Fisheries Biology and Basic Life-Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Procedure
2.3. Data Analysis
3. Results
3.1. Physicochemical Parameters
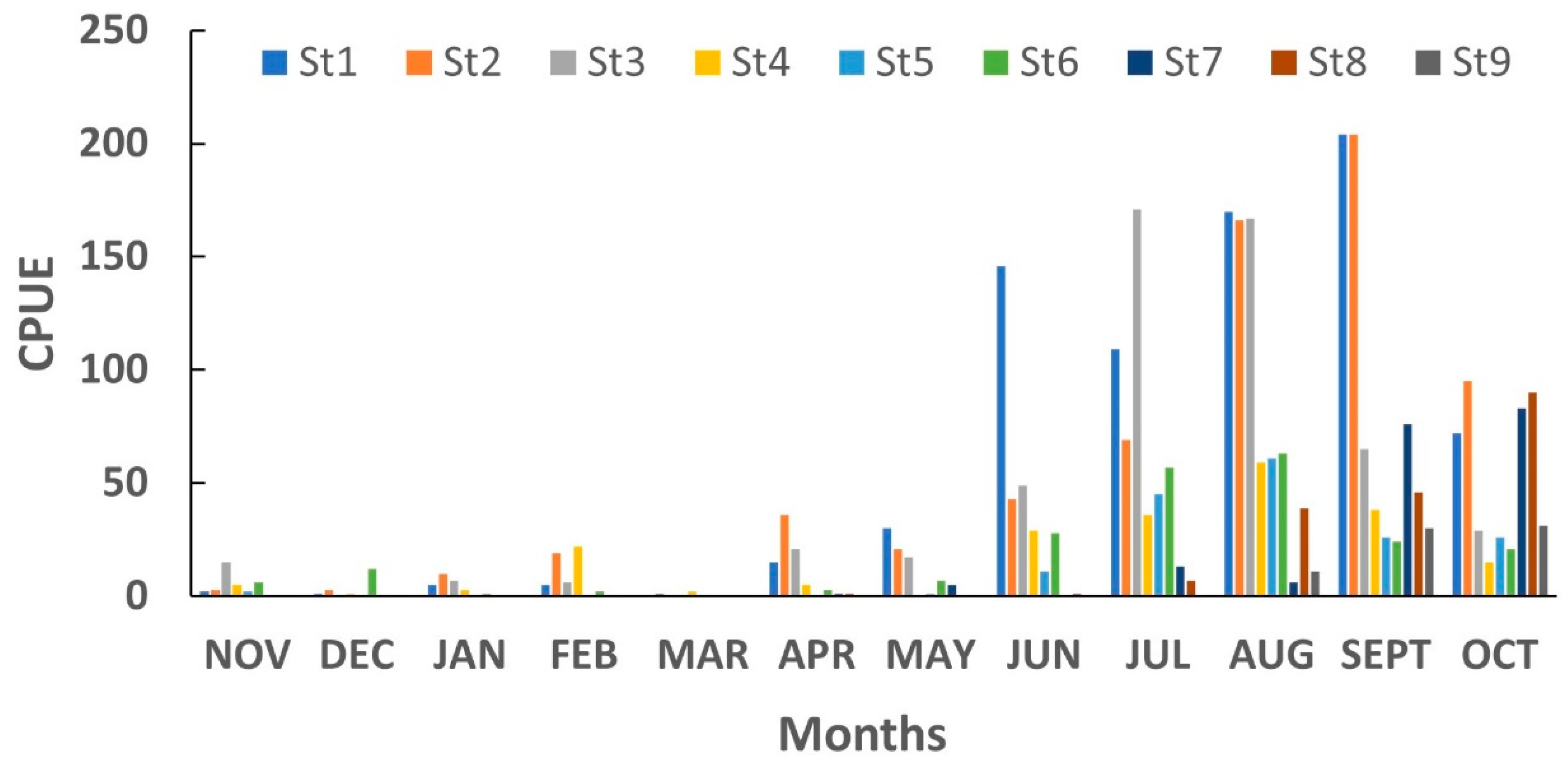
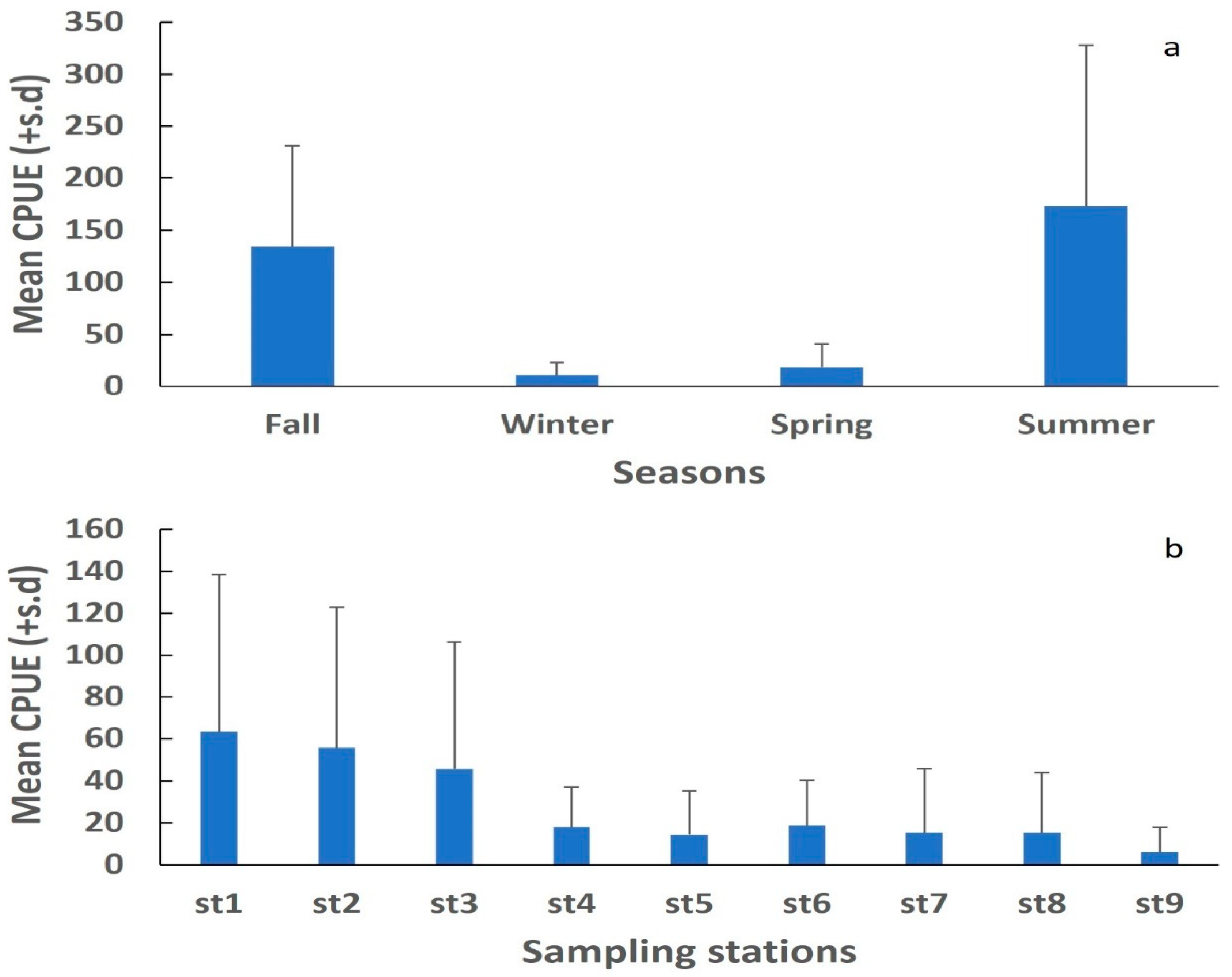
3.2. Catch Per Unit Effort (CPUE)
3.2.1. Seasonal Variation
3.2.2. Spatial Variation
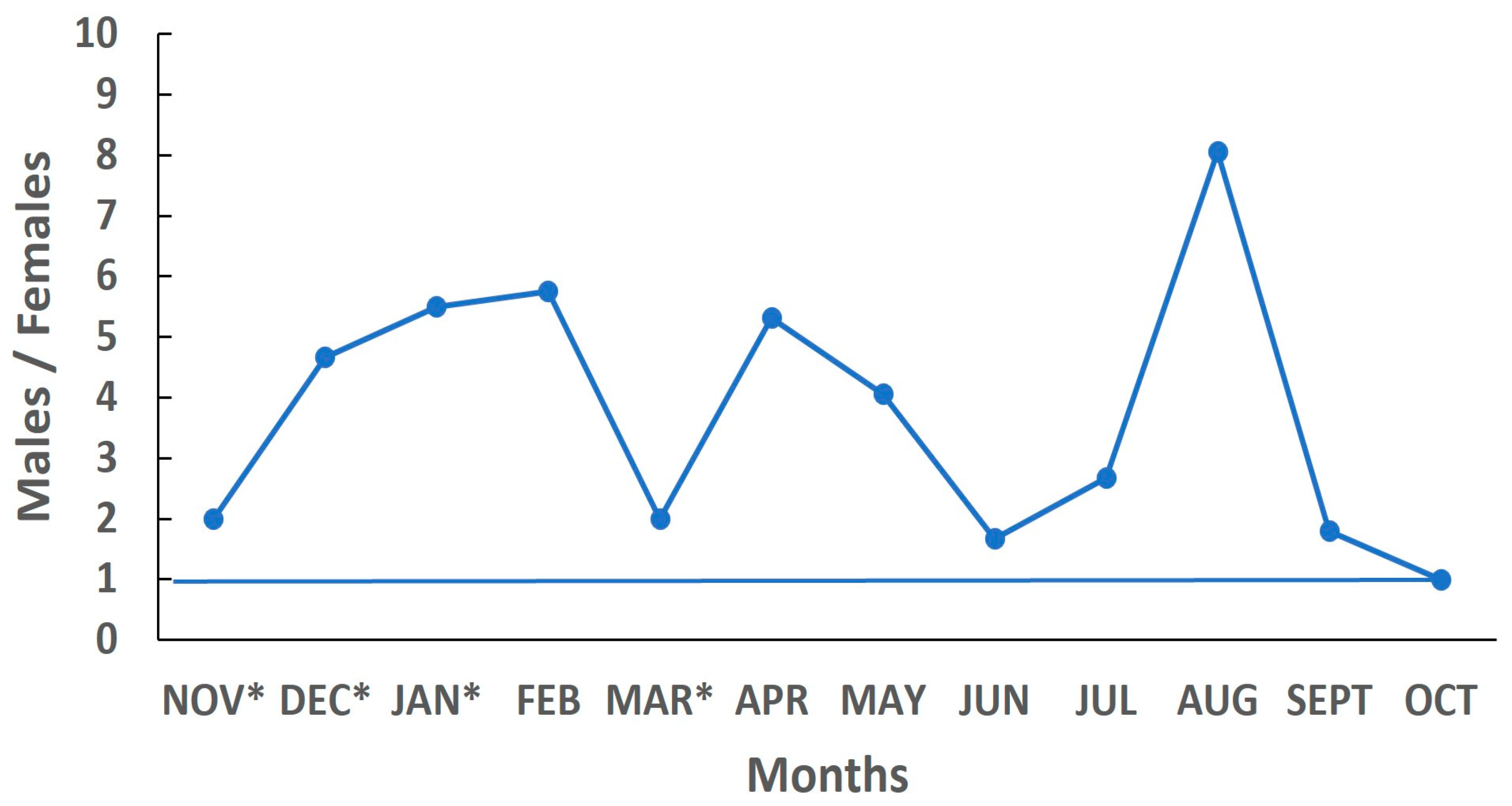
3.3. Sex Ratio
3.4. Population Structure
3.4.1. Age Classes
3.4.2. Size–Frequency Distribution
3.5. Reproductive Aspects
3.5.1. Ovarian Stages
3.5.2. Ovigerous Females
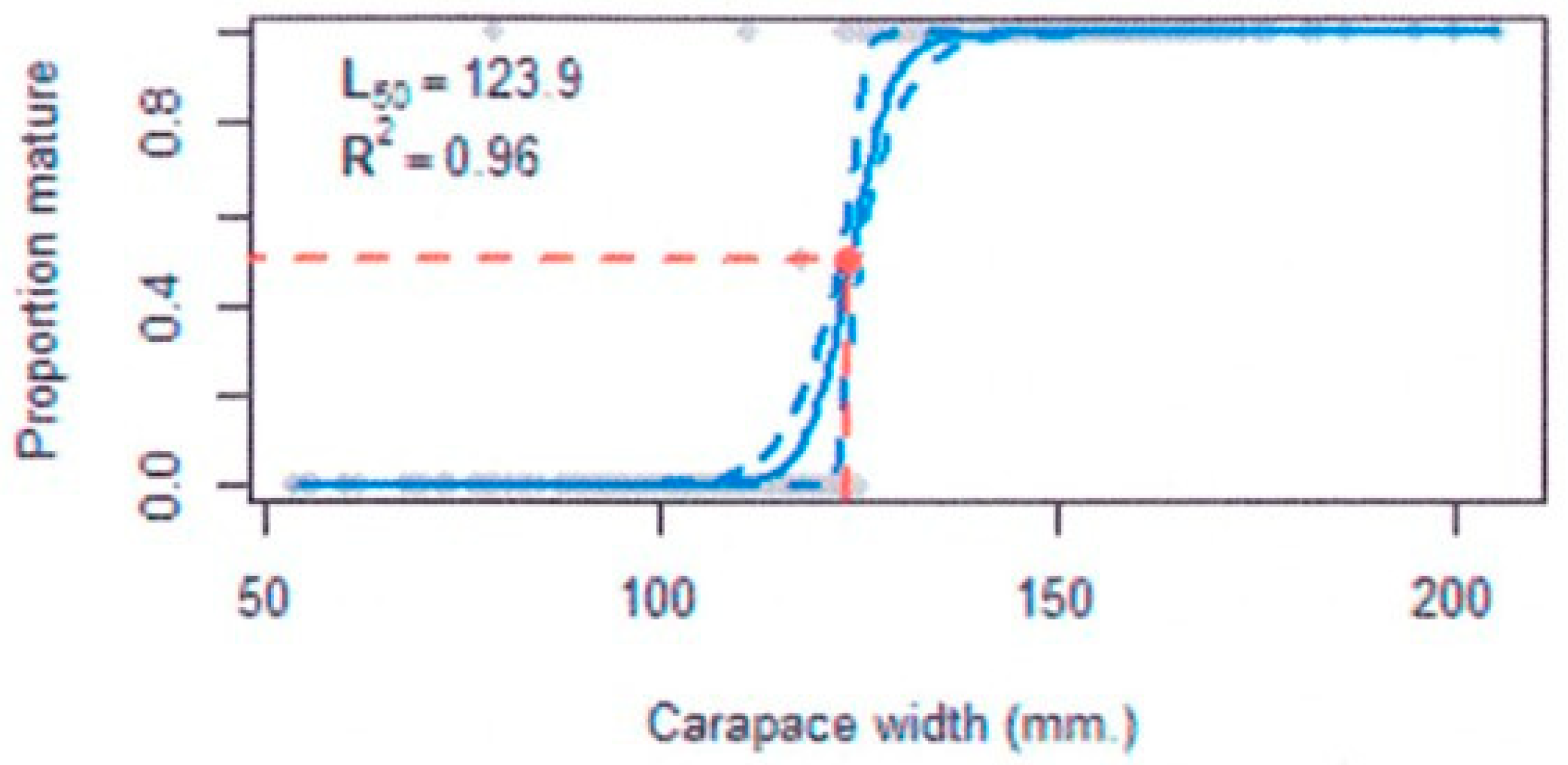
3.5.3. Size at Maturity
4. Discussion
4.1. Physicochemical Parameters
4.2. Catch Per Unit Effort (CPUE)
4.3. Sex Ratio
4.4. Population Structure
4.5. Reproductive Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.B. The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Fish. Bull. 1974, 72, 685–798. [Google Scholar]
- Johnson, D.S. The savory swimmer swims north: A northern range extension of the blue crab Callinectes sapidus? J. Crust. Biol. 2015, 35, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Millikin, M.R.; Williams, A.B. Synopsis of biological data on the blue crab, Callinectes sapidus Rathbun. In FAO Fisheries Synopsis No. 138; NOAA Technical Report NMFS 1; US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Washington, DC, USA, 1984; p. 39. [Google Scholar]
- Mancinelli, G.; Bardelli, R.; Zenetos, A. A global occurrence database of the Atlantic blue crab Callinectes sapidus. Sci. Data 2021, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, E.L. Sur un Callinectes sapidus M. Rathbun trouvé à Rochefort. Bull. Mus. Hist. Nat. Paris 1901, 7, 16–17. [Google Scholar]
- Serbetis, C. Un nouveau Crustacé comestible en Mer Egée Callinectes sapidus Rathb. (Décapode brachyoure). Proc. Gen. Fish. Comm. Mediterran. 1959, 5, 505–507. [Google Scholar]
- Georgiadis, C.; Georgiadis, G. Zur Kenntnis der Crustacea Decapoda des Golfes von Thessaloniki. Crustaceana 1974, 26, 239–248. [Google Scholar] [CrossRef]
- Nehring, S. Invasion history and success of the American blue crab Callinectes sapidus Rathbun, 1896 in European and adjacent waters. In The Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 6, pp. 607–624. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. The occurrence of the American blue crab, Callinectes sapidus Rathbun, in Israel waters. Bull. Res. Counc. of Israel. 1955, 5B, 154–156. [Google Scholar]
- Holthuis, L.B. Report on a collection of Crustacea Decapoda and Stomatopoda from Turkey and the Balkans. Zool. Verh. 1961, 47, 1–67. [Google Scholar]
- Banoub, M.W. Survey of the blue-crab Callinectus sapidus (Rath). In Lake Edku in 1960; Notes and Memoirs, Alexandria Inst. of Hydrobiology: Alexandria, Egypt, 1963; Volume 69, pp. 1–18. [Google Scholar]
- Kevrekidis, Κ.; Antoniadou, C. Abundance and population structure of the blue crab Callinectes sapidus (Decapoda, Portunidae) in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Crustaceana 2018, 69, 641–657. [Google Scholar] [CrossRef]
- Koukouras, A.; Dounas, C.; Turkay, M.; Voultsiadou-Koukoura, E. Decapod crustacean fauna of the Aegean Sea: New information check list, affinities. Senckenberg. Marit. 1992, 22, 217–244. [Google Scholar]
- Kevrekidis, K. Callinectes sapidus (Decapoda, Brachyura): An allochthonous species in Thermaikos Gulf. Fish. News 2010, 340, 44–49. (In Greek) [Google Scholar]
- Kevrekidis, K.; Antoniadou, C.; Avramoglou, K.; Efstathiadis, J.; Chintiroglou, C. Population structure of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay). In Proceedings of the 15th Pan-Hellenic Congress of Ichthyologists, Thessaloniki, Greece, 1–13 October 2013; pp. 113–116, (In Greek with English Abstract). [Google Scholar]
- Nykjaer, L. Mediterranean sea surface warming 1985–2006. Clim. Res. 2009, 39, 11–17. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Perdikaris, C.; Konstantinidis, E.; Gouva, E.; Ergolavou, A.; Klaoudatos, D.; Nathanailides, C.; Paschos, I. Occurrence of the invasive crab species Callinectes sapidus Rathbun,1896 in NW Greece. Walailak J. Sci. Tech. 2016, 13, 503–510. [Google Scholar]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; Guerao, G. A new record of the American blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), from the Mediterranean coast of the Iberian Peninsula. BioInvasions Rec. 2013, 2, 141–143. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Ragkousis, M.; Abdelali, N.; Azzurro, E.; Badreddine, A.; Bariche, M.; Bitar, G.; Crocetta, F.; Denitto, F.; et al. First record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 in the Gulf of Gabès (south-eastern Tunisia). New Alien Mediterranean Biodiversity Records (October 2020). Medit. Mar. Sci. 2020, 21, 631–652. [Google Scholar] [CrossRef]
- Clavero, M.; Franch, N.; Bernardo-Madrid, R.; Lopez, V.; Abello, P.; Queral, J.M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Pollut. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef]
- Yağlıoğlu, D.; Turan, C.; Öğrede, T. First record of blue crab Callinectes sapidus (Rathbun 1896) (Crustacea, Brachyura, Portunidae) from the Turkish Black Sea coast. Black Sea/Medit. Environ. 2014, 20, 13–17. [Google Scholar]
- Vasconcelos, P.; Carvalho, A.N.; Piló, D.; Pereira, F.; Encarnacao, I.; Gaspar, M.B.; Teodosio, M.A. Recent and consecutive records of the Atlantic blue crab (Callinectes sapidus Rathbun, 1896): Rapid westward expansion and confirmed establishment along the southern coast of Portugal. Thalassas 2019, 35, 485–494. [Google Scholar] [CrossRef]
- Chaouti, A.Z.; Belattmania, A.; Nadri, E.; Serrão, A.; Encarnação, J.; Teodósio, M.A.; Reani, A.; Sabour, B. The invasive Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its distributional range southward to Atlantic African shores: First records along the Atlantic coast of Morocco. BioInvasions Rec. 2022, 11, 227–237. [Google Scholar] [CrossRef]
- Glamuzina, L.; Conides, A.; Mancinelli, G.; Glamuzina, B. A comparison of traditional and locally novel fishing gear for the exploitation of the invasive Atlantic blue crab in the Eastern Adriatic Sea. J. Mar. Sci. Eng. 2021, 9, 1019. [Google Scholar] [CrossRef]
- Öndes, F.; Gökçe, G. Distribution and fishery of the blue crab (Callinectes sapidus Rathbun, 1896) in Turkey based on local ecological knowledge of fishers. J. Anatolian Env. Anim. Sci. 2021, 6, 325–332. [Google Scholar] [CrossRef]
- Churchill, E.P., Jr. Life history of the blue crab. Bull. Bur. Fish. 1919, 36, 95–128. [Google Scholar]
- Kennedy, V.S.; Cronin, L.E. The Blue Crab: Callinectes Sapidus; Maryland Sea Grant College: College Park, MD, USA, 2007; p. 800. [Google Scholar]
- Epifanio, C.E. Early life history of the blue crab Callinectes sapidus: A review. J. Shellfish Res. 2019, 38, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hines, A.H.; Lipcius, R.N.; Haddon, A.M. Population dynamics and habitat partitioning by size, sex, and molt stage of blue crabs, Callinectes sapidus, in a subestuary of central Chesapeakey. Mar. Ecol. Prog. Ser. 1987, 36, 55–64. [Google Scholar] [CrossRef]
- Turner, H.V.; Wolcott, D.L.; Wolcott, T.G.; Hines, A.H. Post-mating behavior, intramolt growth, and onset of migration to Chesapeake Bay spawning grounds by adult female blue crabs, Callinectes sapidus Rathbun. J. Exp. Mar. Biol. Ecol. 2003, 295, 107–130. [Google Scholar] [CrossRef]
- Carr, S.D.; Tankersley, R.A.; Hench, J.L.; Forward, R.B.J.R.; Luettich, R.A.J.R. Movement patterns and trajectories of ovigerous blue crabs Callinectes sapidus during the spawning migration. Est. Coast. Shelf Sci. 2004, 60, 567–579. [Google Scholar] [CrossRef]
- Lipcius, R.N.; Eggleston, D.B.; Heck, K.L., Jr.; Seitz, R.D.; Van Montfrans, J. Post-settlement abundance, survival, and growth of postlarvae and young juvenile blue crabs in nursery habitats. Chapter 13. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 535–565. [Google Scholar]
- Hines, A.H. Ecology of juvenile and adult blue crabs. Chapter 14. In The Blue Crab: Callinectes Sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 565–654. [Google Scholar]
- Taylor, D.L.; Fehon, M.M. Blue crab (Callinectes sapidus) population structure in southern New England tidal rivers: Patterns of shallow water, unvegetated habitat use and quality. Estuaries Coasts 2021, 44, 1320–1343. [Google Scholar] [CrossRef]
- Chan, F.T.; Stanislawczyk, K.; Sneekes, A.C.; Dvoretsky, A.; Gollasch, S.; Minchin, D.; David, M.; Jelmert, A.; Albretsen, J.; Bailey, S.A. Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob. Chang. Biol. 2019, 25, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and trends in the rate of introduction of marine non-indigenous species in European Seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- Levine, M.J. Biological invasions. Current Biol. 2008, 18, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, A.H.; Haddon, A.M.; Wiechert, L.A. Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Mar. Ecol. Prog. Ser. 1990, 67, 105–126. [Google Scholar] [CrossRef]
- Belgrad, B.A.; Griffen, B.D. The influence of diet composition on fitness of the blue crab, Callinectes sapidus. PLoS ONE 2016, 11, e0145481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.L.; Fehon, M.M.; Cribari, K.J.; Scro, A.K. Blue crab Callinectes sapidus dietary habits and predation on juvenile winter flounder Pseudopleuronectes americanus in southern New England tidal rivers. Mar. Ecol. Progr. Ser. 2022, 681, 145–167. [Google Scholar] [CrossRef]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun, 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- FAO. General Fisheries Commission for the Mediterranean. In Proceedings of the Report of the Twenty-Second Session of the Scientific Advisory Committee on Fisheries, Online, 22–25 June 2021; No. 1347; FAO Fisheries and Aquaculture Report. FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Enzenroß, R.; Enzenroß, L.; Bingel, F. Occurrence of blue crab, Callinectes sapidus (Rathbun, 1896) (Crustacea, Brachyura) on the Turkish Mediterranean and the adjacent Aegean coast and its size distribution in the bay of Iskenderun. Turk. J. Zool. 1997, 21, 113–122. [Google Scholar] [CrossRef]
- Atar, H.H.; Olmez, M.; Bekcan, S.; Secer, S. Comparison of three different traps for catching blue crab (Callinectes sapidus Rathbun, 1896) in Beymelek lagoon. Turk J. Vet. Anim. Sci. 2002, 26, 1145–1150. [Google Scholar]
- Sumer, C.; Teksam, I.; Karatas, H.; Beyhan, T.; Aydin, C.M. Growth and reproduction biologf the blue crab, Callinectes sapidus Rathbun, 1896, in the Beymelek lagoon (southwestern coast of Turkey). Turk. J. Fish. Aquat. Sci. 2013, 13, 675–684. [Google Scholar] [CrossRef]
- Türeli, C.; Miller, T.J.; Gündogdu, S.; Yeşilyurt, I.N. Growth and mortality of blue crab (Callinectes sapidus) in the north-eastern Mediterranean Sea. J. Fish. Sci. 2016, 10, 55–62. [Google Scholar]
- Türeli, C.; Yeşilyurt, I.N.; Nevşat, I.E. Female reproductive pattern of Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae) in Iskenderun Bay, eastern Mediterranean. J. Middle East 2018, 64, 55–63. [Google Scholar] [CrossRef]
- Mancinelli, G.; Carrozzo, L.; Costantini, M.L.; Rossi, L.; Marini, G.; Pinna, M. Occurrence of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 in two Mediterranean coastal habitats: Temporary visitor or permanent resident? Est. Coast. Shelf Sci. 2013, 135, 46–56. [Google Scholar] [CrossRef]
- Cilenti, L.; Pazienza, G.; Scirocco, T.; Fabbrocini, A.; D’Adamo, R. First record of ovigerous Callinectes sapidus (Rathbun, 1896) in the Gargano Lagoons (south-west Adriatic Sea). BioInvasions Rec. 2015, 4, 281–287. [Google Scholar] [CrossRef]
- Abdel Razek, F.A.; Ismaiel, M.; Ameran, M.A. Occurrence of the blue crab Callinectes sapidus Rathbun, 1896, and its fisheries biology in Bardawil Lagoon, Sinai Peninsula, Egypt. Egypt. J. Aquat. Res. 2016, 42, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Kevrekidis, Κ. Relative growth of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Cah. Biol. Mar. 2019, 60, 395–397. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Desouky, M.G.; Farouk, A.E. Population dynamics and fisheries characteristics of the blue crab Callinectes sapidus (Rathbun, 1896) as an invasive species in Bardawil Lagoon, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Kevrekidis, T. Seasonal variation of the macrozoobenthic community structure at low salinities in a Mediterranean lagoon (Monolimni lagoon, Northern Aegean). Internat. Rev. Hydrobiol. 2004, 89, 407–442. [Google Scholar] [CrossRef]
- Malea, P.; Kevrekidis, T.; Mogias, A. Annual versus perennial growth cycle in Ruppia maritima L.: Temporal variation in population characteristics in Mediterranean lagoons (Monolimni and Drana Lagoons, Northern Aegean Sea). Bot. Mar. 2004, 47, 357–366. [Google Scholar] [CrossRef]
- Olmi, E.J.; Bishop, J.M. Variations in total width-weight relationships of blue crabs, Callinectes sapidus, in relation to sex, maturity, molt stage, and carapace form. J. Crust. Biol. 1983, 3, 575–581. [Google Scholar]
- Cadman, L.R.; Weinstein, M.P. Size-weight relationships of postecdysial juvenile blue crabs (Callinectes sapidus Rathbun) from the Lower Chesapeake Bay. J. Crust. Biol. 1985, 5, 306–310. [Google Scholar] [CrossRef]
- Rugolo, L.J.; Knotts, K.S.; Lange, A.M.; Crecco, V.A. Stock assessment of Chesapeake Bay blue crab (Callinectes sapidus Rathbun). J. Shellfish Res. 1998, 17, 1321–1345. [Google Scholar]
- Hard, W.L. Ovarian growth and ovulation in the mature blue crab, Callinectes sapidus Rathbun. In Chesapeake Biological Laboratory Contribution; Board of Natural resources, Department of Research and Education: Solomons, ML, USA, 1942; Volume 46, p. 17. [Google Scholar]
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised version. User’s guide. In FAO Computerized Information Series (Fisheries). No. 8, Revised Version; FAO: Rome, Italy, 2005; 168p. [Google Scholar]
- Hines, A.H. Ecology of juvenile and adult blue crabs: Summary of discussion of research themes and directions. Bull. Mar. Sci. 2003, 72, 423–433. [Google Scholar]
- Jivoff, P.; Hines, A.H.; Quackenbush, L.S. Reproduction Biology and Embryonic Development. Chapter 7. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 255–298. [Google Scholar]
- Leffler, C.W. Some effects of temperature on the growth and metabolic rate of juvenile blue crabs, Callinectes sapidus, in the laboratory. Mar. Biol. 1972, 14, 104–110. [Google Scholar] [CrossRef]
- Mangum, C.; Towle, D. Physiological adaptation to unstable environments. Am. Sci. 1977, 65, 67–75. [Google Scholar]
- Epifanio, C.E. Biology of larvae. Chapter 12. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 513–533. [Google Scholar]
- Tagatz, M.E. Some relations of temperature acclimation and salinity to thermal tolerance of the blue crab, Callinectes sapidus. Trans. Amer. Fish. Soc. 1969, 98, 713–716. [Google Scholar] [CrossRef]
- Guerin, J.L.; Stickle, W.B. Effects of salinity gradients on the tolerance and bioenergetics of juvenile blue crabs (Callinectes sapidus) from waters of different environmental salinities. Mar. Biol. 1992, 114, 391–396. [Google Scholar] [CrossRef]
- Towle, D.W.; Burnett, L.E. Osmoregulatory, Digestive, and Respiratory Physiology. Chapter 9. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 419–533. [Google Scholar]
- Scalici, M.; Chiesa, S.; Mancinelli, G.; Rontani, P.M.; Voccia, A.; Marzano, F.N. Euryhaline aliens invading Italian inland waters: The case of the Atlantic blue crab Callinectes sapidus Rathbun, 1896. Appl. Sci. 2022, 12, 4666. [Google Scholar] [CrossRef]
- Hines, A.H.; Johnson, E.G.; Darnell, M.Z.; Rittschof, D.; Miller, T.J.; Bauer, L.J.; Rodgers, P.; Aguilar, R. Predicting effects of climate change on Blue Crabs in Chesapeake Bay. In Biology and Management of Exploited Crab Populations under Climate Change; Kruse, G.H., Eckert, G.L., Foy, R.J., Lipcius, R.N., Sainte-Marie, B., Stram, D.L., Woodby, D., Eds.; Alaska Sea Grant; University of Alaska Fairbanks: Fairbanks, AK, USA, 2010; pp. 109–127. [Google Scholar] [CrossRef] [Green Version]
- Brill, R.W.; Bushnell, P.G.; Elton, T.A.; Small, H.J. The ability of blue crab (Callinectes sapidus, Rathbun 1886) to sustain aerobic metabolism during hypoxia. J. Exp. Mar. Biol. Ecol. 2015, 471, 126–136. [Google Scholar] [CrossRef]
- Steele, P.; Bert, T.M. Population ecology of the blue crab, Callinectes sapidus Rathbun. In A Subtropical Estuary: Population Structure, Aspects of Reproduction, and Habitat Partitioning; Florida Marine Research Institute Publication: St. Petersburg, FL, USA, 1994; p. 24. [Google Scholar]
- Miller, R.E.; Sulkin, S.D.; Lippson, R.L. Composition and seasonal abundance of the blue crab, Callinectes sapidus Rathbun, in the Chesapeake and Delaware Canal and adjacent waters. Chesapeake Sci. 1975, 16, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Jensen, O.P.; Miller, T.J. Geostatistical analysis of the abundance and winter distribution patterns of the blue crab Callinectes sapidus in Chesapeake Bay. Trans. Amer. Fish. Soc. 2005, 134, 1582–1598. [Google Scholar] [CrossRef]
- Buchanan, B.A.; Stoner, A.W. Distributional patterns of blue crabs (Callinectes sp.) in a tropical estuarine lagoon. Estuaries 1988, 11, 231–239. [Google Scholar] [CrossRef]
- Murphy, R.F.; Secor, D.H. Fish and blue crab assemblage structure in a US mid Atlantic coastal lagoon complex. Estuaries Coasts 2006, 29, 1121–1131. [Google Scholar] [CrossRef]
- Jivoff, P.R.; Smith, J.M.; Sodi, V.L.; Van Morter, S.M.; Faugno, K.M.; Werda, A.L.; Shaw, M.J. Population structure of adult blue crabs, Callinectes sapidus, in relation to physical characteristics in Barnegat Bay, New Jersey. Estuaries Coasts 2017, 40, 235–250. [Google Scholar] [CrossRef]
- King, R.S.; Hines, A.H.; Doug Craige, F.; Grap, S. Regional, watershed and local correlates of blue crab and bivalve abundances in subestuaries of Chesapeake Bay, USA. J. Exp. Mar Biol. Ecol. 2005, 319, 101–116. [Google Scholar] [CrossRef]
- Bell, G.W.; Eggleston, D.B.; Wolcott, T.G. Behavioral responses of free-ranging blue crabs to episodic hypoxia: I. Movement. Mar. Ecol. Prog. Ser. 2003, 259, 215–225. [Google Scholar] [CrossRef]
- Orth, R.J.; van Montfrans, J. Utilization of marsh and seagrass habitats by early stages of Callinectes sapidus: A latitudinal perspective. Bull. Mar. Sci. 1990, 46, 126–144. [Google Scholar]
- Pardieck, R.A.; Orth, R.J.; Diaz, R.J.; Lipcius, R.N. Ontogenetic changes in habitat use by postlarvae and young juveniles of the blue crab. Mar. Ecol. Prog. Ser. 1999, 187, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Chartosia, N.; Κevrekidis, Κ.; Xinari, I.; Simon, L.; Boubonari, T.; Mogias, A.; Malea, P.; Κevrekidis, T. Preliminary data for the seasonal diet of the blue crab Callinectes sapidus in the estuarine area of Evros River (ΝΕ Aegean Sea). In Proceedings of the 18th Pan-Hellenic Congress of Ichthyologists, Mesologgi, Greece, 3–6 November 2022; pp. 17–22, (In Greek with English Abstract). [Google Scholar]
- Fitz, H.C.; Wiegert, R.G. Local population dynamics of estuarine blue crabs: Abundance, recruitment and loss. Mar. Ecol. Prog. Ser. 1992, 87, 23–40. [Google Scholar] [CrossRef]
- Lycett, K.A.; Shields, J.D.; Sook Chunng, J.; Pitula, J.S. Population structure of the blue crab Callinectes sapidus in the Maryland coastal bays. J. Shellfish Res. 2020, 39, 699–713. [Google Scholar] [CrossRef]
- Carrozzo, L.; Potenza, L.; Carlino, P.; Costantini, M.L.; Rossi, L.; Mancinelli, G. Seasonal abundance and trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in a Mediterranean coastal habitat. Rend. Lincei-Sci. Fis. 2014, 25, 201–208. [Google Scholar] [CrossRef]
- Van Engel, W.A. The blue crab and its fishery in Chesapeake Bay. Part I. Reproduction, early development, growth and migration. Commer. Fish. Rev. 1958, 20, 6–17. [Google Scholar]
- Smith, S.G.; Chang, E.S. Molting and Growth. Chapter 6. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 255–298. [Google Scholar]
- Tankersley, R.A.; Wieber, M.G.; Sigala, M.A.; Kachurak, K.A. Migratory behavior of ovigerous blue crabs Callinectes sapidus: Evidence for selective tidal-stream transport. Biol. Bull. 1998, 195, 168–173. [Google Scholar] [CrossRef]
- Tagatz, M.E. Biology of the blue crab, Callinectes sapidus Rathbun, in the St. Johns River, Florida. Fish. Bull. 1968, 67, 17–33. [Google Scholar]
- Ramach, S.; Darnell, M.Z.; Avissar, N.; Rittscof, D. Habitat use and population dynamics of blue crabs, Callinectes sapidus, in a high-salinity embayment. J. Shell. Res. 2009, 28, 635–640. [Google Scholar] [CrossRef]
- Lipcius, R.N.; Stockhausen, W.T. Concurrent decline of the spawning stock, recruitment, larval abundance, and size of the blue crab in Chesapeake Bay. Mar. Ecol. Prog. Ser. 2002, 226, 45–61. [Google Scholar] [CrossRef]
- Hart, H.R.; Crowley, C.E.; Walters, E.A. Blue crab spawning and recruitment in two Gulf coasts and two Atlantic estuaries in Florida. Mar. Coastal Fish. 2021, 13, 113–130. [Google Scholar] [CrossRef]
- Pereira, M.J.; Branco, J.O.; Christoffersen, M.L.; Freitas, F., Jr.; Fracasso, H.A.A.; Pinheiro, T.C. Population biology of Callinectes danae and Callinectes sapidus (Crustacea: Brachyura: Portunidae) in the south-western Atlantic. J. Mar. Biol. Assoc. UK 2009, 89, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Severino-Rodrigues, E.; Musiello-Fernandes, J.; Moura, A.A.S.; Branco, G.M.P.; Caneo, V.O.C. Fecundity, reproductive seasonality and maturation size of Callinectes sapidus females (Decapoda: Portunidae) in the Southeast coast of Brazil. Rev. Biol. Trop. 2013, 61, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Lipcius, R.N.; van Engel, W.A. Blue crab population dynamics in Chesapeake Bay: Variation in abundance (York River, 1972–1988) and stock-recruit functions. Bull. Mar. Sci. 1990, 46, 180–194. [Google Scholar]
- Aguilar, R.; Hines, A.H.; Wolcott, T.G.; Wolcott, D.L.; Kramer, M.A.; Lipcius, R.N. The timing and route of movement and migration of post-copulatory female blue crabs, Callinectes sapidus Rathbun, from the upper Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 2005, 319, 117–128. [Google Scholar] [CrossRef]



| Relationship | Blue Crab Population | Male Blue Crabs | Female Blue Crabs | |||
|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | |
| CPUE–temperature | 0.93 | * | 0.895 | * | 0.818 | * |
| CPUE–salinity | 0.629 | * | 0.594 | * | 0.497 | n.s |
| CPUE–oxygen | −0.762 | * | −0.720 | * | −0.692 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kevrekidis, K.; Kevrekidis, T.; Mogias, A.; Boubonari, T.; Kantaridou, F.; Kaisari, N.; Malea, P.; Dounas, C.; Thessalou-Legaki, M. Fisheries Biology and Basic Life-Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 462. https://doi.org/10.3390/jmse11030462
Kevrekidis K, Kevrekidis T, Mogias A, Boubonari T, Kantaridou F, Kaisari N, Malea P, Dounas C, Thessalou-Legaki M. Fisheries Biology and Basic Life-Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). Journal of Marine Science and Engineering. 2023; 11(3):462. https://doi.org/10.3390/jmse11030462
Chicago/Turabian StyleKevrekidis, Kosmas, Theodoros Kevrekidis, Athanasios Mogias, Theodora Boubonari, Foteini Kantaridou, Nikoletta Kaisari, Paraskevi Malea, Costas Dounas, and Maria Thessalou-Legaki. 2023. "Fisheries Biology and Basic Life-Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean)" Journal of Marine Science and Engineering 11, no. 3: 462. https://doi.org/10.3390/jmse11030462
APA StyleKevrekidis, K., Kevrekidis, T., Mogias, A., Boubonari, T., Kantaridou, F., Kaisari, N., Malea, P., Dounas, C., & Thessalou-Legaki, M. (2023). Fisheries Biology and Basic Life-Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). Journal of Marine Science and Engineering, 11(3), 462. https://doi.org/10.3390/jmse11030462








