Pathology, Enzyme Activity and Immune Responses after Cryptocaryon irritans Infection of Golden Pompano Trachinotus ovatus (Linnaeus 1758)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. C. irritans Challenge and Sampling
2.3. HE Staining of Tissue Sections
2.4. Determination of Immunity-Related Enzyme Activities
2.5. Cloning of the NEMO Gene
2.6. Bioinformatic Analysis of the NEMO Gene
2.7. Quantitative Real-Time PCR and Statistical Analysis
2.8. Statistical Analysis
3. Result
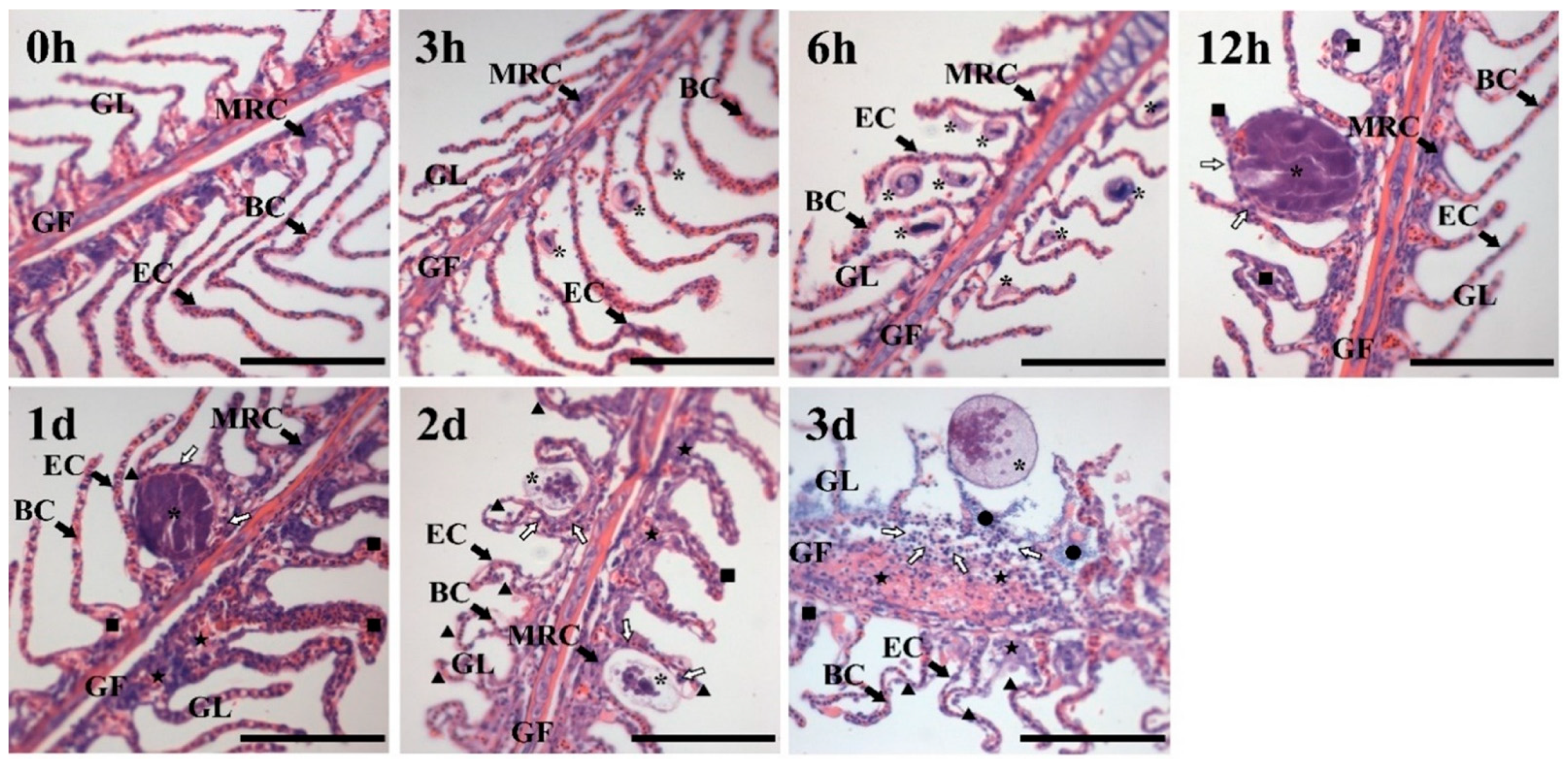
3.1. Histological Analysis
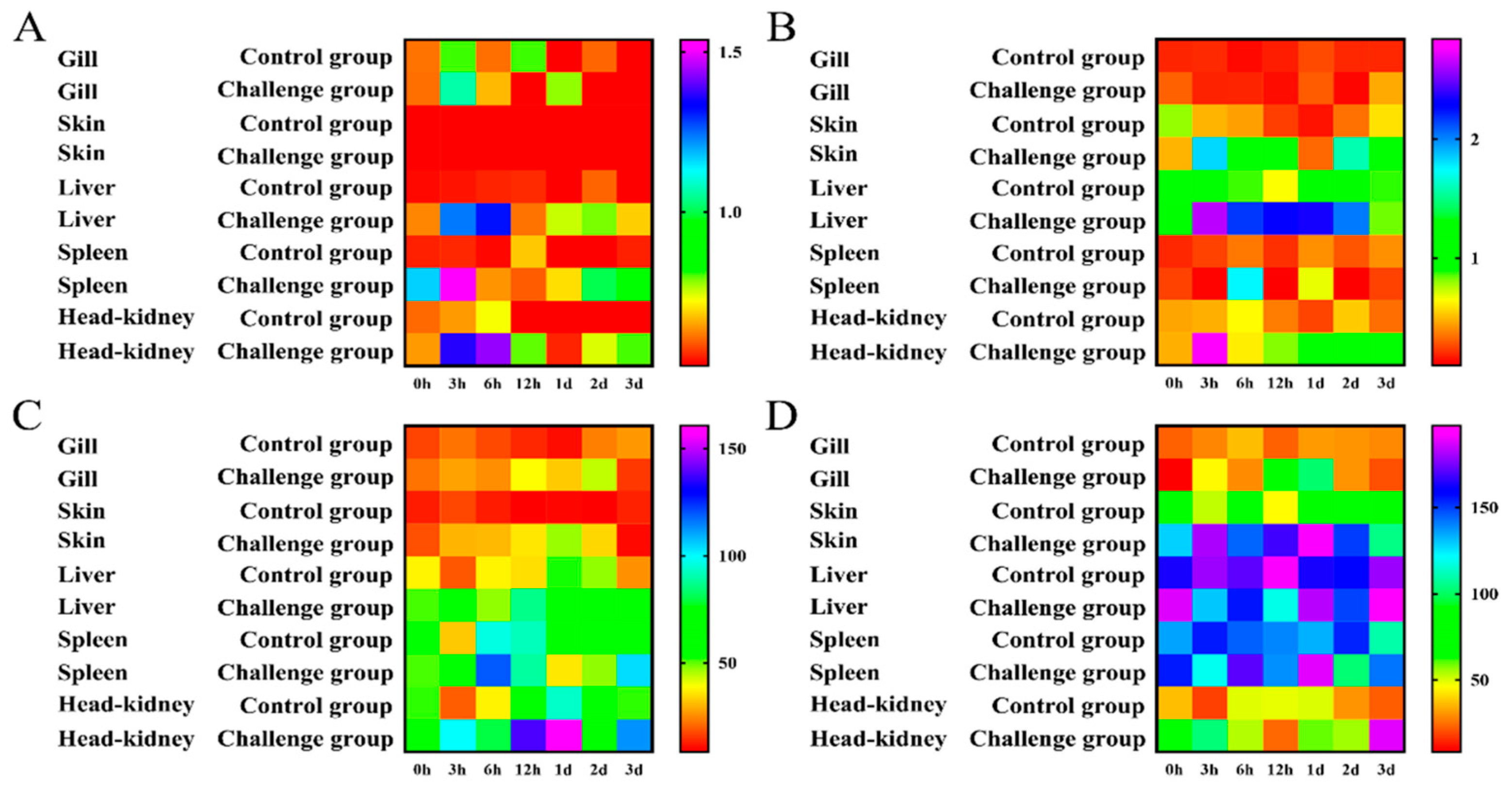
3.2. Immune-Related Enzyme Activity Analysis
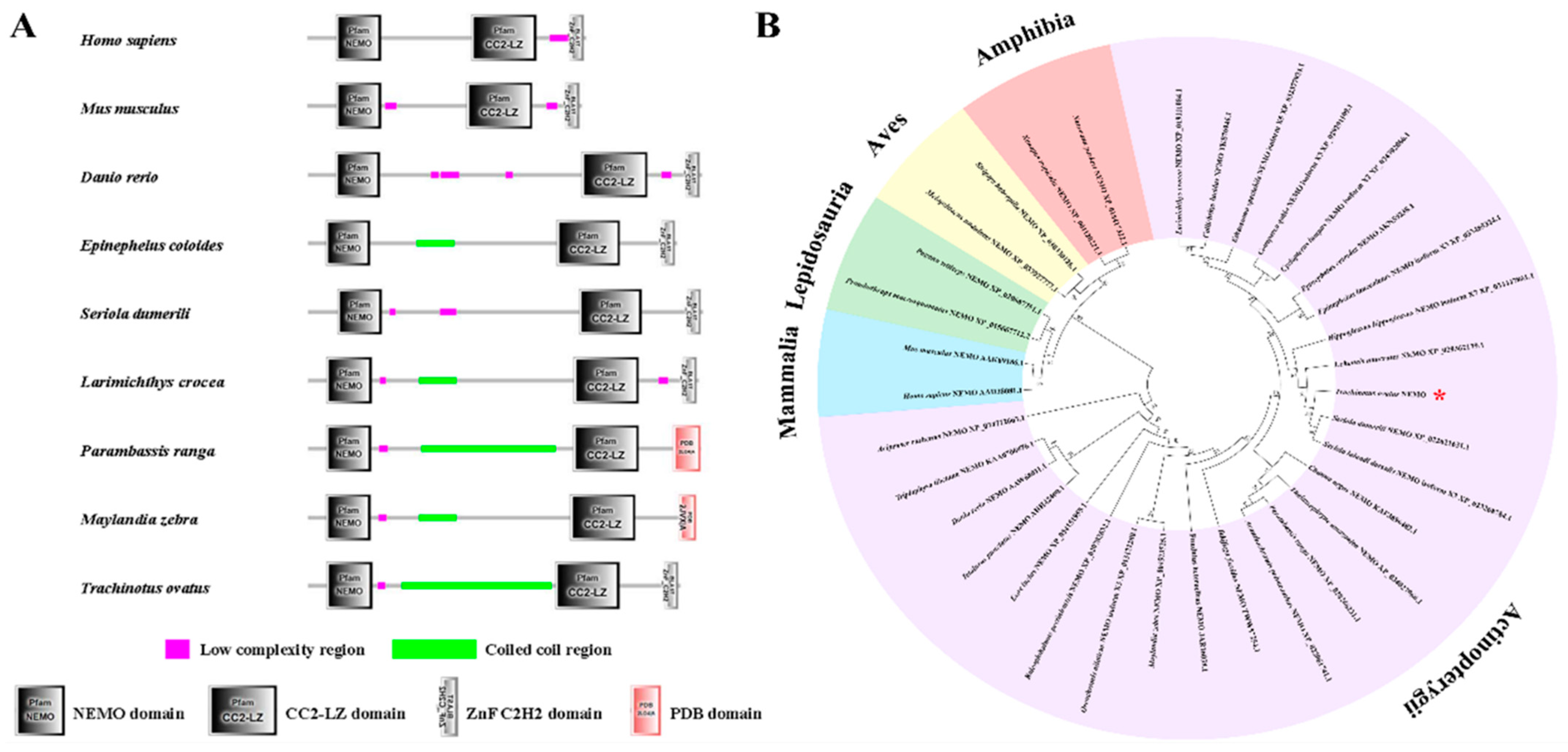
3.3. NEMO Bioinformatic Analysis
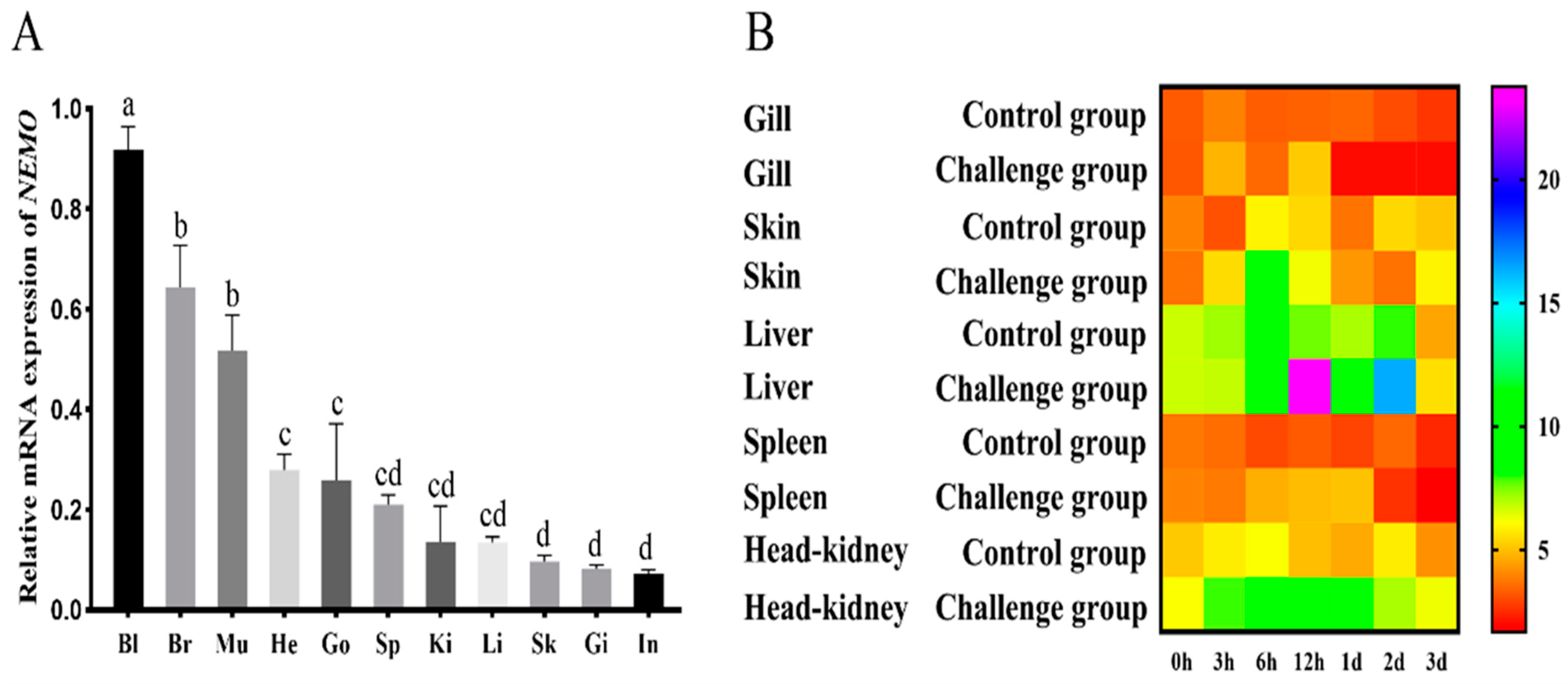
3.4. NEMO Gene Expression Pattern Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colorni, A.; Burgrss, P. Cryptocaryon irritans Brown 1951, the cause of ‘white spot disease’ in marine fish: An update. Aquar. Sci. Conserv. 1997, 1, 217–238. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Jiang, B.; Li, Z.C.; Li, S.Y.; Li, A.X. Quantification of parasite abundance: A novel method to evaluate anti-Cryptocaryon irritans efficacy. Aquaculture 2020, 528, 735482. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, X.M.; Yang, J.L.; Zhang, R.R.; Zhang, Q.; Yang, J.M. The bacteriolytic mechanism of an invertebrate-type lysozyme from mollusk Octopus ocellatus. Fish Shellfish Immunol. 2019, 93, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Liang, W.W.; Chen, M.; Huang, T.; Li, L.P.; Lei, A.Y.; Wang, R.; Yang, X.M.; Luo, H.L.; Ouyang, X.H. Histopathology study on the Cryptocaryon irritans disease of Trachinotus ovatus. J. Fish Res. 2017, 39, 181–187. (In Chinese) [Google Scholar]
- Braue, J.; Murugesan, V.; Holland, S.; Patel, N.; Naik, E.; Leiding, J.; Yacoub, A.T.; Prieto-Granada, C.N.; Greene, J.N. NF-kappaB Essential Modulator Deficiency Leading to Disseminated Cutaneous Atypical Mycobacteria. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, M.J.; D`Acquisto, D.; Madge, L.A.; Glochner, J.; Pober, J.S.; Ghosh, S. Selective Inhibition of NF-κB Activation by a Peptide That Blocks the Interaction of NEMO with the IκB Kinase Complex. Science 2000, 289, 1550–1554. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IκB kinase complex: Master regulator of NF-κB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [Green Version]
- Scheidereit, C. IkappaB kinase complexes: Gateways to NF-kappaB activation and transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Dan, X.M.; Sun, P.; Shi, Z.H.; Gao, Q.X.; Peng, S.M.; Li, A.X. Growth, feed intake and immune responses of orange-spotted grouper (Epinephelus coioides) exposed to low infectious doses of ectoparasite (Cryptocaryon irritans). Fish Shellfish Immunol. 2014, 36, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.H.; Wang, S.P.; Jiang, H.X.; Nie, G.X.; Li, X.J. Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat. Toxicol. 2012, 120, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.K.; Hur, Y.B. Toxic effects of waterborne nitrite exposure on antioxidant responses, acetylcholinesterase inhibition, and immune responses in olive flounders, Paralichthys olivaceus, reared in bio-floc and seawater. Fish Shellfish Immunol. 2020, 97, 581–586. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Liu, M.; Huang, M.X. Molecular cloning, expression and antibacterial activity of goose-type lysozyme gene in Microptenus salmoides. Fish Shellfish Immunol. 2018, 82, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.H.; Guo, W.L.; Lei, Y.; Wang, F.; Wang, S.F.; Sun, Y.; Hu, W.T.; Zhou, Y.C. Antiparasitic efficacy of honokiol against Cryptocaryon irritans in pompano, Trachinotus ovatus. Aquaculture 2019, 500, 398–406. [Google Scholar] [CrossRef]
- Watanabe, Y.; How, K.H.; Zenke, K.; Itoh, N.; Yoshinaga, T. Characterization of the proteases in the parasitic stage of Cryptocaryon irritans, and in vitro and in vivo effects of protease inhibitors on cryptocaryoniasis. Aquaculture 2019, 512, 734311. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, K.C.; Guo, L.; Liu, B.S.; Guo, H.Y.; Zhang, N.; Yang, J.W.; Zhang, D.C. Molecular characterization of GRP94 and HSP90 alpha from Trachinotus ovatus, Linnaeus 1758 and their expression responses to various levels of stocking density stress and Cryptocaryon irritans infection. Aquaculture 2019, 529, 735601. [Google Scholar] [CrossRef]
- Guo, L.; He, P.Y.; Zhu, K.C.; Guo, H.Y.; Liu, B.S.; Zhang, N.; Jiang, J.G.; Zhang, D.C. Functional identification of ToLAAO genes and polymorphism association analysis of Cryptocaryon irritans resistance in Trachinotus ovatus. Aquac. Res. 2021, 53, 208–220. [Google Scholar] [CrossRef]
- Zhu, K.C.; Liu, J.; Liu, B.S.; Guo, H.Y.; Zhang, N.; Guo, L.; Jiang, S.G.; Zhang, D.C. Functional characterization of four ToRac genes and their association with anti-parasite traits in Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2022, 560, 738514. [Google Scholar] [CrossRef]
- Dan, X.M.; Li, A.X.; Lin, X.T.; Teng, N.; Zhu, X.Q. A standardized method to propagate Cryptocaryon irritans on a susceptible host pompano Trachinotus ovatus. Aquaculture 2006, 258, 127–133. [Google Scholar] [CrossRef]
- Yin, F.; Sun, P.; Tang, B.J.; Dan, X.M.; Li, A.X. Immunological, ionic and biochemical responses in blood serum of the marine fish Trachinotus ovatus to poly-infection by Cryptocaryon irritans. Exp. Parasitol. 2015, 154, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Guo, L.; Guo, H.Y.; Zhu, K.C.; Li, S.Q.; Zhang, Y.; Zhang, N.; Liu, B.S.; Jiang, S.G.; Li, J.T. Chromosome-level genome assembly of golden pompano (Trachinotus ovatus) in the family Carangidae. Sci. Data 2019, 6, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.C.; Song, L.; Guo, H.Y.; Guo, L.; Zhang, N.; Liu, B.S.; Jiang, S.G.; Zhang, D.C. Elovl4a participates in LC-PUFA biosynthesis and is regulated by PPAR alpha beta in golden pompano Trachinotus ovatus (Linnaeus 1758). Sci. Rep.-Uk. 2019, 9, 4684. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Jiang, B.; Dan, X.M.; Li, A.X. Advances in the research on mucosal immune response of fish against Cryptocaryon irritans infection. J. Fish. China 2019, 43, 156–167. (In Chinese) [Google Scholar]
- Cheng, T.; Dougherty, W. Ultrastructural evidence for the destruction of Schistosoma mansoni sporocysts associated with elevated lysosomal enzyme levels in Biomphalaria glabrata. J. Parasitol. 1989, 75, 928–941. [Google Scholar] [CrossRef]
- Xing, J.; Zhan, W.B.; Zhou, L. Endoenzymes associated with haemocyte types in the scallop (Chlamys farreri). Fish Shellfish Immunol. 2002, 13, 271–278. [Google Scholar] [CrossRef]
- Huo, D.; Sun, L.; Ru, X.; Zhang, L.; Lin, C.; Liu, S.; Xin, X.; Yang, H. Impact of hypoxia stress on the physiological responses of sea cucumber Apostichopus japonicus: Respiration, digestion, immunity and oxidative damage. Peer J. 2018, 6, e4651. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Ge, X.; Zhu, J.; Xuan, F.; Jiang, X. Identification and mRNA expression of antioxidant enzyme genes associated with the oxidative stress response in the Wuchang bream (Megalobrama amblycephala Yih) in response to acute nitrite exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 159, 69–77. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; He, M.; Liu, A.; Long, H.; Guo, W.; Cao, Z.; Xie, Z.; Zhou, Y. Construction and analysis of the immune effect of Vibrio harveyi subunit vaccine and DNA vaccine encoding TssJ antigen. Fish Shellfish Immunol. 2020, 98, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Dan, X.M.; Zhang, T.W.; Luo, X.C.; Li, A.X. Immune-related genes expression profile in orange-spotted grouper during exposure to Cryptocaryon irritans. Parasite Immunol. 2011, 33, 679–987. [Google Scholar] [CrossRef] [PubMed]
- Rushe, M.; Silvian, L.; Bixler, S.; Chen, L.L.; Cheung, A.; Bowes, S.; Cuervo, H.; Berkowitz, S.; Zheng, T.; Guckian, K.; et al. Structure of a NEMO/IKK-associating domain reveals architecture of the interaction site. Structure 2008, 16, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barczewski, A.H.; Ragusa, M.J.; Mierke, D.F.; Pellegrini, M. The IKK-binding domain of NEMO is an irregular coiled coil with a dynamic binding interface. Sci. Rep. 2019, 9, 2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.W.; Luo, X.C.; Dan, X.M.; Huang, X.Z.; Qiao, W.; Zhong, Z.P.; Li, A.X. Orange-spotted grouper (Epinephelus coioides) TLR2, MyD88 and IL-1beta involved in anti-Cryptocaryon irritans response. Fish Shellfish Immunol. 2011, 30, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.C.; Liu, B.S.; Zhang, N.; Guo, H.Y.; Guo, L.; Jiang, S.G.; Zhang, D.C. Interferon regulatory factor 2 plays a positive role in interferon gamma expression in golden pompano, Trachinotus ovatus (Linnaeus 1758). Fish Shellfish Immunol. 2020, 96, 107–113. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequences (5′→3′) |
|---|---|
| NEMO-ORF-R | ATGGTGCAGCCACAACCTG |
| NEMO-ORF-L | CTGTATACAGTCCATGA |
| NEMO-qRT-R | GGCTCACTGGAGACTGTT |
| NEMO-qRT-L | GAGGAAATCTGCCTTGTA |
| EF-1α-qRT-R | CCCCTTGGTCGTTTTGCC |
| EF-1α-qRT-L | GCCTTGGTTGTCTTTCCGCTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.-Y.; Li, W.-F.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Liu, B.; Yang, J.-W.; Zhang, D.-C. Pathology, Enzyme Activity and Immune Responses after Cryptocaryon irritans Infection of Golden Pompano Trachinotus ovatus (Linnaeus 1758). J. Mar. Sci. Eng. 2023, 11, 262. https://doi.org/10.3390/jmse11020262
Guo H-Y, Li W-F, Zhu K-C, Liu B-S, Zhang N, Liu B, Yang J-W, Zhang D-C. Pathology, Enzyme Activity and Immune Responses after Cryptocaryon irritans Infection of Golden Pompano Trachinotus ovatus (Linnaeus 1758). Journal of Marine Science and Engineering. 2023; 11(2):262. https://doi.org/10.3390/jmse11020262
Chicago/Turabian StyleGuo, Hua-Yang, Wen-Fu Li, Ke-Cheng Zhu, Bao-Suo Liu, Nan Zhang, Bo Liu, Jing-Wen Yang, and Dian-Chang Zhang. 2023. "Pathology, Enzyme Activity and Immune Responses after Cryptocaryon irritans Infection of Golden Pompano Trachinotus ovatus (Linnaeus 1758)" Journal of Marine Science and Engineering 11, no. 2: 262. https://doi.org/10.3390/jmse11020262





