Clionaterpene, a New Cadinene Sesquiterpene from the Marine Sponge Cliona sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extaction and Isolation
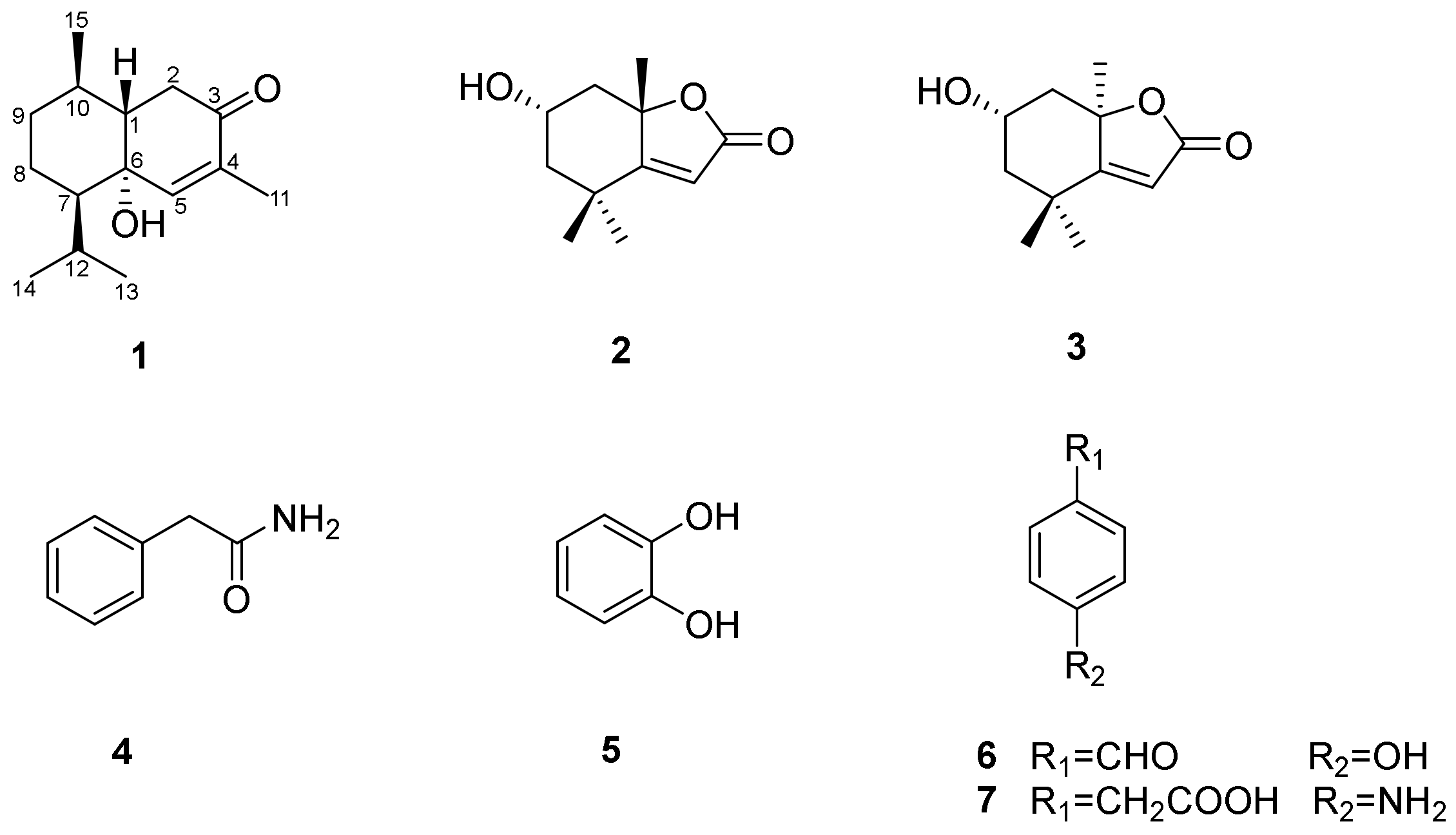
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, R.J. Tetracetyl clionamide, a 6-bromotryptophan derivative from the sponge Cliona celata. Tetrahedron Lett. 1978, 19, 2541–2544. [Google Scholar] [CrossRef]
- Stonard, R.J.; Andersen, R.J. Celenamides A and B, linear peptide alkaloids from the sponge Cliona celata. J. Org. Chem. 1980, 45, 3687–3691. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O.; Petrucci, F.; Bavestrello, G.; Calcinai, B.; Cerrano, C.; Di, M.P.; Ianaro, A. Polychlorinated androstanes from the burrowing sponge Cliona nigricans. Org. Lett. 2004, 6, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Gaspar, H.; Silva, J.; Alves, C.; Martins, A.; Teodoro, F.; Susano, P.; Pinteus, S.; Pedrosa, R. Unravelling the anti-inflammatory and antioxidant potential of the marine sponge Cliona celata from the Portuguese coastline. Mar. Drugs 2021, 19, 632. [Google Scholar] [CrossRef] [PubMed]
- Lenis, L.A.; Nuñez, L.; Jiménez, C.; Riguera, R. Isonitenin and acetylhomoagmatine new metabolites from the sponges Spongia officinalis and Cliona celata collected at the Galician coast (NW Spain). Nat. Prod. Lett. 1996, 8, 15–23. [Google Scholar] [CrossRef]
- Sawangwong, P.; Wattanadilok, R.; Kijjoa, A. Secondary metabolites from a marine sponge Cliona patera. Biochem. Syst. Ecol. 2008, 36, 493–496. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D. New steroidal hydroxyketones and closely related diols from the marine sponge Cliona copiosa. J. Nat. Prod. 1992, 55, 1588–1594. [Google Scholar] [CrossRef]
- Kouchaksaraee, R.M.; Li, F.; Nazemi, M.; Farimani, M.M.; Tasdemir, D. Molecular networking-guided isolation of new etzionin-type diketopiperazine hydroxamates from the persian gulf sponge Cliona celata. Mar. Drugs 2021, 19, 439. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Maldonado, M.E.; Rivera, E.; Porras, B. The fatty acid 4,8,12-trimethyltridecanoic as a common constituent of the phospholipids of the sponge families Spirastrellidae and Clionidae. Biochem. Syst. Ecol. 1989, 17, 311–314. [Google Scholar] [CrossRef]
- Martin, G.E.; Sanduja, R.; Alam, M. Two-dimensional NMR studies of marine natural products. 2. Utilization of two-dimensional proton double quantum coherence NMR spectroscopy in natural products structure elucidation on-determination of long-range couplings in plumericin. J. Org. Chem. 1985, 50, 2383–2386. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.; Sharma, U.; Singh, D.; Dobhal, M.; Singh, S. Anti-mycobacterial activity of plumericin and isoplumericin against MDR Mycobacterium tuberculosis. Pulm. Pharmacol. Ther. 2013, 26, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wen, Y.; Long, G. Analysis on Cadinane sesquiterpenoidnene composition in Vetiveria zizanioides. J. Anhui Agric. Sci. 2009, 37, 5636–5638. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, Y.; Peng, Y.; Chen, T.; Chen, J.; Yang, J.; Gong, T.; Zhu, P. Advances in biosynthesis of cadinane sesquiterpenes. Chin. J. Biotechnol. 2021, 37, 1952–1967. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, X.; Lin, J.; Xu, W.; Chen, G.; Zhao, B.; Xu, S. Spongiains A-C: Three new spongian diterpenes with ring a rearrangement from the marine sponge Spongia sp. Tetrahedron 2019, 75, 3802–3808. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, X.; Zhao, B.; Xu, S. (+)- and (−)-Spongiterpene, a pair of new valerenane sesquiterpene enantiomers from the marine sponge Spongia sp. Nat. Prod. Res. 2019, 35, 2178–2183. [Google Scholar] [CrossRef]
- Lai, W.; Qin, S.; Zou, G.; Liao, X.; Chen, G.; Zhang, H.; Zhao, B.; Xu, S. Sinulaspirolactam A, a novel aza-spirocyclic valerenane sesquiterpenoid from soft coral Sinularia sp. J. Asian Nat. Prod. Res. 2019, 21, 494–501. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Huang, L.; Liao, X.; Jiang, Z.; Xu, S.; Zhao, B. Two new halogenated metabolites from the red alga Laurencia sp. J. Asian Nat. Prod. Res. 2022; in press. [Google Scholar] [CrossRef]
- Su, W.; Zhao, J.; Hu, J.; Yang, M.; Jacob, M.; Cai, X.; Zeng, R. Two new bicyclic sesquiterpenes from the stems of Kadsura heteroclita. Nat. Prod. Res. 2014, 28, 1197–1201. [Google Scholar] [CrossRef]
- Park, K.E.; Kim, Y.A.; Jung, H.A.; Lee, H.J.; Ahn, J.W.; Lee, B.J.; Seo, Y.W. Three norisoprenoids from the brown alga Sargassum thunbergii. J. Korean Chem. Soc. 2004, 48, 394–398. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Q.; Lu, D.; Leng, L.; Li, P.; Liu, J. Chemical constituents in Lepidium meyenii cultivated in Jilin. Chin. Tradit. Herb. Drugs 2014, 45, 2457–2460. [Google Scholar] [CrossRef]
- Xu, L.; Ying, Z.; Wei, W.; Hao, D.; Wang, H. A novel alkaloid from Portulaca oleracea L. Nat. Prod. Res. 2017, 31, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lin, X.; Zhou, X.; Pang, X. Study on the secondary metabolites from South China Sea deep-water-derived fungus Penicillium brocae SCSIO 05793. Chin. J. Mar. Drugs 2017, 36, 23–28. [Google Scholar] [CrossRef]
- Yang, J.; He, S.; Ding, L. Study on chemical constituents from sponge-derived fungus Penicillium sp. HPQJ10. Chin. Tradit. Herb. Drugs 2017, 48, 5105–5111. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, B.; Huang, M.; Tang, J.; Li, Y.; Guo, L.; He, R.; Hu, D.; Yao, X.; Gao, H. Tripodalsporormielones A-C, unprecedented cage-like polyketides with complex polyvdent bridged and fused ring systems. Acta Pharm. Sin. B 2021, 11, 3648–3654. [Google Scholar] [CrossRef]
- Hwang, B.S.; Jeong, Y.T.; Lee, S.; Jeong, E.J.; Rho, J.R. Densazalin, a new cytotoxic diazatricyclic alkaloid from the marine sponge Haliclona densaspicula. Molecules 2021, 26, 3164. [Google Scholar] [CrossRef]
- Liu, T.; Liao, X.; Xu, S.; Zhao, B. Solieritide A, a new polyketide from the red alga Solieria sp. Nat. Prod. Res. 2021, 35, 3780–3786. [Google Scholar] [CrossRef]




| No. | δH | δC |
|---|---|---|
| 1 | 2.01 | 49.0 |
| 2 | a 2.41 dd (18.0, 6.0) b 2.31 dd (18.0, 13.8) | 34.7 |
| 3 | - | 199.7 |
| 4 | - | 133.7 |
| 5 | 6.87 q (1.2) | 153.5 |
| 6 | - | 74.9 |
| 7 | 1.53 | 46.3 |
| 8 | 1.52 | 20.4 |
| 9 | a 1.58 | 28.8 |
| b 1.18 m | ||
| 10 | 2.11 m | 29.2 |
| 11 | 1.80 d (1.2) | 15.6 |
| 12 | 2.03 m | 27.0 |
| 13 | 0.99 d (7.2) | 18.6 |
| 14 | 0.90 d (7.2) | 23.8 |
| 15 | 0.87 d (7.2) | 18.4 |
| Possible Isomer | 1A | 1B |
|---|---|---|
| sDP4+ | 96.70% | 3.30% |
| uDP4+ | 97.84% | 2.16% |
| DP4+ | 99.92% | 0.08% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liang, H.; Wang, Z.; Liao, X.; Xu, S.; Zhao, B. Clionaterpene, a New Cadinene Sesquiterpene from the Marine Sponge Cliona sp. J. Mar. Sci. Eng. 2023, 11, 131. https://doi.org/10.3390/jmse11010131
Zhou Y, Liang H, Wang Z, Liao X, Xu S, Zhao B. Clionaterpene, a New Cadinene Sesquiterpene from the Marine Sponge Cliona sp. Journal of Marine Science and Engineering. 2023; 11(1):131. https://doi.org/10.3390/jmse11010131
Chicago/Turabian StyleZhou, Ying, Huixian Liang, Zhaocong Wang, Xiaojian Liao, Shihai Xu, and Bingxin Zhao. 2023. "Clionaterpene, a New Cadinene Sesquiterpene from the Marine Sponge Cliona sp." Journal of Marine Science and Engineering 11, no. 1: 131. https://doi.org/10.3390/jmse11010131
APA StyleZhou, Y., Liang, H., Wang, Z., Liao, X., Xu, S., & Zhao, B. (2023). Clionaterpene, a New Cadinene Sesquiterpene from the Marine Sponge Cliona sp. Journal of Marine Science and Engineering, 11(1), 131. https://doi.org/10.3390/jmse11010131





