Anomalous Coloration of Indo-Pacific Humpback Dolphins off Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Field Survey
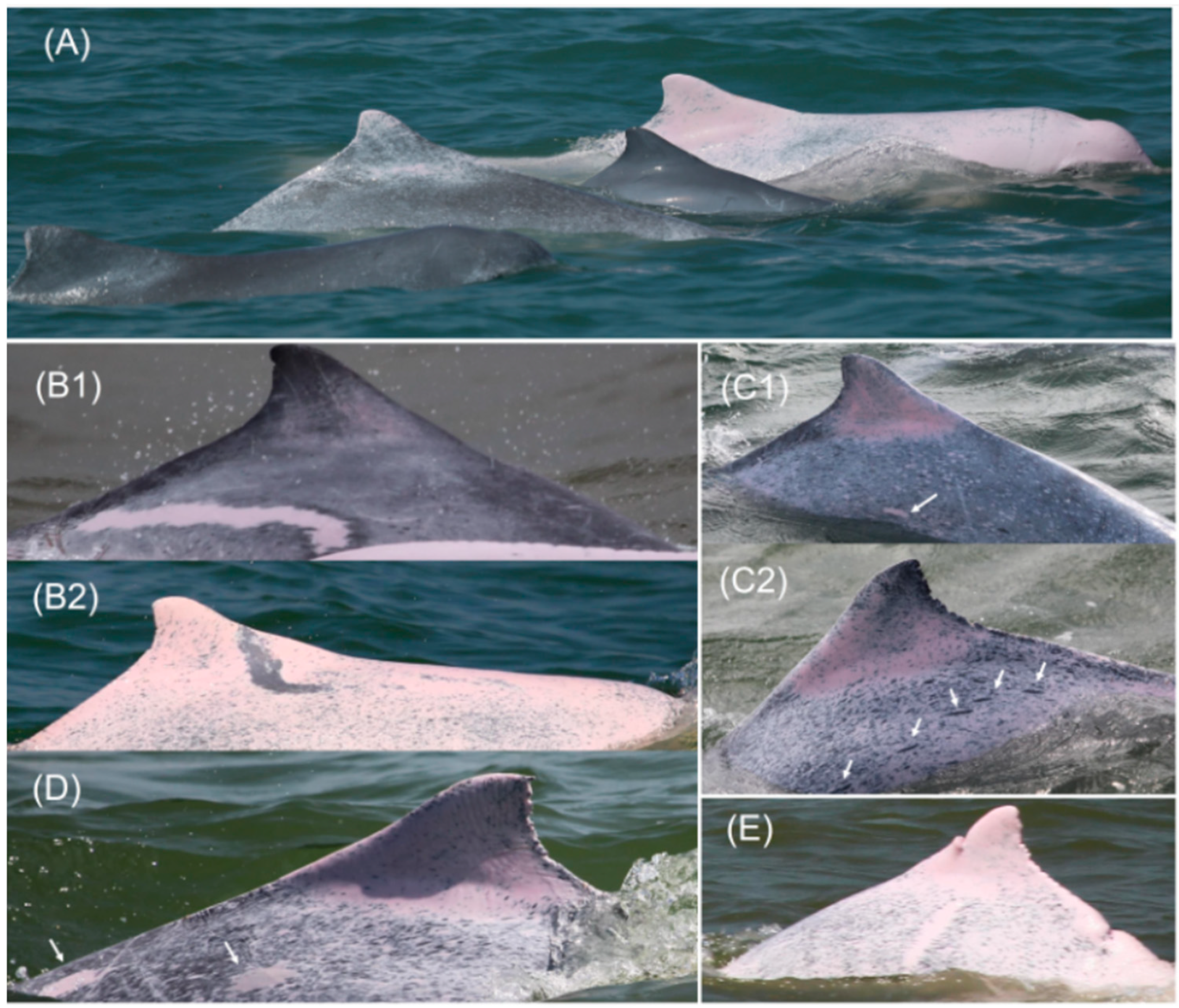
2.2. Anomalous Pigmentation
3. Results
3.1. Frequency and Spatial Distribution of Anomalies
3.2. Ontogeny of Hypopigmentation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jefferson, T.A.; Curry, B.E. Humpback dolphins: A brief introduction to the genus Sousa. Adv. Mar. Biol. 2015, 72, 1–16. [Google Scholar]
- Mendez, M.; Jefferson, T.A.; Kolokotronis, S.-O.; Krützen, M.; Parra, G.J.; Collins, T.; Minton, G.; Baldwin, R.; Berggren, P.; Särnblad, A.; et al. Integrating multiple lines of evidence to better understand the evolutionary divergence of humpback dolphins along their entire distribution range: A new dolphin species in Australian waters? Mol. Ecol. 2013, 22, 5936–5948. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.A. Population biology of the Indo-Pacific hump-backed dolphin in Hong Kong waters. Wildl. Monogr. 2000, 64, 1–65. [Google Scholar]
- Guo, L.; Lin, W.; Zeng, C.; Luo, D.; Wu, Y. Investigating the age composition of Indo-Pacific humpback dolphins in the Pearl River Estuary based on their pigmentation pattern. Mar. Biol. 2020, 167, 50. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Hung, S.K.; Robertson, K.M.; Archer, F.I. Life history of the Indo-Pacific humpback dolphin in the Pearl River Estuary, southern China. Mar. Mammal Sci. 2012, 28, 84–104. [Google Scholar] [CrossRef]
- Wu, F.; Wang, X.; Ding, X.; Dai, Y.; Zhao, L.; Zhu, Q. Occurrences of Xiamen Indo-Pacific humpback dolphins (Sousa chinensis Osbeck, 1765), in waters of Weitou Bay, China. Acta Theriol. Sin. 2019, 39, 608. [Google Scholar]
- Wu, F.; Wang, X.; Ding, X.; Miao, X.; Zhu, Q. Distribution pattern of Indo-Pacific humpback dolphins (Sousa chinensis) along coastal waters of Fujian Province, China. Aquat. Mamm. 2014, 40, 341–349. [Google Scholar]
- Tang, X.; Lin, W.; Karczmarski, L.; Lin, M.; Chan, S.C.; Liu, M.; Xue, T.; Wu, Y.; Zhang, P.; Li, S. Photo-identification comparison of four Indo-Pacific humpback dolphin populations off southeast China. Integr. Zool. 2021, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sakornwimon, W.; Lin, W.; Zhang, P.; Chantra, R.; Dai, Y.; Aierken, R.; Wu, F.; Li, S.; Kittiwattanawong, K.; et al. Early divergence and differential population histories of the Indo-Pacific humpback dolphin in the Pacific and Indian Oceans. Integr. Zool. 2021, 16, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.A.; Chivers, S.J.; Barlow, J. Reproductive Maturity and Seasonality of Male Spotted Dolphins, Stenella attenuata, in the Eastern Tropical Pacific. Mar. Mammal Sci. 1985, 1, 273–293. [Google Scholar] [CrossRef]
- O’Corry-Crowe, G.M. Beluga whale: Delphinapterus leucas. In Encyclopedia of Marine Mammals; Elsevier: Oxford, UK, 2009; pp. 108–112. [Google Scholar]
- Lodi, L.; Borobia, M. Anomalous colouration in an Atlantic spotted dolphin (Stenella frontalis) from southeastern Brazil. Braz. J. Aquat. Sci. Technol. 2013, 17, NB1–NB3. [Google Scholar] [CrossRef]
- Stockin, K.A.; Visser, I.N. Anomalously Pigmented Common Dolphins (Delphinus sp.) off Northern New Zealand. Aquat. Mamm. 2005, 31, 43–51. [Google Scholar] [CrossRef]
- Filatova, O.A.; Fedutin, I.D.; Titova, O.V.; Siviour, B.; Burdin, A.M.; Hoyt, E. White Killer Whales (Orcinus orca) in the Western North Pacific. Aquat. Mamm. 2016, 42, 350–356. [Google Scholar] [CrossRef]
- Funasaka, N.; Kirihata, T.; Kato, H.; Ohsumi, S. The first record of a true albino common bottlenose dolphin Tursiops truncatus from Japan. Mammal Study 2015, 40, 19–22. [Google Scholar] [CrossRef]
- Katona, S.K.; Whitehead, H.P. Identifying Humpback Whales using their natural markings. Polar Rec. 1981, 20, 439–444. [Google Scholar] [CrossRef]
- Pirotta, V.; Franklin, W.; Mansfield, L.; Lowe, J.; Peterson, O. Sighting records of “Migaloo” the white humpback whale provide evidence of Australian site fidelity and use of New Zealand waters as a migratory route. Aust. Zool. 2022; preprint. [Google Scholar] [CrossRef]
- Polanowski, A.; Robinson-Laverick, S.M.; Paton, D.; Jarman, S.N. Variation in the Tyrosinase Gene Associated with a White Humpback Whale (Megaptera novaeangliae). J. Hered. 2011, 103, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Methion, S.; López, B.D. First record of atypical pigmentation pattern in fin whale Balaenoptera physalus in the Atlantic Ocean. Dis. Aquat. Org. 2019, 135, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, N.; Kirihata, T.; Hosono, M.; Kato, H.; Ohsumi, S. Three Cases of Anomalously White Risso’s Dolphins Grampus griseus in Japan. Mammal Study 2017, 42, 173–178. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, R.; Liu, B.; Chen, S.; Lin, M.; Liu, M.; Liu, W.; Li, S. Low Survivals and Rapid Demographic Decline of a Threatened Estuarine Delphinid. Front. Mar. Sci. 2022, 9, 782680. [Google Scholar] [CrossRef]
- Le Net, R.; Larrat, S.; Michaud, R.; Lair, S. Pathological and epidemiological investigation of skin lesions in belugas (Delphinapterus leucas) from the St. Lawrence Estuary, Quebec, Canada. Mar. Mammal Sci. 2021, 38, 653–681. [Google Scholar] [CrossRef]
- Oiso, N.; Fukai, K.; Kawada, A.; Suzuki, T. Piebaldism. J. Dermatol. 2012, 40, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.C.; Lamoreux, M.L. The Color Loci of Mice–A Genetic Century. Pigment. Cell Res. 2003, 16, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Hay, L.; Jackson, I.J. Ex vivo live imaging of melanoblast migration in embryonic mouse skin. Pigment. Cell Melanoma Res. 2010, 23, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.; Menendez, L.; Cunningham, M.L.; Lovvorn, H.N.; Dalton, S. Using induced pluripotent stem cells as a tool to understand neurocristopathies. In Neural Crest Cells; Trainor, P.A., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 441–459. [Google Scholar]
- Martinez-Levasseur, L.M.; Gendron, D.; Knell, R.J.; O’Toole, E.; Singh, M.; Acevedo-Whitehouse, K. Acute sun damage and photoprotective responses in whales. Proc. R. Soc. B Boil. Sci. 2010, 278, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.; Francisco, A.; de Souza, S.P.; Siciliano, S. Rough-Toothed Dolphins (Steno bredanensis) Along Southeastern Brazil: Report of an Anomalous Pigmented Juvenile and Description of Social and Feeding Behaviors. Aquat. Mamm. 2019, 45, 30–36. [Google Scholar] [CrossRef]



| Categories | Size (in Length) | Shape | Skin Lesion |
|---|---|---|---|
| Normal pigmentation | Ranges from a few mm to 5 cm | Regular dot, spindle, or oval shape | Not relevant |
| Anomalous pigmentation | Usually >20 cm | Irregular patchy shape | Not relevant |
| Physical scars | Irregular size | Stripe, usually with concave skin | Presence and relevant |
| Categories | XM | ST | LZP | DRE | HN | PRD | ||
|---|---|---|---|---|---|---|---|---|
| West | Mid | East | ||||||
| Dolphin records | 48 | 14 | 692 | 204 | 212 | 973 | 603 | 889 |
| Hypopigmentation | 1 | 0 | 0 | 0 | 1 | 4 | 20 | 0 |
| Hyperpigmentation | 0 | 0 | 4 | 1 | 0 | 6 | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Chen, S.; Zheng, R.; Serres, A.; Liu, B.; Lin, M.; Liu, M.; Li, S. Anomalous Coloration of Indo-Pacific Humpback Dolphins off Southern China. J. Mar. Sci. Eng. 2023, 11, 348. https://doi.org/10.3390/jmse11020348
Lin W, Chen S, Zheng R, Serres A, Liu B, Lin M, Liu M, Li S. Anomalous Coloration of Indo-Pacific Humpback Dolphins off Southern China. Journal of Marine Science and Engineering. 2023; 11(2):348. https://doi.org/10.3390/jmse11020348
Chicago/Turabian StyleLin, Wenzhi, Shenglan Chen, Ruiqiang Zheng, Agathe Serres, Binshuai Liu, Mingli Lin, Mingming Liu, and Songhai Li. 2023. "Anomalous Coloration of Indo-Pacific Humpback Dolphins off Southern China" Journal of Marine Science and Engineering 11, no. 2: 348. https://doi.org/10.3390/jmse11020348





