Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Sample Collection and Strain Identification
2.3. Whole-Genome Sequencing and In Silico Analysis
2.4. Metabolic Profile of the Strain by LC-MS and Screening of Desired Compounds
2.5. Scale Up and Isolation of Active Compounds
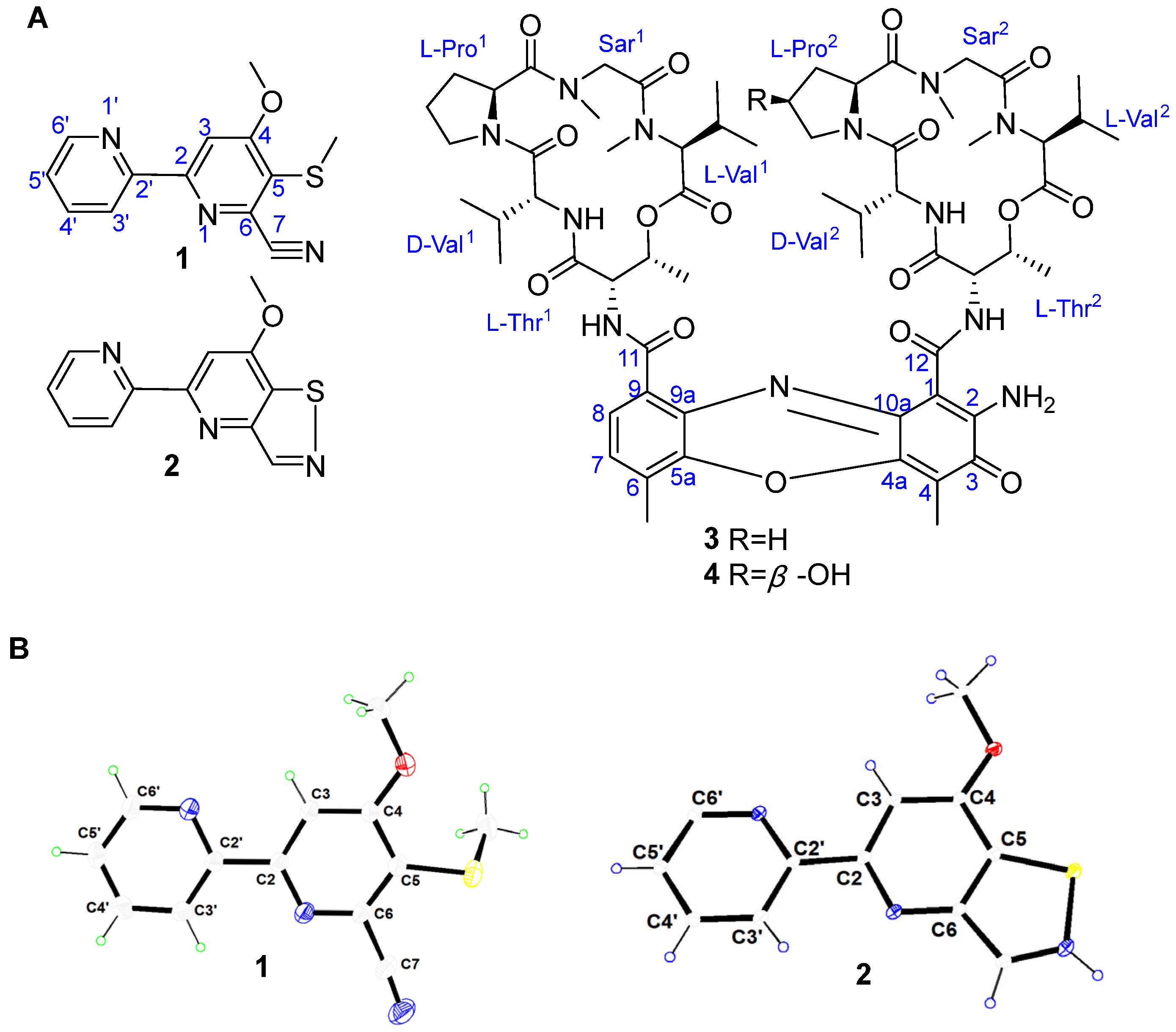
2.6. X-ray Crystallography of Compounds 1 and 2
3. Results and Discussion
3.1. Isolation and Identification of Strain
3.2. Genome Sequencing and Elucidation of Strain SCSIO PteL053
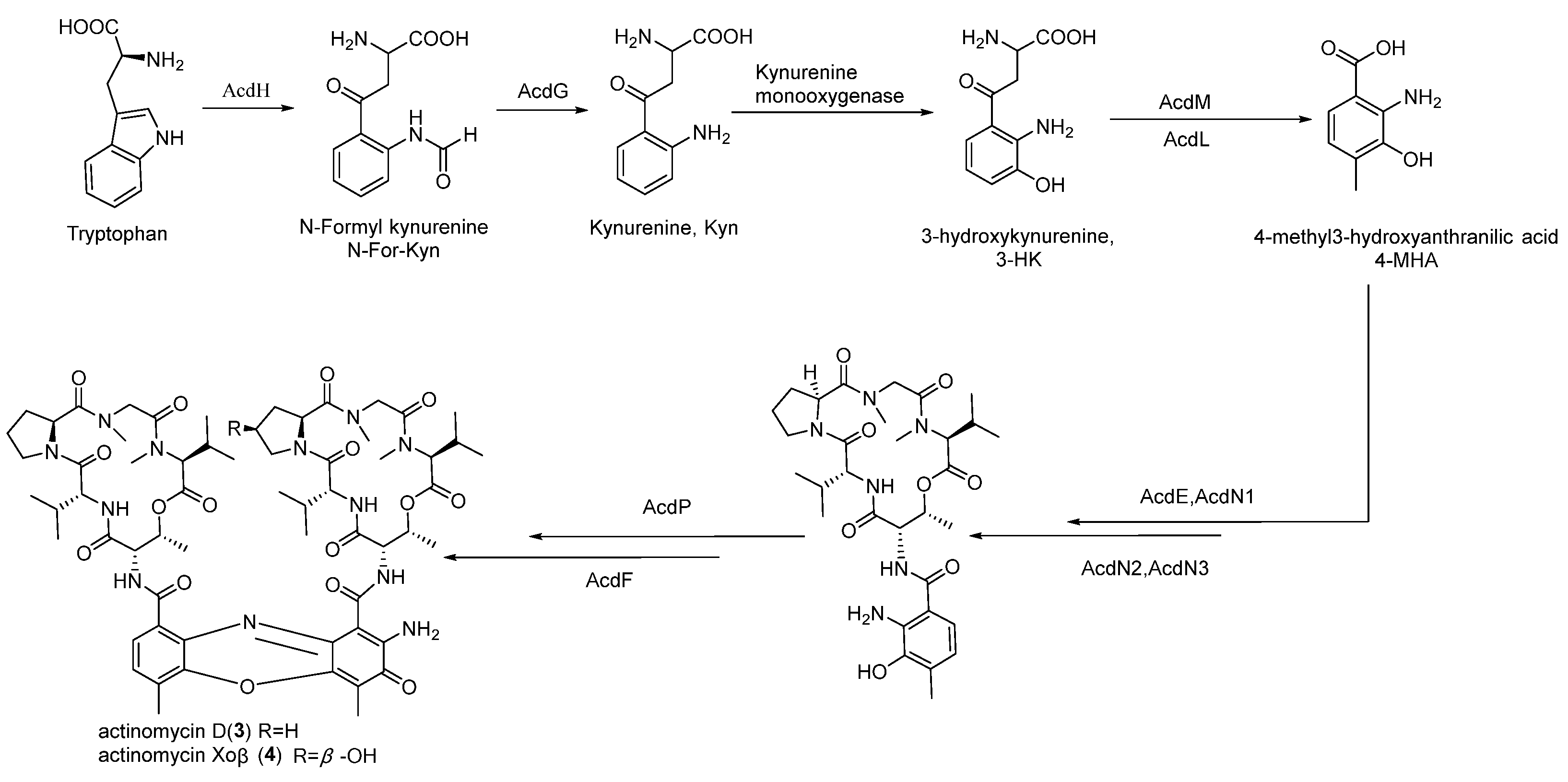
3.3. Production, Isolation, and Elucidation of 2,2′-BP and Actinomycin Analogs
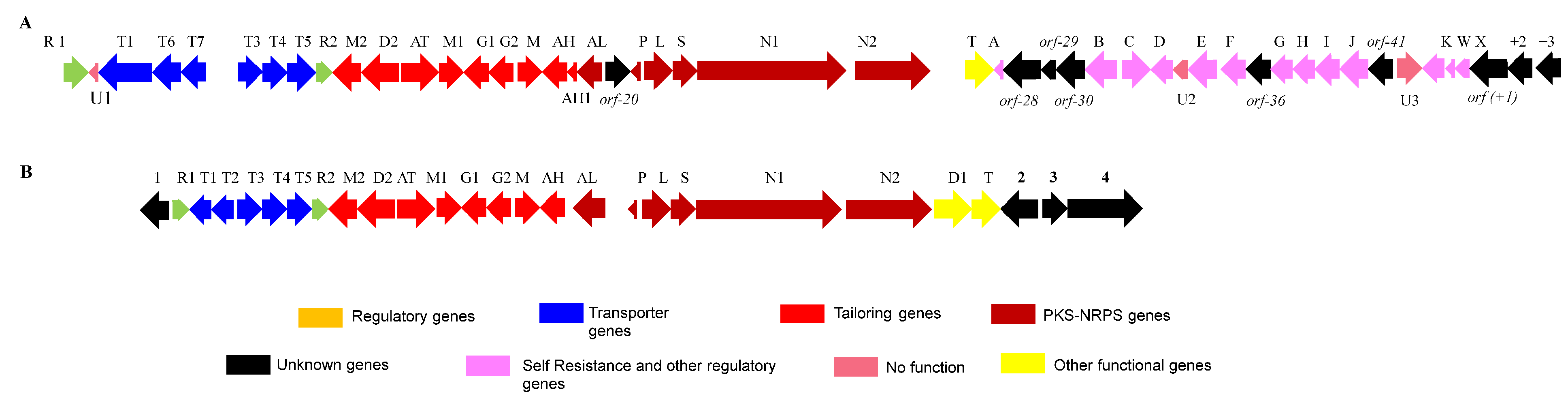
3.4. Bioinformatics Analysis of the Putative 2,2′-BP BGC
3.5. Bioinformatics Analysis of the Putative Actinomycin BGC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Ling, C.Y.; Zhou, Z.B.; Dong, Y.L.; Sun, C.L.; Song, X.Y.; Wong, N.K.; Ju, J.H. Chemical Diversity of Metabolites and Antibacterial Potential of Actinomycetes Associated with Marine Invertebrates from Intertidal Regions of Day Bay and Nansha Islands. Microbiology 2020, 89, 483–492. [Google Scholar] [CrossRef]
- Vander Meij, A.; Worsley, S.F.; Hutchings, M.I.; Van Wwzel, G.P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.A.M.; Moore, B.S. Chasing the treasures of the sea-bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260. [Google Scholar] [CrossRef]
- Zazopoulos, E.; Huang, K.; Staffa, A.; Liu, W.; Bachmann, B.O.; Nonaka, K.; Ahlert, J.; Thorson, J.S.; Shen, B.; Farnet, C.M. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat. Biotechnol. 2003, 21, 187–190. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, C.; Qin, X.; Wei, X.; Liu, Q.; Ju, J. Genome mining of Streptomyces olivaceus SCSIO T05: Discovery of olimycins A and B and assignment of absolute configurations. Tetrahedron 2018, 74, 199–203. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Z.; Zhang, C.; Liu, Z.; He, J.; Liu, Q.; Zhang, T.; Ju, J.; Ma, J. Genome Mining of Streptomyces atratus SCSIO ZH16: Discovery of Atratumycin and Identification of Its Biosynthetic Gene cluster. Org. Lett. 2019, 21, 1453–1457. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Qin, X.; Ma, J.; Sun, C.; Huang, H.; Li, Q.; Ju, J. Genome Mining for Mycemycin: Discovery and Elucidation of related Methylation and Chlorination Biosynthetic Chemistries. Org. Lett. 2018, 20, 7633–7636. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Q.; Liu, W. Discovery of caerulomycin/ collismycin-type 2,2′-bipyridine natural products in the genomic era. J. Ind. Microbiol. Biotechnol. 2019, 46, 459–468. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Y.; Xie, Y.; Zhang, C.; Ma, J.; Sun, C.; Ju, J. Identification of the actinomycin D Biosynthetic Pathway from Marine-Derived Streptomyces costaricanus SCSIO ZS0073. Mar. Drugs 2019, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Sun, C.; Xia, Y.; Wang, F.; Fu, L.; Ma, J.; Li, Q.; Ju, J. Mutasynthesis of Antibacterial Halogenated Actinomycin Analogues. J. Nat. Prod. 2021, 84, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.; D’Angio, G.; Evans, A.; Mitus, A. Clinical studies of actinomycin D with special reference to Wilms tumor in children. J. Urol. 2002, 168, 2560–2562. [Google Scholar] [CrossRef]
- Mondick, J.T.; Gibiansky, L.; Gastonguay, M.R.; Skolnik, J.M.; Cole, M.; Veal, G.J.; Boddy, A.V.; Adamson, P.C.; Barrett, J.S. Population pharmacokinetic investigation of actinomycin D in children and young adults. J. Clin. Pharmacol. 2008, 48, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids. Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef]
- Keller, U.; Lang, M.; Crnovcic, I.; Pfening, F.; Schauwecker, F. The actinomycin biosynthetic gene cluster of Streptomyces chrysomallus: Agenetic hall of mirrors for synthesis of a molecule with mirror symmetry. J. Bacteriol. 2010, 192, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E.; et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Ju, J.; Ma, J. Genome sequencing of Streptomycsatratus SCSIO ZH16 and activation, production of nocardamine via metabolic engineering. Front. Microbiol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Low, Z.J.; Pang, L.M.; Ding, Y.; Cheang, Q.W.; Le mai Hoang, K.; Thitran, H.; Li, J.; Liu, X.W.; Kanagasundaram, Y.; Yang, L.; et al. Identification of a biosynthetic gene cluster for the polyene macrolactam sceliphrolactam in a Streptomyces strain isolated from mangrove sediment. Sci. Rep. 2018, 8, 1594. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.; Choi, H.; Lee, J. Collismycin C from the Micronesian marine Bacterium Streptomyces sp. MC025 Inhibits Staphylococcus aureus Biofilm Formation. Mar. Drugs 2017, 15, 387. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Jiang, X.; Sun, J.; Zhang, C.; Zhang, Y.; Lu, C.; Ju, J. Antibacterial secondary metabolites produced by mangrove-derived actinomycete Streptomyces costaricanus SCSIO ZS0073. Nat. Prod. Res. Dev. 2017, 29, 410–414. [Google Scholar]
- Garcia, I.; Vior, N.M.; Brana, A.F.; Gonzalez-sabin, J.; Rohr, J.; Moris, F.; Mendez, C.; Salas, J.A. Elucidating the biosynthetic pathway for the polyketide-nonribosomal peptide Collismycin A: Mechanism for formation of the 2,2′-bipyridyl ring. Chem. Biol. 2012, 19, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, F.; Schauwecker, F.; Keller, U. Molecular characterization of the genes of actinomycin synthatase I and of a 4-methyl-3-hydroxyanthranilic acid carrier protein involved in the assembly of the acylpeptide chain of actinomycin in Streptomyces. J. Biol. Chem. 1999, 274, 12508–12516. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, D.; Mootz, H.D.; Linne, U.; Marahiel, M.A. Regeneration of misprimednonribosomal peptide synthetases by type II thioesterases. Proc. Natl. Acad. Sci. USA 2002, 99, 14083–14088. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Liao, R.; Tang, Z.; Guo, S.; Wu, Z.; Liu, W. Caerulomycin and collismycin antibiotics share a trans-acting flavoprotein-dependent assembly line for 2.2′-bipyridine formation. Nat. Commun. 2021, 12, 3124. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohnishi, Y.; Horinouchi, S. GriC and GriD constitute a carboxylic acid reductase involved in grixazone biosynthesis in Streptomyces griseus. J. Antibiot. 2007, 60, 380–387. [Google Scholar] [CrossRef]
- Garcia, I.; Vior, N.M.; Gonzalez-sabin, J.; Brana, A.F.; Rohr, J.; Moris, F.; Mendez, C.; Salas, J.A. Engineering the biosynthesis of the polyketide-nonribosomal peptide Collismycin A for generation of analogs with neuroprotective activity. Chem. Biol. 2013, 20, 1022–1032. [Google Scholar] [CrossRef]
- Qu, X.; Pang, B.; Zhang, Z.; Chen, M.; Wu, Z.; Zhao, Q.; Zhang, Q.; Wang, Y.; Liu, Y.; Liu, W. Caerulomycins and collismycins share a common paradigm for 2,2′ bipyridine biosynthesis via an unusual hybrid polyketide-peptide assembly logic. J. Am. Chem. Soc. 2012, 134, 9038–9041. [Google Scholar] [CrossRef]
- Holm, L.; Sander, C. An evolutionary treasure: Unification of a broad set of amidohydrolases related to urease. Proteins 1997, 28, 72–82. [Google Scholar] [CrossRef]
- Fleming, F.F. Nitrile- containing natural products. Nat. Prod. Rep. 1999, 16, 597–606. [Google Scholar] [CrossRef]
- Bezerragomes, P.; Nett, M.; Dahse, H.M.; Sattler, I.; Martin, K.; Hertweck, C. Bezerramycins A-C, antiproliferative ohenoxazinones from Streptomyces griseus featuring carboxy, Carboxamide or nitrile substituents. Eur. J. Org. Chem. 2010, 2, 231–235. [Google Scholar]
- Olano, C.; Moss, S.J.; Brana, A.F.; Sheridan, R.M.; Math, V.; Weston, A.J.; Mendez, C.; Leadlay, P.F.; Wilkinson, B.; Salas, J.A. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tu 4055: Insights into nitrile formation. Mol. Microbiol. 2004, 52, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Vior, N.M.; Olano, C.; Garcia, I.; Mendez, C.; Salas, J.A. Collismycin A biosynthesis in Streptomyces sp. CS40 is regulated by iron levels through two pathway- specific regulators. Microbiology 2014, 160, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Egea, P.U.; Medina, M.; Garcia, E.; Perez, J.; Fernandez, R.I.; Medarde, A.; Canedo, L.M.; Romero, F.; Castro, A.; et al. Use of Collismycin and Derivatives There of as Oxidative Stress Inhibitors. U.S. Patent WO2007017146A3, 26 July 2007. [Google Scholar]
- Xu, Y.; Mitra, B. A highly active, soluble mutant of the membrane associated (S)- mandelate degydrogenase from Pseudomonas putida. Biochemistry 1999, 38, 12367–12376. [Google Scholar] [CrossRef] [PubMed]
- Crnovcic, I.; Ruckert, C.; Semsary, S.; Lang, M.; Kalinowski, M.; Keller, U. Genetic interrelations in the actinomycin biosynthetic gene clusters of Streptomyces antibioticus IMRU 3720 and Streptomyces chrysomallus ATCC11523, producers of actinomycin X and actinomycin C. Adv. Appl. Bioinformatics Chem. 2017, 10, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Gao, G.; Lu, J.; Long, Q.; Chen, X.; Zhang, F.; Xu, M.; Liu, K.; Wang, Y.; Deng, Z.; et al. Engineered Streptomyces lividans strains for optimal identification and expression of cryptic biosynthetic gene clusters. Front. Microbiol. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Garnier, T.; Eiglmeier, K.; Camus, J.C.; Medina, N.; Mansoor, H.; Pryor, M.; Duthoy, S.; Grondin, S.; Lacroix, C.; Monsempe, C.; et al. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 2003, 100, 7877–7882. [Google Scholar] [CrossRef]
- Baltz, R.H. Function MbtH homologs in nonribosomal peptide biosynthesis and applications in secondary metabolite discovery. J. Ind. Microbiol. Biotechnol. 2011, 38, 1747–1760. [Google Scholar] [CrossRef]
- Felnage, E.A.; Barkei, J.J.; Park, H.; Podevels, A.H.; McMahon, M.D.; Drott, D.W.; Thomas, M.G. MbtH-like proteins as integral components of bacterial nonribosomal peptide synthetases. Biochemistry 2010, 49, 8815–8817. [Google Scholar] [CrossRef]
- Imker, H.J.; Krahn, D.; Clerc, J.; Kaiser, M.; Walsh, C.T. N-Acylation during glidobactin biosynthesis by the tridomainnonribosomal peptide synthetase module GlbF. Chem. Biol. 2010, 17, 1077–1083. [Google Scholar] [CrossRef]
- Peschke, U.; Schmidt, H.; Zhang, H.Z.; Pieperserg, W. Molecular characterization of the lincomycin-production gene cluster of Strptomyceslincolnensis 78-11. Mol. Microbiol. 1995, 16, 1137–1156. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.L.; Martinez-Bueno, M.; Molina-Henares, A.J.; Teran, W.; Watanabe, K.; Zhang, X.; Gallegos, M.T.; Brennan, R.; Tobes, R. The tetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005, 69, 326–356. [Google Scholar] [CrossRef] [Green Version]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Mo, X.; Wang, Z.; Wang, B.; Ma, J.; Huang, H.; Tian, X.; Zhang, C.; Ju, J. Cloning and characterization of the biosynthetic gene cluster of the bacterial RNA polymerase inhibitor titrandamycin from marine-derived Streptomyces sp. SCSIO 1666. Biochem. Biophys. Res. Commun. 2011, 406, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, C.R.; Colombo, A.L. Genetic engineering of doxorubicin production in Streptomyces peucetius: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Russell, J. Biochemical coupling between the DrrA and DrrB proteins of the doxorubicin efflux pump of Streptomyces peucetius. J. Biol. Chem. 1998, 273, 17933–17939. [Google Scholar] [CrossRef]
- Husain, I.; Van Houten, B.; Thomas, D.C.; Sancar, A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J. Biol. Chem. 1986, 261, 4895–4901. [Google Scholar] [CrossRef]
- Florez, L.V.; Biedermann, P.H.W.; Engl, T.; Kaltenpoth, M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 2015, 32, 904–936. [Google Scholar] [CrossRef] [Green Version]



| Features | Value |
|---|---|
| Genome size (bp) | 8,040,759 |
| GC content (%) | 71.60 |
| Protein-coding genes | 7077 |
| Size of protein-coding gene (bp) | 7,075,212 |
| rRNAs | 19 |
| tRNAs | 67 |
| ORF | Size | Proposed Function | ID/SI | Protein Homolog and Origin |
|---|---|---|---|---|
| clsR1 | 185 | Putative TetR transcriptional regulator | 98/98 | ClmR1, (CCC55902.1); Streptomyces sp. CS40 |
| clsU1 | 118 | Uncharacterized protein | 50/64 | CUP04914.1; Flavonifractorplautii |
| clsT1 | 581 | Putative ABC transporter, ATPase, and permease | 99/100 | ClmT1, (CCC55903.1; Steptomyces sp. CS40 |
| clsT6 | 586 | ABC transporter, ATP-binding protein | 99/99 | MdlB, WP_032768595.1; Streptomyces sp. CNS654 |
| clsT7 | 569 | ABC transporter, ATP-binding protein | 100/100 | MdlB, WP_032768595.1; Streptomyces sp. CNS654 |
| clsT3 | 322 | Putative ABC transporter, solute-binding protein | 96/98 | ClmT3, CCC55905.1; Steptomyces sp. CS40 |
| clsT4 | 327 | Putative ABC transporter, permease component | 99/99 | ClmT4, CCC55906.1; Steptomyces sp. CS40 |
| clsT5 | 261 | Putative ABC transporter, ATP-binding protein | 98/99 | ClmT5, CCC55907.1; Steptomyces sp. CS40 |
| clsR2 | 168 | LuxR family transcriptional regulator | 98/98 | ClmR2, CCC55908.1; Streptomyces sp. CS40 |
| clsM2 | 342 | O-methyltransferase | 96/98 | ClmM2, CCC55909.1; Streptomyces sp. CS40 |
| clsD2 | 532 | Putative dehydrogenase | 97/98 | ClmD2, CCC55910.1; Streptomyces sp. CS40 |
| clsAT | 544 | Putative aminotransferase | 98/98 | ClmAT, CCC55911.1; Streptomyces sp. CS40 |
| clsM1 | 328 | S-Methyltransferas | 97/98 | ClmM1, CCC55912.1; Streptomyces sp. CS40 |
| clsG1 | 462 | GriD-like dehydrogenase component | 96/98 | ClmG1, CCC55913.1; Streptomyces sp. CS40 |
| clsG2 | 343 | GriC-like dehydrogenase component | 98/99 | ClmG2, CCC55914.1; Streptomyces sp. CS40 |
| clsM | 397 | Putative FAD-dependent oxidoreductase | 97/98 | ClmM, CCC55915.1; Streptomyces sp. CS40 |
| clsAH | 413 | Amidohydrolase family protein | 96/98 | ClmAH, CCC55916.1; Streptomyces sp. CS40 |
| clsAH1 | 89 | Amidohydrolase family protein | 99/99 | WP_032768579.1; Streptomyces sp. CNS654 |
| clsAL | 562 | Putative acyl CoA ligase | 98/98 | ClmAL, CCC55917.1; Streptomyces sp. CS40 |
| orf-20 | 63 | Hypothetical protein | 45/54 | PAZ10747.1; Streptomyces sp. SA15 |
| clsP | 86 | Putative free-standing acyl carrier protein | 96/97 | ClmP, CCC55918.1; Streptomyces sp. CS40 |
| clsL | 458 | L-Lysine 2-aminotransferase | 97/98 | ClmL, CCC55919.1; Streptomyces sp. CS40 |
| clsS | 393 | Monomeric sarcosine oxidase | 97/97 | ClmS, CCC55920.1; Streptomyces sp. CS40 |
| clsN1 | 2552 | Non-ribosomal peptide synthetase/polyketide synthase hybrid protein | 97/98 | ClmN1, CCC55921.1; Streptomyces sp. CS40 |
| clsN2 | 1331 | Putative non-ribosomal peptide synthetase | 82/84 | ClmN2, CCC55922.1; Streptomyces sp. CS40 |
| clsT | 242 | Putative type II thioesterase | 98/99 | ClmT, CCC55924.1; Streptomyces sp. CS40 |
| clsA | 375 | PD-(D/E)XK nuclease family protein | 50/62 | NEB63020.1; Streptomyces diastaticus |
| orf-28 | 257 | Hypothetical protein | 97/98 | WP_032768566.1; Streptomyces sp. CNS654 |
| orf-29 | 302 | Hypothetical protein | 98/98 | WP_032768565.1; Streptomyces sp. CNS654 |
| orf-30 | 302 | Hypothetical protein | 95/96 | WP_032768565.1; Streptomyces sp. CNS654 |
| clsB | 224 | GNAT family N-acetyltransferase | 100/100 | RimI, WP_050487033.1; Streptomyces sp. CNS654 |
| clsC | 354 | Alpha/beta hydrolase | 100/100 | AXE1, WP_100562610.1; Streptomyces sp. CB02613 |
| clsD | 167 | Helix-turn-helix transcriptional winged regulator | 99/99 | MarR, WP_093443708.1; Streptomyces sp.Cmuel-A718b |
| clsU2 | - | Unknown function | - | - |
| clsE | 436 | 3-phytase | 99/99 | SCF80346.1; Streptomyces sp. Cmuel-A718b |
| clsF | 275 | Endo alpha-1,4 polygalactosaminidase | 99/100 | WP_032768511.1; Streptomyces sp. CNS654 |
| orf-37 | 220 | hypothetical protein | 98/99 | WP_032768508.1; Streptomyces sp. CNS654 |
| clsG | 308 | NAD(P)-dependent oxidoreductase | 98/99 | WcaG, WP_032768507.1; Streptomyces sp. CNS654 |
| clsH | 308 | NAD(P)-dependent oxidoreductase | 81/82 | WcaG, WP_032768507.1; Streptomyces sp. CNS654 |
| clsI | 237 | Nucleotidyltransferase family protein | 100/100 | GCD1, WP_032768506.1; Streptomyces sp. CNS654 |
| orf-41 | 333 | SDR family NAD(P)-dependent oxidoreductase | 99/99 | WP_032768503.1; Streptomyces sp. CNS654 |
| clsJ | 468 | Hypothetical protein | 99/99 | WP_032768501.1; Streptomyces sp. CNS654 |
| clsU3 | - | Unknown function | - | - |
| clsK | 129 | DUF3492 domain-containing protein | 100/100 | RfaB, WP_032768499.1; Streptomyces sp. CNS654 |
| clsW | 60 | DUF3492 domain-containing protein | 93/93 | RfaB, WP_043252613.1; Streptomyces vinaceus |
| clsX | 41 | DUF3492 domain-containing protein | 100/100 | RfaB, WP_032768499.1; Streptomyces sp. CNS654 |
| orf (+1) | 524 | Hypothetical protein | 84/85 | HSNSD, WP_032768497.1; Streptomyces sp. CNS654 |
| orf (+2) orf(+3) | 176 175 | Hypothetical protein Hypothetical protein | 93/94 100/100 | HSNSD, WP_096629522.1; Streptomyces sp. WZ.A104, WP_032768496.1; Streptomyces sp. CNS654 |
| ORF | Size | Proposed Function | ID/SI | Protein Homologue and Origin |
|---|---|---|---|---|
| acdU3 | 210 | Hypothetical protein | 30/49 | GLYR1; Drosophila pseudoobscura (Q29NG1.2) |
| acdU4 | 187 | Hypothetical protein | 27/36 | SWS; Drosophila mojavensis (B4L535.1) |
| acdD | 66 | MbtH protein | 63/77 | MbtH; Mycobacterium tuberculosis variant bovisAF2122/97(P59965.1) |
| acdE | 78 | 4-MHA carrier protein | 25/54 | AflC; Aspergillus parasiticus SU-1 (Q12053.1) |
| acdN1 | 410 | Acyl–CoA ligase | 44/60 | Schizosaccharomyces pombe 972h- (O74976.1) |
| acdN2 | 2537 | Non-ribosomal peptide synthetase | 44/60 | DhbF; Bacillus subtilis subsp. subtilis str. 168 (P45745.4) |
| acdN3 | 4250 | Non-ribosomal peptide synthetase | 29/45 | Metarhiziumrobertsii ARSEF 23 (E9FCP4.2) |
| acdF | 210 | Hypothetical protein(DA) | 29/45 | Schizosaccharomyces pombe 972h (O13799.1) |
| acdG | 299 | Arylformamidase | 34/50 | Afmid; Danio rerio (Q566U4.2) |
| acdH | 285 | Tryptophan 2,3-dioxygenase | 43/58 | KynA; Polaromonas sp. JS666 (Q126P7.1) |
| acdL | 420 | Kynureninase | 44/62 | KynU; Pseudomonas fluorescens (P83788.1) |
| acdM | 347 | Methyltransferase | 54/70 | AcmL; Streptomyces lavendulae NRRL 11,002 (ABI22137.1) |
| acdP | 435 | Cytochrome P450 | 49/67 | CypA; Saccharopolyspora erythraea NRRL 2338 (P33271.1) |
| adnU | 63 | Ferredoxin | 45/60 | SoyB; Streptomyces griseus (P26910.1) |
| acdO | 216 | LmbU-like protein | 99/99 | LmbU: Streptomyces parvus (WP_167533479) |
| acdR | 281 | TetR family transcriptional regulator | 35/53 | TetR; Vibrio anguillarum (P51560.1) |
| acdQ | 289 | Siderophore-interacting protein | 33/48 | Mb2919c; Mycobacterium tuberculosis variant bovis AF2122/97 (P65050.1) |
| acdT1 | 327 | ABC transporter, ATP-binding protein | 46/60 | DrrA; Streptomyces peucetius(P32010.1) |
| acdT2 | 255 | ABC-2-type transporter | 27/44 | DrrB; Streptomyces peucetius (P32011.1) |
| acdT3 | 753 | ATP-binding protein | 53/70 | UvrA; Methanothermobacterthermautotrophicus str. Delta H (O26543.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindarajan, G.; Yao, Z.; Zhou, Z.; Zheng, X.; Ma, J.; Kumar, P.S.; Ju, J.; Sun, C. Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis. J. Mar. Sci. Eng. 2023, 11, 396. https://doi.org/10.3390/jmse11020396
Govindarajan G, Yao Z, Zhou Z, Zheng X, Ma J, Kumar PS, Ju J, Sun C. Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis. Journal of Marine Science and Engineering. 2023; 11(2):396. https://doi.org/10.3390/jmse11020396
Chicago/Turabian StyleGovindarajan, Ganesan, Ziwei Yao, Zhenbin Zhou, Xiaohong Zheng, Junying Ma, Pachaiyappan Saravana Kumar, Jianhua Ju, and Changli Sun. 2023. "Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis" Journal of Marine Science and Engineering 11, no. 2: 396. https://doi.org/10.3390/jmse11020396
APA StyleGovindarajan, G., Yao, Z., Zhou, Z., Zheng, X., Ma, J., Kumar, P. S., Ju, J., & Sun, C. (2023). Genome Sequencing of Streptomyces griseus SCSIO PteL053, the Producer of 2,2′-Bipyridine and Actinomycin Analogs, and Associated Biosynthetic Gene Cluster Analysis. Journal of Marine Science and Engineering, 11(2), 396. https://doi.org/10.3390/jmse11020396







