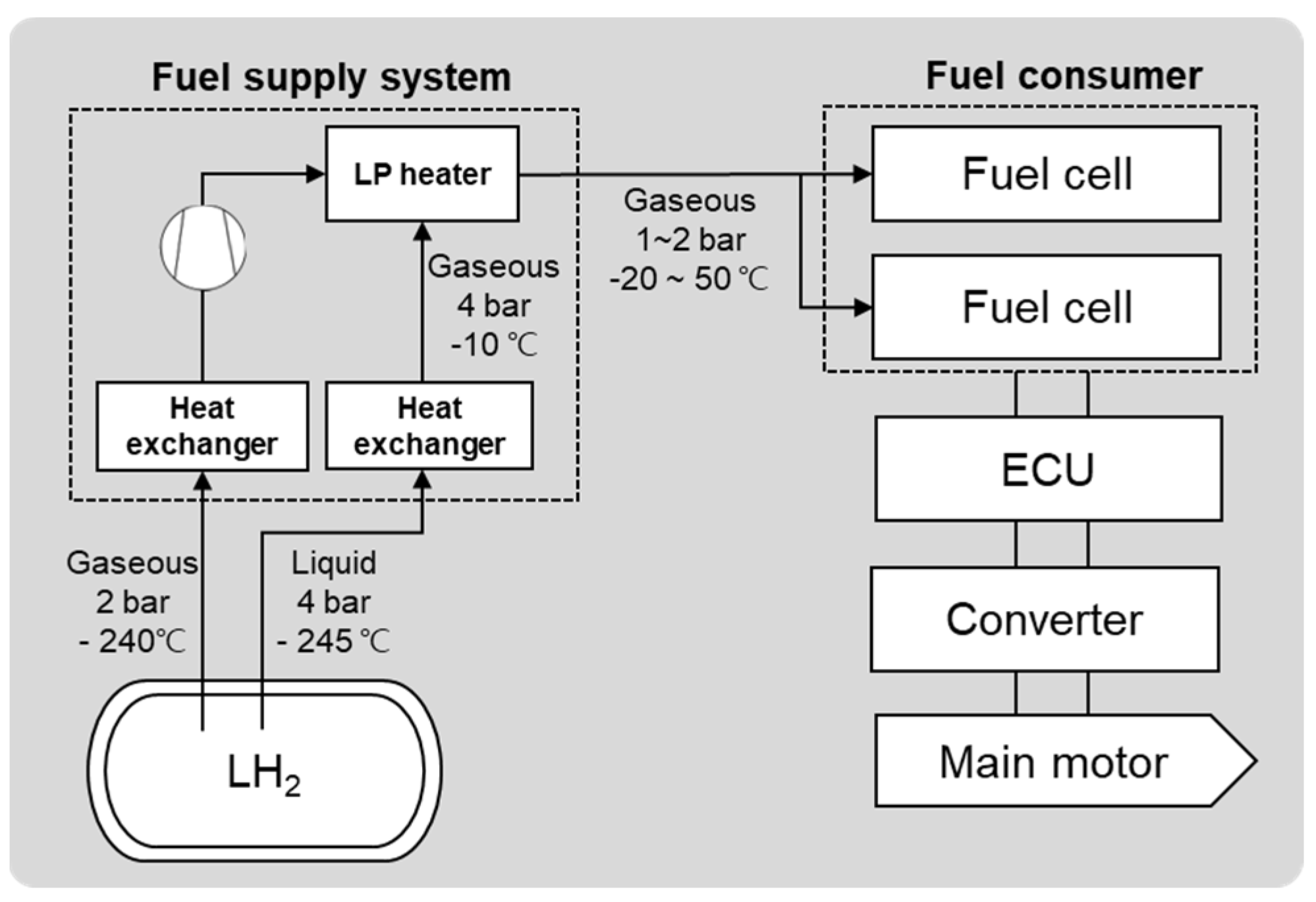
| Category | Material | Sensitivity of Hydrogen Embrittlement | ||
|---|---|---|---|---|
| Extreme | Moderate/Small | Negligible | ||
| Al alloy | Al 6061-T6 | ✓ | ||
| Fe | Steel | ✓ | ||
| Carbon steel | ✓ | |||
| Stainless steel | A286 | ✓ | ||
| 304ELC | ✓ | |||
| 310 | ✓ | |||
| 316 | ✓ | |||
| 410 | ✓ | |||
| Ti | Ti | ✓ | ||
| Code | Chemical Composition (%) | ||||
|---|---|---|---|---|---|
| C | Si | Mn | P | S | |
| KS D 3631 | <0.30 | <0.35 | <0.95 | <0.040 | <0.035 |
| Classification | List of Standards | |
|---|---|---|
| Material compatibility | ANSI/CSA CHMC1-2014 (R2018) Test Methods For Evaluating Material Compatibility In Compressed hydrogen Applications—Metals | |
| ASME BPVC Section VIII Division 3 KD-10 Special Requirements for Vessels in Hydrogen Service | ||
| SAE J2579 (2018) Standard for Fuel Systems in Fuel Cell and Other Hydrogen Vehicles | ||
| ISO 11114-4 (2017) Transportable gas cylinders—Compatibility of cylinder and valve materials with gas contents—Part 4: Test methods for selecting steels resistant to hydrogen embrittlement | ||
| Test method | H2 charging | ASTM G142-98 (2022) Standard Test Method for Determination of Susceptibility of Metals to Embrittlement in Hydrogen Containing Environments at High Pressure, High Temperature, or Both |
| ISO 16573-1 (2020) Steel—Measurement method for the evaluation of hydrogen embrittlement resistance of high-strength steels—Part 1: Constant load test ISO 16573-2 (2022) Steel—Measurement method for the evaluation of hydrogen embrittlement resistance of high-strength steels—Part 2: Slow strain rate test | ||
| Fatigue crack growth | ASTM E647 (2015) Standard Test Method for Measurement of Fatigue Crack Growth Rates | |
| Fatigue life | ASTM E466 (2021) Standard Practice for Conducting Force-Controlled Constant Amplitude Axial Fatigue Tests of Metallic Materials ASTM E606/E606M (2021) Standard Test Method for Strain-Controlled Fatigue Testing | |
| Fracture toughness | ASTM E1820 (2022) Standard Test Method for Measurement of Fracture Toughness | |
| ASTM E1681-03 (2020) Standard Test Method for Determining Threshold Stress Intensity Factor for Environment-Assisted Cracking of Metallic Materials (KIH) | ||
| ASTM E399 (2022) Standard Test Method for Linear–Elastic Plane-Strain Fracture Toughness of Metallic Materials (KIH) | ||
| Test Item | Testing Temp. Range | Remarks | ||
|---|---|---|---|---|
| Under 150 K | 150~270 K | Over 270 K | ||
| Tensile test | Low temperature | H2 environment and low temperature | H2 environment | - |
| Fatigue life test | - | - | H2 environment | Not necessary to consider evaluation at low temperature |
| Fracture mechanics test | Not reported | H2 environment and low temperature | H2 environment | Not reported under 150 K with H2 environment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Chun, K.W. A Comprehensive Review on Material Compatibility and Safety Standards for Liquid Hydrogen Cargo and Fuel Containment Systems in Marine Applications. J. Mar. Sci. Eng. 2023, 11, 1927. https://doi.org/10.3390/jmse11101927
Kim M-S, Chun KW. A Comprehensive Review on Material Compatibility and Safety Standards for Liquid Hydrogen Cargo and Fuel Containment Systems in Marine Applications. Journal of Marine Science and Engineering. 2023; 11(10):1927. https://doi.org/10.3390/jmse11101927
Chicago/Turabian StyleKim, Myung-Sung, and Kang Woo Chun. 2023. "A Comprehensive Review on Material Compatibility and Safety Standards for Liquid Hydrogen Cargo and Fuel Containment Systems in Marine Applications" Journal of Marine Science and Engineering 11, no. 10: 1927. https://doi.org/10.3390/jmse11101927
APA StyleKim, M.-S., & Chun, K. W. (2023). A Comprehensive Review on Material Compatibility and Safety Standards for Liquid Hydrogen Cargo and Fuel Containment Systems in Marine Applications. Journal of Marine Science and Engineering, 11(10), 1927. https://doi.org/10.3390/jmse11101927









