Spatial Distribution and Genetic Diversity of Turbot (Scophthalmus maximus, Linnaeus, 1758) in Bulgarian Black Sea Waters Relative to Fishing Pressure and Their Abiotic Environment
Abstract
:1. Introduction
2. Materials and Methods
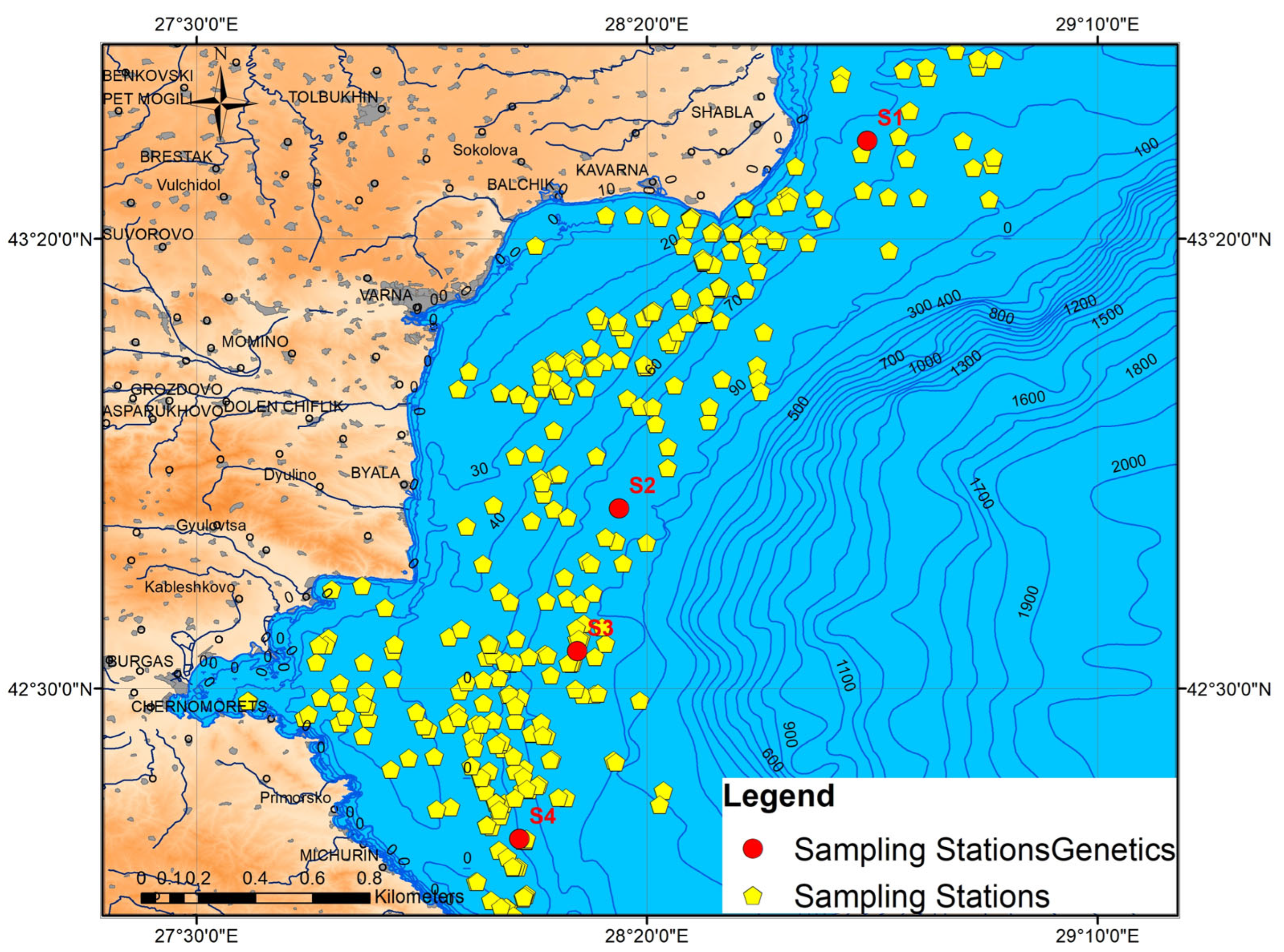
2.1. Study Area
2.2. Spatial Distribution Modeling
2.2.1. Spatial Modeling Software
2.2.2. Species Presence Data
2.2.3. Selection of Abiotic Variables
2.2.4. Model Evaluation and Validation
2.3. Mitochondrial DNA Analysis
2.3.1. Sample Collection and DNA Extraction
2.3.2. PCR and Sequence Analysis of Mitochondrial DNA (COIII)
2.3.3. Statistical Analyses
3. Results
3.1. Species Distribution Modeling
3.1.1. SDM of S. maximus and Variables’ Contribution to Species Spatial Dispersal
3.1.2. Evaluation of SDMs Performance
3.1.3. Model Validation with the Field-Measured Catch per Unit Effort (CPUE)
3.2. Mitochondrial DNA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grant, W.S. Status and trends in genetic resources of capture fisheries. In Proceedings of the Workshop on Status and Trends in Aquatic Genetic Resources: A Basis for International Policy, Victoria, BC, Canada, 8–10 May 2006. [Google Scholar]
- Neves, J.M.; Nolen, Z.J.; Fabré, N.N.; Mott, T.; Pereira, R.J. Genomic methods reveal independent demographic histories despite strong morphological conservatism in fish species. Heredity 2021, 127, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusoff, N.I.S.; Mat Jaafar, T.N.A.; Vilasri, V.; Mohd Nor, S.A.; Seah, Y.G.; Habib, A.; Wong, L.L.; Danish-Daniel, M.; Sung, Y.Y.; Mazlan, A.G.; et al. Genetic diversity, population structure and historical demography of the two-spined yellowtail stargazer (Uranoscopus cognatus). Sci. Rep. 2021, 11, 13357. [Google Scholar] [CrossRef]
- Scientific, Technical and Economic Committee for Fisheries (STECF). The 2017 Annual Economic Report on the EU Fishing Fleet (STECF-17-12); PUBSY No. JRC107883; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-73426-7. [Google Scholar] [CrossRef]
- Carson, S.; Shackell, N.; Mills Flemming, J. Local overfishing may be avoided by examining parameters of a spatio-temporal model. PLoS ONE 2017, 12, e0184427. [Google Scholar] [CrossRef] [PubMed]
- Parra, H.E.; Pham, C.K.; Menezes, G.M.; Rosa, A.; Tempera, F.; Morato, T. Predictive modeling of deep-sea fish distribution in the Azores. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 49–60. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Liu, M.; Tan, I.Z.; Chung, A.; Terrasa, B.; Guijarro, B.; Ordines, F.; Ramírez-Amaro, S.; Massutí, E.; Schunter, C. Declining population sizes and loss of genetic diversity in commercial fishes: A simple method for a first diagnostic. Front. Mar. Sci. 2022, 9, 872537. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2020; General Fisheries Commission for the Mediterranean: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Ovando, D.; Hilborn, R.; Monnahan, C.; Rudd, M.; Sharma, R.; Thorson, J.T.; Rousseau, Y.; Ye, Y. Improving estimates of the state of global fisheries depends on better data. Fish Fish. 2021, 22, 1377–1391. [Google Scholar] [CrossRef]
- Kenchington, E.L. The effects of fishing on species and genetic diversity. In Responsible Fisheries in the Marine Ecosystem; Sinclair, M., Valdimarsson, G., Eds.; CABI Publishing: Oxfordshire, UK, 2003; pp. 235–253. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Palumbi, S.R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014, 23, 29–39. [Google Scholar] [CrossRef]
- Bryndum-Buchholz, A.; Tittensor, D.P.; Lotze, H.K. The status of climate change adaptation in fisheries management: Policy, legislation and implementation. Fish Fish. 2021, 22, 1248–1273. [Google Scholar] [CrossRef]
- Barbut, L.; Groot Crego, C.; Delerue-Ricard, S.; Vandamme, S.; Volckaert, F.A.; Lacroix, G. How larval traits of six flatfish species impact connectivity. Limnol. Oceanogr. 2019, 64, 1150–1171. [Google Scholar] [CrossRef]
- Rodriguez-Mendoza, R.; Saborido-Rey, F. The potential use of genomic methods in bottom trawl surveys to improve stock assessments in Europe. Front. Mar. Sci. 2023, 10, 1095171. [Google Scholar] [CrossRef]
- Hemmer-Hansen, J.; Nielsen, E.; Frydenberg, J.; Loeschcke, V. Adaptive divergence in a high gene flow environment: Hsc70 variation in the European flounder (Platichthys flesus L.). Heredity 2007, 99, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, S.G.; Maes, G.E.; Raeymaekers, J.A.M.; Cottenie, K.; Imsland, A.K.; Hellemans, B.; Volckaert, F.A.M. Regional environmental pressure influences population differentiation in turbot (Scophthalmus maximus). Mol. Ecol. 2014, 23, 618–636. [Google Scholar] [CrossRef] [PubMed]
- Niţă, V.; Diaconescu, Ş.; Zaharia, T.; Maximov, V.; Nicolae, C.; Micu, D. The characterization of the main habitat types populated by the Black Sea Turbot in its different stages of development. Aquac. Aquar. Conserv. Legis. 2011, 4, 552–570. Available online: http://www.bioflux.com.ro/aacl (accessed on 1 March 2023).
- STECF. Advice for Sprat and Turbot in Theblack Sea, Commission Staff Working Paper. 2007. Available online: https://stecf.jrc.ec.europa.eu/documents/43805/55543/07-10_Black+Sea+advice+for+sprat+and+turbot.pdf (accessed on 8 September 2023).
- Yeaman, S.; Whitlock, M.C. The genetic architecture of adaptation under migration–selection balance. Evolution 2011, 65, 1897–1911. [Google Scholar] [CrossRef]
- Orsini, L.; Vanoverbeke, J.; Swillen, I.; Mergeay, J.; De Meester, L. Drivers of population genetic differentiation in the wild: Isolation by dispersal limitation, isolation by adaptation and isolation by colonization. Mol. Ecol. 2013, 22, 5983–5999. [Google Scholar] [CrossRef]
- Vilas, R.; Bouza, C.; Vera, M.; Millán, A.; Martínez, P. Variation in anonymous and EST-microsatellites suggests adaptive population divergence in turbot. Mar. Ecol. Prog. Ser. 2010, 420, 231–239. [Google Scholar] [CrossRef]
- Vilas, R.; Vandamme, S.G.; Vera, M.; Bouza, C.; Maes, G.E.; Volckaert, F.A.; Martínez, P. A genome scan for candidate genes involved in the adaptation of turbot (Scophthalmus maximus). Mar. Genom. 2015, 23, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Diopere, E.; Vandamme, S.G.; Hablützel, P.I.; Cariani, A.; Van Houdt, J.; Rijnsdorp, A.; Tinti, F.; FishPopTrace Consortium; Volckaert, F.A.M.; Maes, G.E. Seascape genetics of a flatfish reveals local selection under high levels of gene flow. ICES J. Mar. Sci. 2018, 75, 675–689. [Google Scholar] [CrossRef]
- Do Prado, F.D.; Vera, M.; Hermida, M.; Bouza, C.; Pardo, B.G.; Vilas, R.; Blanco, A.; Fernández, C.; Maroso, F.; Maes, G.E.; et al. Parallel evolution and adaptation to environmental factors in a marine flatfish: Implications for fisheries and aquaculture management of the turbot (Scophthalmus maximus). Evol. Appl. 2018, 11, 1322–1341. [Google Scholar] [CrossRef]
- Tserkova, F.; Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Klisarova, D.; Gerdzhikov, D.; Maximov, V.; Radu, G. Stock Assessment of Scophtalmus maximus in Bulgarian Black Sea zone during autumn 2015. Proc. Inst. Fish. Resour. 2017, 28, 24–33. [Google Scholar]
- Ivanova, P.; Dzhembekova, N.; Atanassov, I.; Rusanov, K.; Raykov, V.; Zlateva, I.; Yankova, M.; Raev, Y.; Nikolov, G. Genetic diversity and morphological characterisation of three turbot (Scophthalmus maximus L., 1758) populations along the Bulgarian Black Sea coast. Nat. Conserv. 2021, 43, 123–146. [Google Scholar] [CrossRef]
- Turan, C.; Ivanova, P.P.; Raykov, V.S.; Gurlek, M.; Erguden, D.; Yaglioglu, D.; Karan, S.; Dodgu, S.A.; Uyan, A.; Ozturk, B.; et al. Genetics structure analysis of turbot (Scophthalmus maximus, Linnaeus, 1758) in the Black and Mediterranean Seas for application of innovative Management Strategies. Front. Mar. Sci. 2019, 6, 740. [Google Scholar] [CrossRef]
- Syphard, A.D.; Franklin, J. Differences in spatial predictions among species distribution modeling methods vary with species traits and environmental predictors. Ecography 2009, 32, 907–918. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Yu, H.; Cooper, A.R.; Infante, D.M. Improving species distribution model predictive accuracy using species abundance: Application with boosted regression trees. Ecol. Model. 2020, 432, 109202. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modeling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Li, W.; Guo, Q.; Elkan, C. Can we model the probability of presence of species without absence data? Ecography 2011, 34, 1096–1105. [Google Scholar] [CrossRef]
- Buebos-Esteve, D.E.; Mamasig, G.D.N.S.; Ringor, A.M.D.; Layog, H.N.B.; Murillo, L.C.S.; Dagamac, N.H.A. Modeling the potential distribution of two immortality flora in the Philippines: Applying MaxEnt and GARP algorithms under different climate change scenarios. Model. Earth Syst. Environ. 2023, 9, 2857–2876. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S. Principal bioclimatic variables of ten dominant plant species in Korea wetland using the Maxent model. Ecol. Eng. 2022, 183, 106729. [Google Scholar] [CrossRef]
- Gonzaleza, C.R.; Arciaa, Y.A.; Bussa, E.F. Remote Sensing and modeling tools exploration for habitat delimitation of leishmaniasis transmitting vectors. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2021, XLVI-4/W2-2021, 139–145. [Google Scholar] [CrossRef]
- Gülçin, D.; Arslan, E.S.; Örücü, Ö.K. Effects of climate change on the ecological niche of common hornbeam (Carpinus betulus L.). Ecol. Inform. 2021, 66, 101478. [Google Scholar] [CrossRef]
- Jan, A.; Arismendi, I.; Giannico, G.; Flitcroft, R. Habitat overlap among native and introduced cold-water fishes in the Himalayas. Sci. Rep. 2023, 13, 15033. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. MaxEnt modelling and impact of climate change on habitat suitability variations of economically important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Mukherjeeq, S.S.; Purohit, S.; Bag, S.; Aditya, G.; Hossain, A. Species Distribution Modeling of Variable Tawny Rajah (Charaxes bernardus hierax) in some unusual habitats of West Bengal, India: Implications for conservation. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Oyarzun, M.C. Predicting Archaeological Site Locations in Northeastern California’s High Desert using the Maxent Model. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 2016. [Google Scholar]
- Solanou, M.; Valavanis, V.D.; Karachle, P.K.; Giannoulaki, M. Looking at the Expansion of Three Demersal Lessepsian Fish Immigrants in the Greek Seas: What Can We Get from Spatial Distribution Modeling? Diversity 2023, 15, 776. [Google Scholar] [CrossRef]
- Soliman, M.M.; Al-Khalaf, A.A.; El-Hawagry, M.S. Effects of Climatic Change on Potential Distribution of Spogostylum ocyale (Diptera: Bombyliidae) in the Middle East Using Maxent Modelling. Insects 2023, 14, 120. [Google Scholar] [CrossRef]
- Tadesse, Z.; Nemomissa, S.; Lemessa, D. Predicting the distributions of Pouteria adolfi-friederici and Prunus africana iconic tree species under current and future climate change scenarios in Ethiopia. bioRxiv 2021. [Google Scholar] [CrossRef]
- Yousaf, A.; Hadi, R.; Khan, N.; Ibrahim, F.; Moin, H.; Rahim, S.; Hussain, M. Identification of suitable habitat for Taxus wallichiana and Abies pindrow in moist temperate forest using maxent modelling technique. Saudi J. Biol. Sci. 2022, 29, 103459. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Petrova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Klisarova, D.; Gerdjikov, D.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Spring 2017; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2017; Available online: https://www.ifrvarna.com/images/files/Publikacii/4.%20Bottom%20Trawl%20Surveys%20In%20The%20Bulgarian%20Black%20Sea%20Area,%20Spring%202017.pdf (accessed on 20 March 2023).
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Klisarova, D.; Gerdjikov, D.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2017; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2018. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Klisarova, D.; Gerdjikov, D.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Spring 2018; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2018. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2018; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2019. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Spring 2019; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2019. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2019; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2020. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Summer 2020; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2020. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2020; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2021. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Spring-Summer 2021; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2021. [Google Scholar]
- Petrova-Pavlova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2021; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2022. [Google Scholar]
- Damalas, D.; Katsanevakis, S.; Maravelias, C.D.; Karageorgis, A.P. Habitat selection of flatfish in relation to Spatial, temporal and environmental parameters in the Aegean Sea. In Proceedings of the 9th Symposium on Oceanography & Fisheries, Patras, Greece, 13–16 May 2009. [Google Scholar]
- Lauria, V.; Vaz, S.; Martin, C.S.; Mackinson, S.; Carpentier, A. What influences European plaice (Pleuronectes platessa) distribution in the eastern English Channel? Using habitat modelling and GIS to predict habitat utilization. ICES J. Mar. Sci. 2011, 68, 1500–1510. [Google Scholar] [CrossRef]
- Baptista, V.; Blasco, I.P.; Bueno-Pardo, J.; Teodosio, M.; Leitão, F. Environmental variability and fishing effects on artisanal flatfish fisheries along the Portuguese coast. Front. Mar. Sci. 2022, 9, 844158. [Google Scholar] [CrossRef]
- De Raedemaecker, F.; Brophy, D.; O’Connor, I.; O’Neill, B. Dependence of RNA: DNA ratios and Fulton’s K condition indices on environmental characteristics of plaice and dab nursery grounds. Estuar. Coast. Shelf Sci. 2012, 1, 60–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Brzezinski, D.; Chang, J.H.; Stepanek, K.; Chen, Y. Spatial structuring of fish community in association with environmental variables in the coastal Gulf of Maine. J. Northw. Atl. Fish. Sci. 2010, 43, 47–64. [Google Scholar] [CrossRef]
- Araújo, F.G.; de Azevedo, M.C.C.; de Araújo Silva, M.; Pessanha, A.L.M.; Gomes, I.D.; da Cruz-Filho, A.G. Environmental influences on the demersal fish assemblages in the Sepetiba Bay, Brazil. Estuaries 2002, 25, 441–450. [Google Scholar] [CrossRef]
- Essington, T.E.; Anderson, S.C.; Barnett, L.A.; Berger, H.M.; Siedlecki, S.A.; Ward, E.J. Advancing statistical models to reveal the effect of dissolved oxygen on the spatial distribution of marine taxa using thresholds and a physiologically based index. Ecography 2022, 8, e06249. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Santos, P.T. Factors affecting the distribution of epibenthic biodiversity in the Cávado estuary (NW Portugal). J. Integr. Coast. Zone Manag. 2013, 13, 101–111. [Google Scholar] [CrossRef]
- Claireaux, G.; Lefrançois, C. Linking environmental variability and fish performance: Integration through the concept of scope for activity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 2031–2204. [Google Scholar] [CrossRef]
- Craig, J.K. Aggregation on the edge: Effects of hypoxia avoidance on the spatial distribution of brown shrimp and demersal fishes in the Northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 445, 75–95. [Google Scholar] [CrossRef]
- Vestfals, C.D.; Ciannelli, L.; Hoff, G.R. Changes in habitat utilization of slope-spawning flatfish across a bathymetric gradient. ICES J. Mar. Sci. 2016, 73, 1875–1889. [Google Scholar] [CrossRef]
- Madec, G. NEMO Reference Manual 3_6_STABLE: “NEMO Ocean Engine” Note du Pôle de Modélisation; Institut Pierre-Simon Laplace (IPSL): Guyancourt, France, 2016; ISSN 1288-1619. [Google Scholar]
- Capet, A.; Meysman, F.J.; Akoumianaki, I.; Soetaert, K.; Grégoire, M. Integrating sediment biogeochemistry into 3D oceanic models: A study of benthic-pelagic coupling in the Black Sea. Ocean Model. 2016, 101, 83–100. [Google Scholar] [CrossRef]
- Grégoire, M.; Raick, C.; Soetaert, K. Numerical modeling of the central Black Sea ecosystem functioning during the eutrophication phase. Prog. Oceanogr. 2008, 76, 286–333. [Google Scholar] [CrossRef]
- Grégoire, M.; Soetaert, K. Carbon, nitrogen, oxygen and sulfide budgets in the Black Sea: A biogeochemical model of the whole water column coupling the oxic and anoxic parts. Ecol. Model. 2010, 221, 2287–2301. [Google Scholar] [CrossRef]
- Lima, L.; Aydogdu, A.; Escudier, R.; Masina, S.; Ciliberti, S.A.; Azevedo, D.; Peneva, E.L.; Causio, S.; Cipollone, A.; Clementi, E.; et al. Black Sea Physical Reanalysis (CMEMS BS-Currents) (Version 1) [Data Set]. CMEMS. 2020. Available online: https://data.marine.copernicus.eu/product/BLKSEA_MULTIYEAR_PHY_007_004/description (accessed on 15 February 2023). [CrossRef]
- Jansen, E.; Martins, D.; Stefanizzi, L.; Ciliberti, S.A.; Gunduz, M.; Ilicak, M.; Lecci, R.; Cretí, S.; Causio, S.; Aydoğdu, A.; et al. Black Sea Physical Analysis and Forecast (Copernicus Marine Service BS-Currents, EAS5 System) (Version 1) [Data Set]. CMEMS. 2022. Available online: https://data.marine.copernicus.eu/product/BLKSEA_ANALYSISFORECAST_PHY_007_001/description (accessed on 15 February 2023). [CrossRef]
- The MathWorks Inc. MATLAB, Version: 2020a; The MathWorks Inc.: Natick, MA, USA, 2020; Available online: https://www.mathworks.com (accessed on 18 February 2023).
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Valles-Jiménez, R. Estudios Sobre la Estructura Genética del Camarón Blanco (Litopenaeus vannamei), del Pacífico Oriental Inferidos del Análisis de Microsatélites y ADN Mitocondrial. Ph.D. Thesis, Centro de Investigaciones Biológicas Del Noroeste, S.C., La Paz, Mexico, 2005; 74p. Available online: http://dspace.cibnor.mx:8080/handle/123456789/122 (accessed on 18 April 2023).
- Kumar, S.; Stecher, G.; Tamura, K. MEGAX: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2002, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Jiang, Y.; Li, Z.; Li, X.; Xu, W.; Wei, H.; Li, Y.; Li, X. Genetic diversity and variation of seven Chinese grass shrimp (Palaemonetes sinensis) populations based on the mitochondrial COI gene. BMC Ecol. Evol. 2021, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Z.; Zhang, Y.; Gao, T.; Zou, J.; Zhang, X. Evidence for intra-mitochondrial variation in population genetic structure of Platycephalus sp.1 in the northwestern Pacific. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2019, 30, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, A. Population genetic diversity of yellow barbell (Carasobarbus luteus) from Kueik, Euphrates and Tigris Rivers based on mitochondrial DNA D-loop sequences. Turkish J. Fish. Aquat. Sci. 2019, 20, 79–86. [Google Scholar] [CrossRef]
- Yorisue, T.; Iguchi, A.; Yasuda, N.; Yoshioka, Y.; Sato, T.; Fujita, Y. Evaluating the effect of overharvesting on genetic diversity and genetic population structure of the coconut crab. Sci. Rep. 2020, 10, 10026. [Google Scholar] [CrossRef]
- Murray, J.W.; Top, Z.; Özsoy, E. Hydrographic properties and ventilation of the Black Sea. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, S663–S689. [Google Scholar] [CrossRef]
- Konovalov, S.K.; Murray, J.W.; Luther III, G.W. Black Sea Biogeochemistry. Oceanography 2005, 18, 24–35. [Google Scholar] [CrossRef]
- Olden, J.D.; Neff, B.D. Cross-correlation bias in lag analysis of aquatic time series. Mar. Biol. 2001, 138, 1063–1070. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Tompkins, D.M.; Poulin, R. Climate warming may cause a parasite-induced collapse in coastal amphipod populations. Oecologia 2005, 146, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Traill, L.W.; Lim, M.L.; Sodhi, N.S.; Bradshaw, C.J. Mechanisms driving change: Altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 2010, 79, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Reiss, H.; Cunze, S.; König, K.; Neumann, H.; Kröncke, I. Species distribution modelling of marine benthos: A North Sea case study. Mar. Ecol. Prog. Ser. 2011, 442, 71–86. [Google Scholar] [CrossRef]




| Season | Demersal Trawl Surveys (Turbot Stock Assessment) | Pelagic Trawl Surveys (Multi-Species Stock Assessment) | Other Sampling |
|---|---|---|---|
| Spring | 2017 (May) 2018 (May–June) 2021 (May) | 2019 (April) 2021 (April)—genetic markers | |
| Summer | 2020 (July–August) | ||
| Autumn | 2017 (December) | 2017 (December) | |
| 2018 (December) | 2018 (December) | ||
| 2019 (December) | 2019 (October–November) | ||
| 2020 (December) | 2020 (November) |
| Sampling Location | Station Coordinates (DD) | Date | Depth | |
|---|---|---|---|---|
| Latitude °N | Longitude °E | |||
| S1. Shabla | 43.514 | 28.741 | March 2019 | 70–80 m |
| S2. Shkorpilovtsi | 42.833 | 28.282 | April 2020/2021 | 70–80 m |
| S3. Nesebar | 42.569 | 28.204 | April 2020/2021 | 70–80 m |
| S4. Tsarevo | 42.221 | 28.097 | April 2021 | 70–80 m |
| Variable | Percentage Contribution | Permutation Importance |
|---|---|---|
| Temperature (temp_eez) | 90.20 | 56.4 |
| Dissolved oxygen (do_eez) | 7.5 | 35.1 |
| Salinity (sal_eez) | 1.8 | 3.1 |
| Current speed (curr_eez) | 0.5 | 5.4 |
| Sampling Location | mtDNA COIII | ||||||
|---|---|---|---|---|---|---|---|
| Genetic Polymorphism | Neutrality Test | ||||||
| N | S | h | Hd (s.d.) | π (s.d.) | Tajima’s D | Fu’s Fs | |
| S1. Shabla | 30 | 8 | 8 | 0.418 (0.114) | 0.00128 (0.00044) | −2.123 * | −5.509 |
| S2. Nesebar | 34 | 12 | 12 | 0.783 (0.057) | 0.00233 (0.00037) | −1.889 * | −7.824 |
| S3. Shkorpilovtsi | 29 | 5 | 6 | 0.594 (0.095) | 0.00146 (0.00033) | −0.974 | −2.297 |
| S4. Tsarevo | 40 | 9 | 8 | 0.363 (0.098) | 0.00139 (0.00045) | −1.990 * | −4.485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlateva, I.; Ivanova, P.; Dzhembekova, N.; Doncheva, V.; Popov, I.; Slabakova, V.; Raev, Y.; Raykov, V.; Dimitrov, D. Spatial Distribution and Genetic Diversity of Turbot (Scophthalmus maximus, Linnaeus, 1758) in Bulgarian Black Sea Waters Relative to Fishing Pressure and Their Abiotic Environment. J. Mar. Sci. Eng. 2023, 11, 1982. https://doi.org/10.3390/jmse11101982
Zlateva I, Ivanova P, Dzhembekova N, Doncheva V, Popov I, Slabakova V, Raev Y, Raykov V, Dimitrov D. Spatial Distribution and Genetic Diversity of Turbot (Scophthalmus maximus, Linnaeus, 1758) in Bulgarian Black Sea Waters Relative to Fishing Pressure and Their Abiotic Environment. Journal of Marine Science and Engineering. 2023; 11(10):1982. https://doi.org/10.3390/jmse11101982
Chicago/Turabian StyleZlateva, Ivelina, Petya Ivanova, Nina Dzhembekova, Valentina Doncheva, Ivan Popov, Violeta Slabakova, Yordan Raev, Violin Raykov, and Dimitar Dimitrov. 2023. "Spatial Distribution and Genetic Diversity of Turbot (Scophthalmus maximus, Linnaeus, 1758) in Bulgarian Black Sea Waters Relative to Fishing Pressure and Their Abiotic Environment" Journal of Marine Science and Engineering 11, no. 10: 1982. https://doi.org/10.3390/jmse11101982





