Epizoans on Floating Golden Tide Macroalgae in the Southern Yellow Sea
Abstract
:1. Introduction
2. Materials and Methods
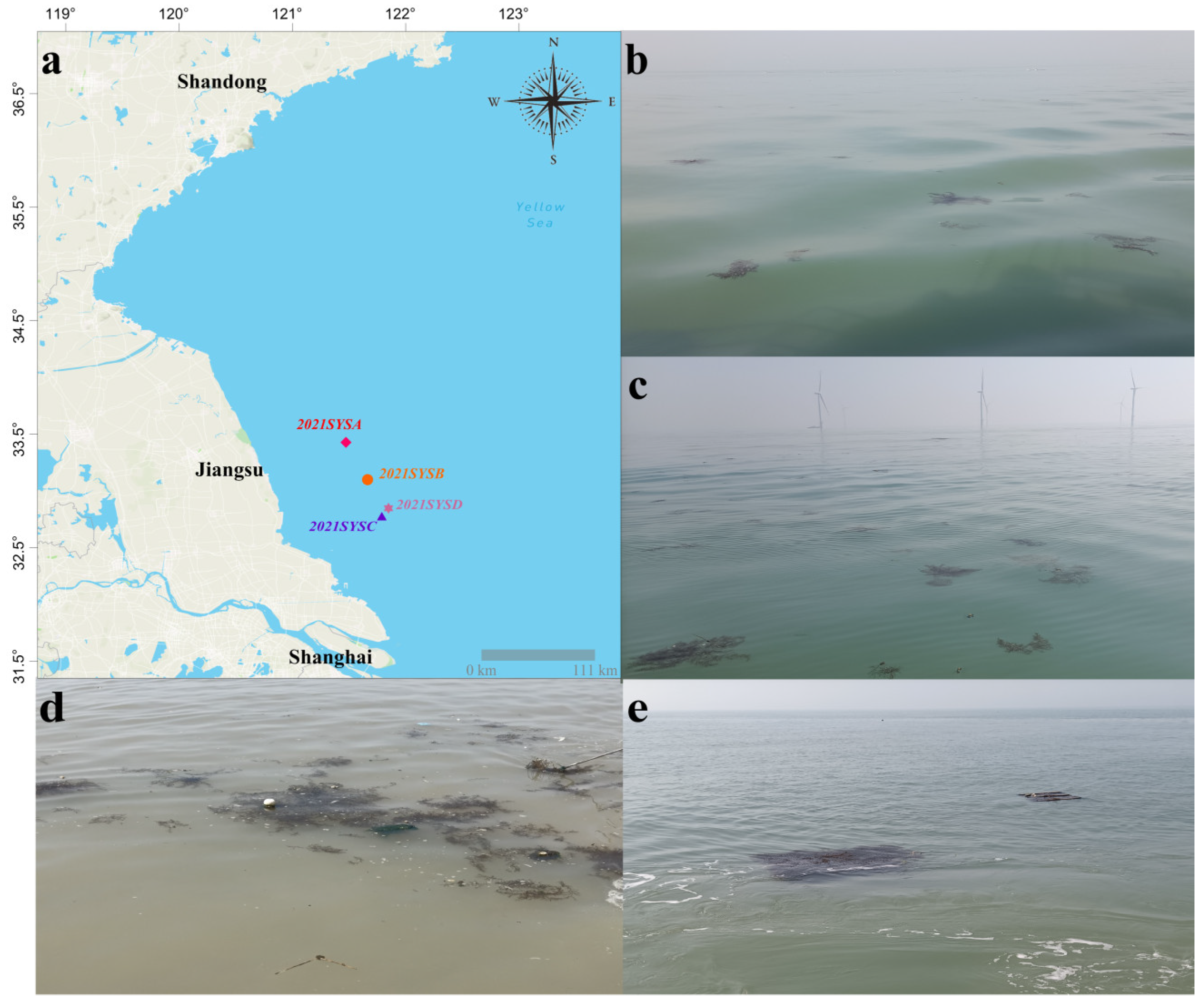
2.1. Sample Collection of Macroalgae and Epizoans
2.2. Morphological and Molecular Identification of Macroalgae
2.3. Morphological and Molecular Identification of Epizoans
3. Results
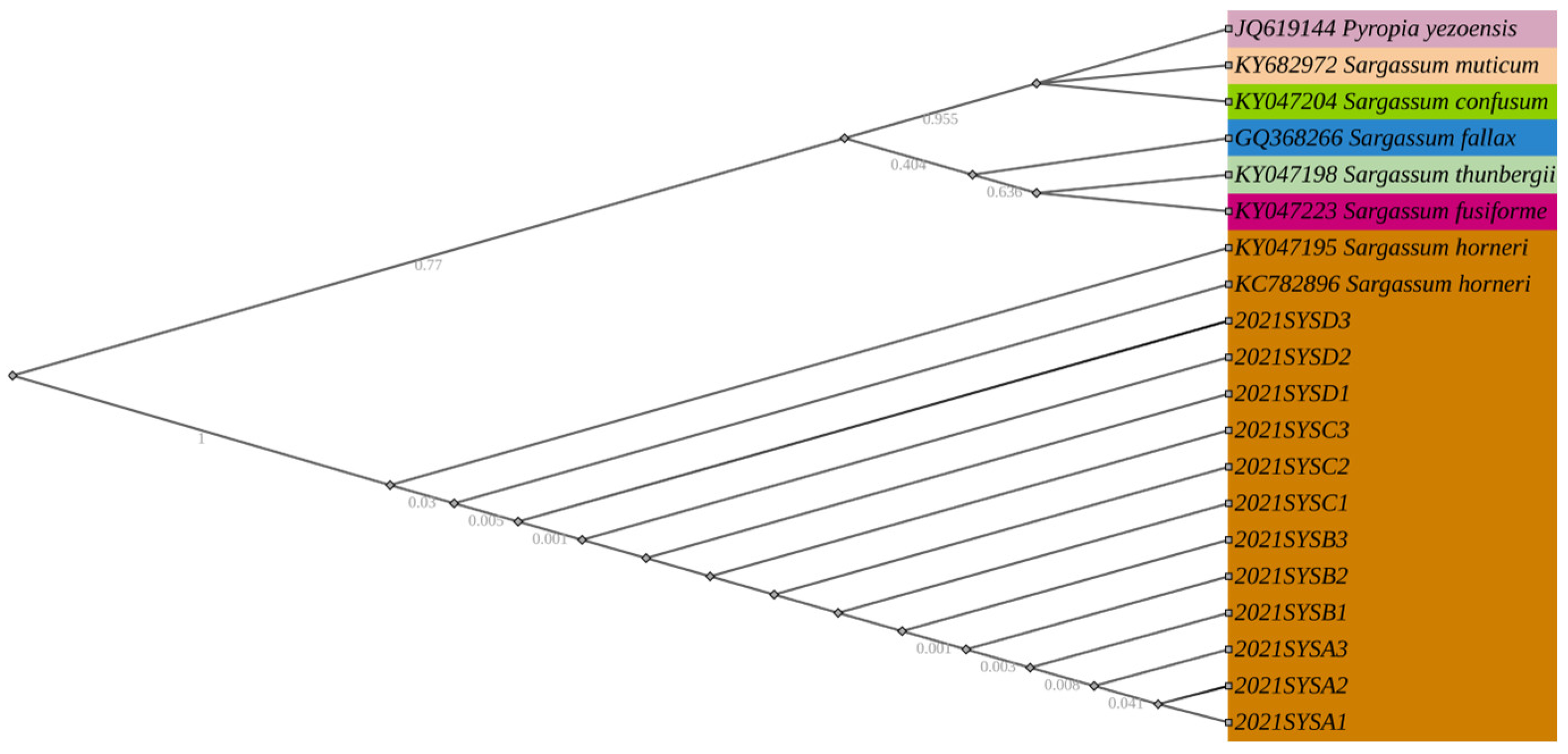
3.1. Identification of Macroalgae
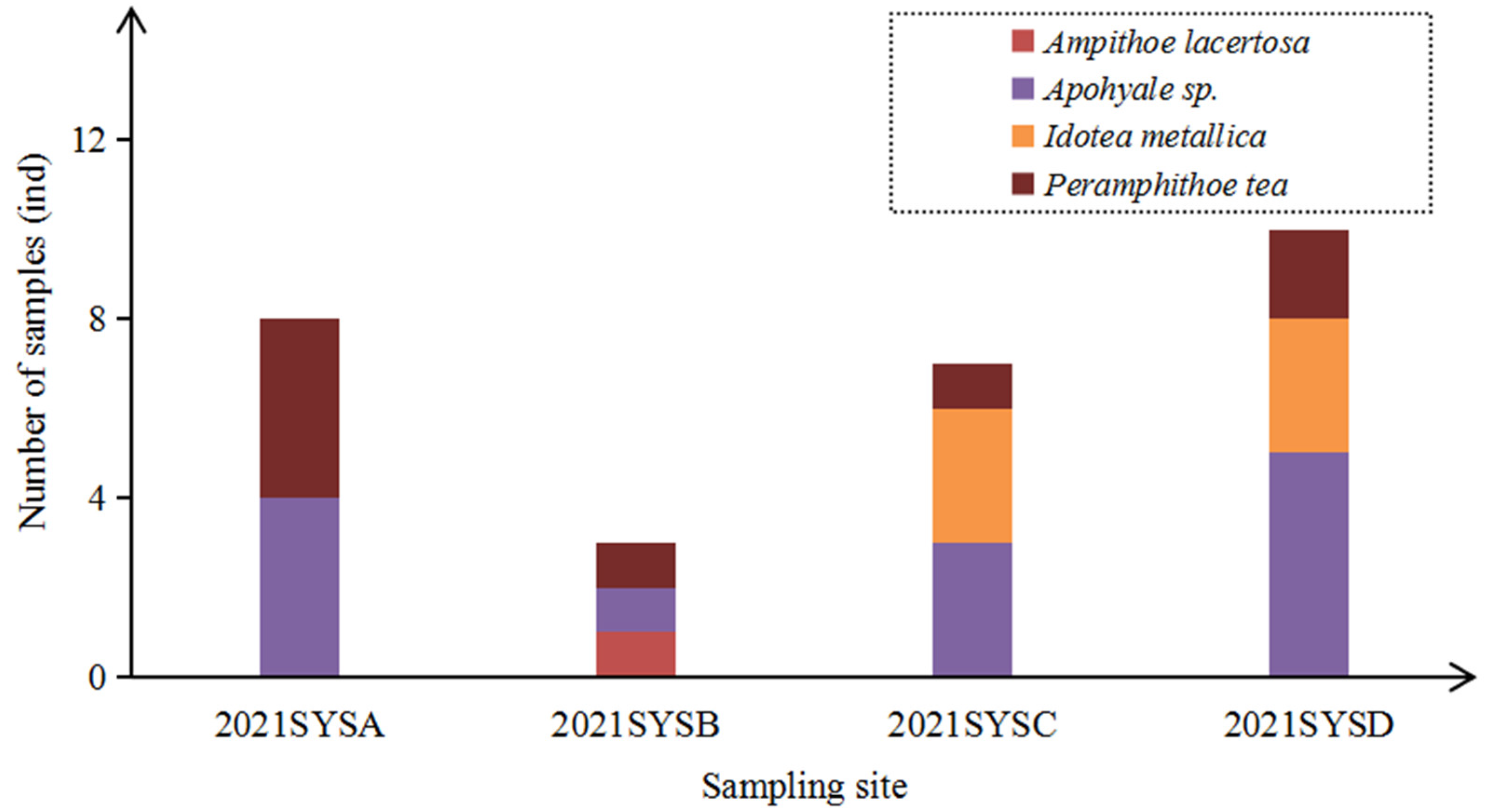
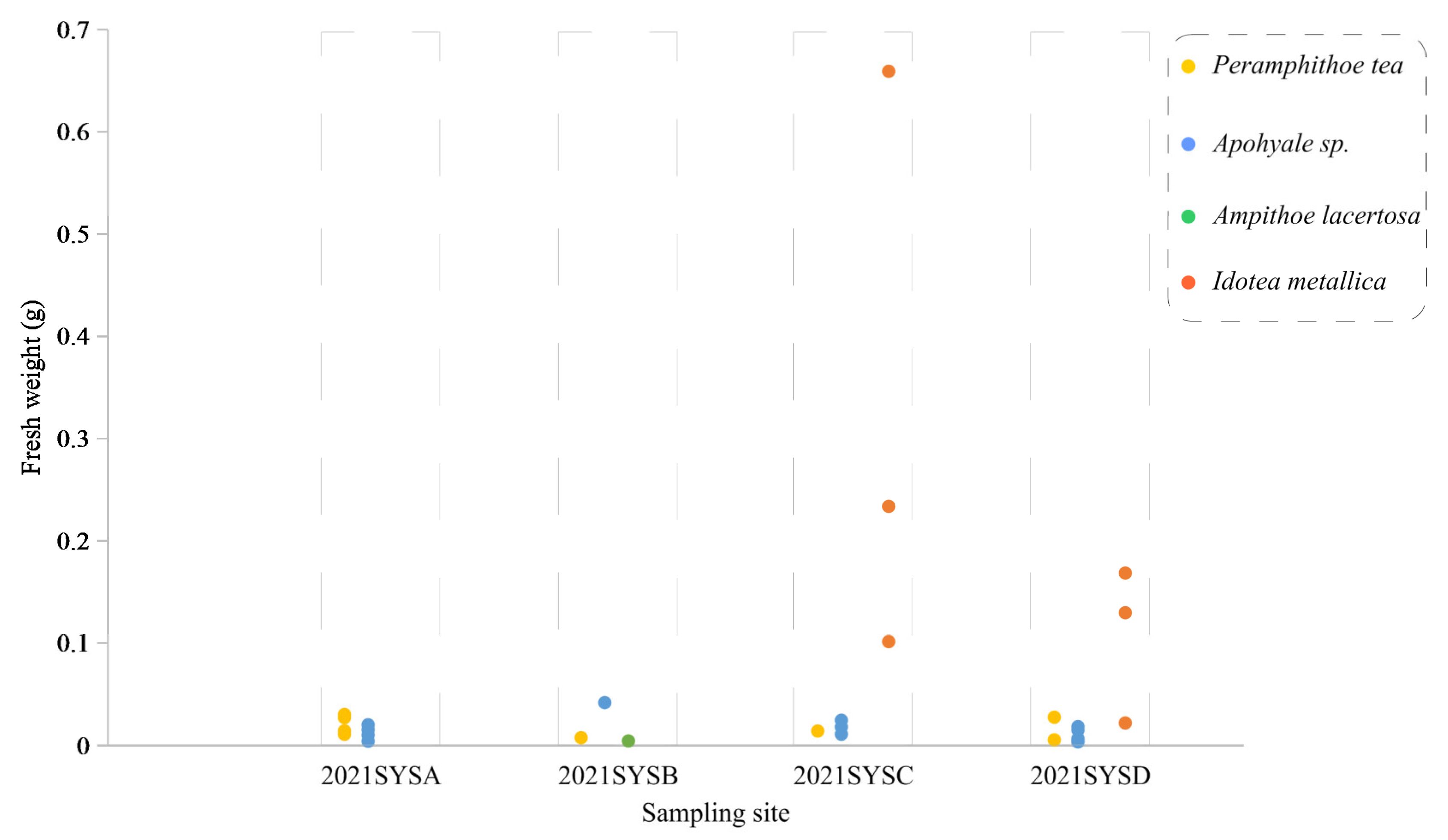
3.2. Species Composition and Biological Parameters of Epizoans
4. Discussion
4.1. Golden Tides in the SYS and the Origin of Blooms
4.2. Potential Effects of Epizoans Entering the SYS
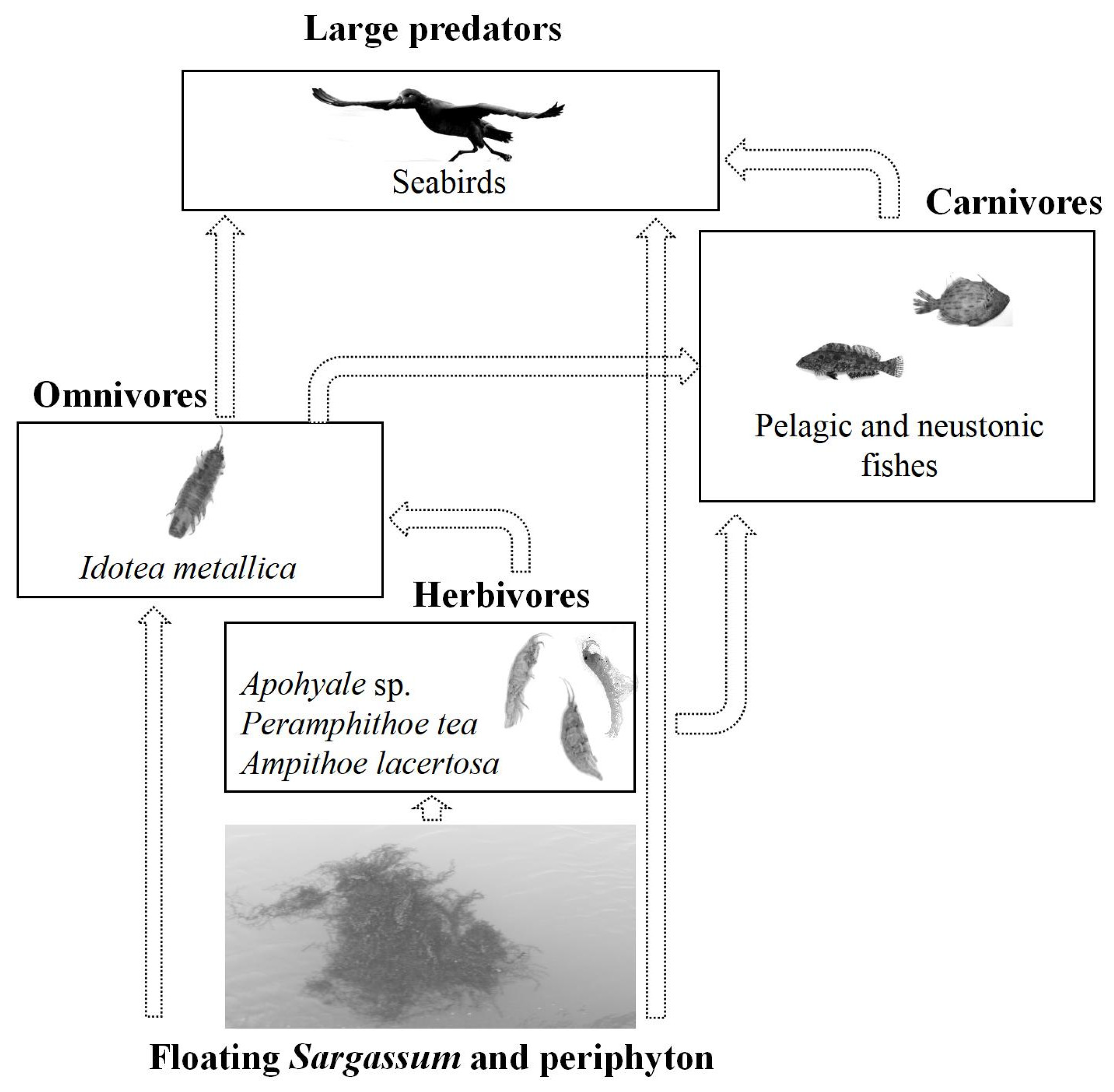
4.3. Relevance of Epizoans in Marine Ecosystems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez, C. Boom-bust of Sargassum muticum in northern Spain: 30 years of invasion. Eur. J. Phycol. 2020, 55, 285–295. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.C.; Gu, W.H. Macroalgal blooms caused by marine nutrient changes resulting from human activities. J. Appl. Ecol. 2020, 57, 766–776. [Google Scholar] [CrossRef]
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal. Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Kang, E.J.; Han, A.R.; Kim, J.H.; Kim, I.N.; Lee, S.; Min, J.O.; Nam, B.R.; Choi, Y.J.; Edwards, M.S.; Diaz-Pulido, G.; et al. Evaluating bloom potential of the green-tide forming alga Ulva ohnoi under ocean acidification and warming. Sci. Total Environ. 2021, 769, 144443. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, R.; Golden, N.; Schrofner, E.; Knöller, K.; Fenton, O.; Serrão, E.; Morrison, L. Biomass and nutrient dynamics of major green tides in Ireland: Implications for biomonitoring. Mar. Pollut. Bull. 2022, 175, 113318. [Google Scholar] [CrossRef]
- Guo, X.N.; Zhu, A.; Chen, R.S. China’s algal bloom suffocates marine life. Science 2021, 373, 751. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, C.X.; Xia, J.; Sun, Y.Q.; Tong, Y.C.; Zhang, J.H.; Zhao, S.; Zhuang, M.M.; He, P.M. Epizoic Ulva attached to intertidal animals in the Subei intertidal zone are not the additional source of the famed Yellow Sea green tides. J. Sea. Res. 2021, 174, 102065. [Google Scholar] [CrossRef]
- Liu, J.L.; Xia, J.; Zhuang, M.M.; Zhang, J.H.; Yu, K.F.; Zhao, S.; Sun, Y.Q.; Tong, Y.C.; Xia, L.H.; Qin, Y.T.; et al. Controlling the source of green tides in the Yellow Sea: NaClO treatment of Ulva attached on Pyropia aquaculture rafts. Aquaculture 2021, 535, 736378. [Google Scholar] [CrossRef]
- Vazquez-Delfin, E.; Freile-Pelegrin, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Mendez-Rodriguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Liu, J.L.; Tong, Y.C.; Xia, J.; Sun, Y.Q.; Zhao, X.H.; Sun, J.Y.; Zhao, S.; Zhuang, M.M.; Zhang, J.H.; He, P.M. Ulva macroalgae within local aquaculture ponds along the estuary of Dagu River, Jiaozhou Bay, Qingdao. Mar. Pollut. Bull. 2022, 174, 113243. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huo, Y.Z.; Zhang, J.H.; Cui, J.J.; Wang, Y.; Yang, L.L.; Zhou, Q.Y.; Lu, Y.W.; Yu, K.F.; He, P.M. Variations of dominant free-floating Ulva species in the source area for the world’s largest macroalgal blooms, China: Differences of ecological tolerance. Harmful Algae 2018, 74, 58–66. [Google Scholar] [CrossRef]
- Hiraoka, M. Massive Ulva Green Tides Caused by Inhibition of Biomass Allocation to Sporulation. Plants 2021, 10, 2482. [Google Scholar] [CrossRef]
- Liu, D.Y.; Keesing, J.K.; Xing, Q.U.; Shi, P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef]
- Han, H.B.; Li, Y.; Ma, X.J.; Song, W.; Wang, Z.L.; Zhang, X.L. Factors influencing the spatial and temporal distributions of green algae micro-propagules in the coastal waters of Jinmenghaiwan, Qinhuangdao, China. Mar. Pollut. Bull. 2022, 175, 113328. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Liu, J.L.; Xia, J.; Tong, Y.C.; Li, C.X.; Zhao, S.; Zhuang, M.M.; Zhao, X.H.; Zhang, J.H.; He, P.M. Research development on resource utilization of green tide algae from the Southern Yellow Sea. Energy Rep. 2022, 8, 295–303. [Google Scholar] [CrossRef]
- Wu, H.L.; Feng, J.C.; Li, X.S.; Zhao, C.Y.; Liu, Y.H.; Yu, J.T.; Xu, J.T. Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. Mar. Pollut. Bull. 2019, 146, 639–644. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shi, J.T.; Gao, S.; Huo, Y.Z.; Cui, J.J.; Shen, H.; Liu, G.Y.; He, P.M. Annual patterns of macroalgal blooms in the Yellow Sea during 2007–2017. PLoS ONE 2019, 14, e0210460. [Google Scholar] [CrossRef]
- Liu, J.L.; Xia, J.; Zhuang, M.M.; He, P.M.; Sun, Y.Q.; Tong, Y.C.; Zhao, S.; Zhang, J.H. Golden seaweed tides accumulated in Pyropia aquaculture areas are becoming a normal phenomenon in the Yellow Sea of China. Sci. Total Environ. 2021, 774, 145726. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.L.; Liu, D.Y.; Fu, M.Z.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef]
- Gower, J.F.R.; King, S.A. Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic Ocean mapped using MERIS. Int. J. Remote Sens. 2011, 32, 1917–1929. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.Y.; Oh, H.J.; Kim, S.; Yun, S.H.; Kang, J.H.; Park, S.R.; Lee, H.J. The origin and population genetic structure of the ‘golden tide’ seaweeds, Sargassum horneri, in Korean waters. Sci. Rep. 2019, 9, 7757. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, Z.; Zhou, X.; Xu, J. Changes in morphological plasticity of Ulva prolifera under different environmental conditions: A laboratory experiment. Harmful Algae 2016, 59, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, T.; Shi, H.; Gao, K.; Xu, J.; Wang, J.; Wang, R.; Li, X.; Gao, G. Microplastics in bloom-forming macroalgae: Distribution, characteristics and impacts. J. Hazard. Mater. 2020, 397, 122752. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, T.; Wang, J.; Huang, W.; Wang, R.; Xu, J.; Fu, G.; Gao, G. Spatio-temporal features of microplastics pollution in macroalgae growing in an important mariculture area, China. Sci. Total Environ. 2020, 719, 137490. [Google Scholar] [CrossRef]
- An, D.Y.; Yu, D.F.; Zheng, X.Y.; Zhou, Y.; Meng, L.; Xing, Q.G. Monitoring the Dissipation of the Floating Green Macroalgae Blooms in the Yellow Sea (2007–2020) on the Basis of Satellite Remote Sensing. Remote Sens. 2021, 13, 3811. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.X.; Liu, D.X.; Sun, Z.W.; Tang, R.K.; Ma, X.; Feng, Z.H. Loading of microplastics by two related macroalgae in a sea area where gold and green tides occur simultaneously. Sci. Total Environ. 2022, 814, 152809. [Google Scholar] [CrossRef]
- Liu, F.; Pan, J.; Zhang, Z.S.; Moejes, F.W. Organelle genomes of Sargassum confusum (Fucales, Phaeophyceae): mtDNA vs cpDNA. J. Appl. Phycol. 2018, 30, 2715–2722. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wan, J.H.; Zhang, J.; Ma, Y.J.; Wang, L.; Zhao, J.H.; Wang, Z.Z. Spatial-Temporal Distribution of Golden Tide Based on High–Resolution Satellite Remote Sensing in the South Yellow Sea. J. Coastal Res. 2019, 90, 221–227. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ding, X.W.; Zhuang, M.M.; Wang, S.Y.; Chen, L.; Shen, H.; He, P.M. An increase in new Sargassum (Phaeophyceae) blooms along the coast of the East China Sea and Yellow Sea. Phycologia 2019, 58, 374–381. [Google Scholar] [CrossRef]
- Zhuang, M.M.; Liu, J.L.; Ding, X.W.; He, J.Z.; Zhao, S.; Wu, L.J.; Gao, S.; Zhao, C.Y.; Liu, D.Y.; Zhang, J.H.; et al. Sargassum blooms in the East China Sea and Yellow Sea: Formation and management. Mar. Pollut. Bull. 2021, 162, 111845. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Neviere, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabie, A.; Lebrun, T.; Megarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J.; Li, X.; Li, J. Complete mitochondrial genome of the brown alga Sargassum horneri (Sargassaceae, Phaeophyceae): Genome organization and phylogenetic analyses. J. Appl. Phycol. 2015, 27, 469–478. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.F.; Wang, Y.; Lin, Z.; Moejes, F.W.; Sun, S. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol. Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, Z.Y.; Zhong, Z.H.; Zhuang, L.C.; Bi, Y.X.; Qin, S. Limited Genetic Connectivity Among Sargassum horneri (Phaeophyceae) Populations in the Chinese Marginal Seas Despite Their high Dispersal Capacity. J. Phycol. 2020, 56, 994–1005. [Google Scholar] [CrossRef]
- Mei, X.Y.; Wu, C.H.; Zhao, J.; Yan, T.; Jiang, P. Community Structure of Bacteria Associated with Drifting Sargassum horneri, the Causative Species of Golden Tide in the Yellow Sea. Front. Microbiol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Duffy, J.E.; Hay, M.E. Strong impacts of grazing amphipods on the organization of a benthic community. Ecol. Monogr. 2000, 70, 237–263. [Google Scholar] [CrossRef]
- Jiang, R.J.; Zhang, S.Y.; Bi, Y.X.; Wang, Z.H. Food sources of small invertebrates in the macroalgal bed of Gouqi Island. J. Fish. China 2015, 39, 1487–1498. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhuang, M.M.; Zhao, L.J.; Liu, Y.K.; Wen, Q.L.; Fu, M.L.; Yu, K.F.; Zhang, J.H.; He, P.M. Taxonomy and Genetic Diversity of Amphipods Living on Ulva lactuca L. from Gouqi Coast, China. Pac. Sci. 2020, 74, 137–146. [Google Scholar] [CrossRef]
- McDonald, P.S.; Bingham, B.L. Comparing macroalgal food and habitat choice in sympatric, tube-building amphipods, Ampithoe lacertosa and Peramphithoe humeralis. Mar. Biol. 2010, 157, 1513–1524. [Google Scholar] [CrossRef]
- Quillien, N.; Nordstrom, M.C.; Le Bris, H.; Bonsdorff, E.; Grall, J. Green tides on inter- and subtidal sandy shores: Differential impacts on infauna and flatfish. J. Mar. Biol. Assoc. UK 2018, 98, 699–712. [Google Scholar] [CrossRef]
- Catenazzi, A.; Donnelly, M.A. Role of supratidal invertebrates in the decomposition of beach-cast green algae Ulva sp. Mar. Ecol. Prog. Ser. 2007, 349, 33–42. [Google Scholar] [CrossRef]
- Schaal, G.; Leclerc, J.C.; Droual, G.; Leroux, C.; Riera, P. Biodiversity and trophic structure of invertebrate assemblages associated with understorey red algae in a Laminaria digitata bed. Mar. Biol. Res. 2016, 12, 513–523. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Proc. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Chen, L.P.; Chang, Y.G.; Li, J.; Wang, J.M.; Liu, J.L.; Zhi, Y.C.; Li, X.J. Application of DNA Barcoding in the Classification of Grasshoppers (Orthoptera: Acridoidea)—A Case Study of grasshoppers from Hebei Province, China. Zootaxa 2018, 4497, 99–110. [Google Scholar] [CrossRef]
- Mattio, L.; Payri, C. Assessment of five markers as potential barcodes for identifying Sargassum subgenus Sargassum species (Phaeophyceae, Fucales). Cryptogam Algol. 2011, 31, 467–485. [Google Scholar]
- Yip, Z.T.; Quek, R.Z.B.; Huang, D.W. Historical biogeography of the widespread macroalga Sargassum (Fucales, Phaeophyceae). J. Phycol. 2019, 56, 300–309. [Google Scholar] [CrossRef]
- Yip, Z.T.; Quek, R.Z.B.; Low, J.K.Y.; Wilson, B.; Bauman, A.G.; Chou, L.M.; Todd, P.A.; Huang, D.W. Diversity and phylogeny of Sargassum (Fucales, Phaeophyceae) in Singapore. Phytotaxa 2018, 369, 200–210. [Google Scholar] [CrossRef]
- Donald, K.M.; Preston, J.; Williams, S.T.; Reid, D.G.; Winter, D.; Alvarez, R.; Buge, B.; Hawkins, S.J.; Templado, J.; Spencer, H.G. Phylogenetic relationships elucidate colonization patterns in the intertidal grazers Osilinus Philippi, 1847 and Phorcus Risso, 1826 (Gastropoda: Trochidae) in the northeastern Atlantic Ocean and Mediterranean Sea. Molec. Phylogenet. Evol. 2012, 62, 35–45. [Google Scholar] [CrossRef]
- Linse, K.; Jackson, J.A.; Malyutina, M.V.; Brandt, A. Shallow-Water Northern Hemisphere Jaera (Crustacea, Isopoda, Janiridae) Found on Whale Bones in the Southern Ocean Deep Sea: Ecology and Description of Jaera tyleri sp. nov. PLoS ONE 2014, 9, e93018. [Google Scholar] [CrossRef]
- Ng, P.K.; Chiou, Y.S.; Liu, L.C.; Sun, Z.M.; Shimabukuro, H.; Lin, S.M. Phylogeography and genetic connectivity of the marine macro-alga Sargassum ilicifolium (Phaeophyceae, Ochrophyta) in the northwestern Pacific. J. Phycol. 2019, 55, 7–24. [Google Scholar] [CrossRef]
- Tseng, C.K.; Lu, B.R. Marine Algal Flora of China Tomus III Phaeophyta Fucales; Science Press: Beijing, China, 2000; pp. 43–44. [Google Scholar]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Phil. Trans. R. Soc. B 2005, 360, 1879–1888. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Kurihara, A.; Conklin, K.Y.; Sauvage, T.; Presting, G.G. The Hawaiian Rhodophyta Biodiversity Survey (2006–2010): A summary of principal findings. BMC Plant Biol. 2010, 10, 258. [Google Scholar] [CrossRef]
- Du, G.Y.; Wu, F.F.; Guo, H.; Xue, H.F.; Mao, Y.X. DNA barcode assessment of Ceramiales (Rhodophyta) in the intertidal zone of the northwestern Yellow Sea. Chin. J. Oceanol. Limnol. 2015, 33, 685–695. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Niwa, K.; Sano, F.; Sakamoto, T. Molecular evidence of allodiploidy in F1 gametophytic blades from a cross between Neopyropia yezoensis and a cryptic species of the Neopyropia yezoensis complex (Bangiales, Rhodophyta) by the use of microsatellite markers. Aquac. Rep. 2020, 18, 100489. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Ivica, L.; Peer, B. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Zhao, X.B.; Pang, S.J.; Shan, T.F.; Liu, F. Applications of three DNA barcodes in assorting intertidal red macroalgal flora in Qingdao, China. J. Ocean Univ. China 2013, 12, 139–145. [Google Scholar] [CrossRef]
- Silberfeld, T.; Leigh, J.W.; Verbruggen, H.; Cruaud, C.; De Reviers, B.; Rousseau, F. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the “brown algal crown radiation”. Mol. Phylogenet. Evol. 2010, 56, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Genovese, G.; Morabito, M.; Faggio, C.; Pagano, M.; Spanò, A.; Zammuto, V.; Minicante, S.A.; Manghisi, A.; Cigala, R.M.; et al. Potential Antibacterial Activity of Marine Macroalgae against Pathogens Relevant for Aquaculture and Human Health. J. Pure Appl. Microbiol. 2017, 11, 1695–1706. [Google Scholar] [CrossRef]
- Wei, C.D.; Chen, Y.S. Fauna of Zhejiang Crustacea; Zhejiang Science & Technology Press: Hangzhou, China, 1991; 481p. [Google Scholar]
- Jiang, N.C.; Lu, J.P. Field Internship Guidance of Zhejiang Coastal Zoology; Zhejiang Universuty Press: Zhejiang, China, 2005; 184p. [Google Scholar]
- Ren, X.Q. Crustacea Amphipoda Gammaridea I, Fauna Sinica Invertebrata; Science Press: Beijing, China, 2006; 588p. [Google Scholar]
- Miao, X.X.; Xiao, J.; Fan, S.L.; Zang, Y.; Zhang, X.L.; Wang, Z.L. Assessing Herbivorous Impacts of Apohyale sp. on the Ulva prolifera Green Tide in China. Front. Plant Sci. 2021, 12, 795560. [Google Scholar] [CrossRef]
- Yoshino, H.; Yamaji, F.; Ohsawa, T.A. Genetic structure and dispersal patterns in Limnoria nagatai (Limnoriidae, Isopoda) dwelling in non-buoyant kelps, Eisenia bicyclis and E. arborea, in Japan. PLoS ONE 2018, 3, e0198451. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Ding, G.; Zhou, T.T.; Yang, W.J.; Li, J.; Peng, X.; Tang, B.P. Genetic Diversity of Oratosquilla oratoria Populations in Yellow Sea. Chin. J. Zool. 2013, 48, 232–240. [Google Scholar] [CrossRef]
- Lobo, J.; Ferreira, M.S.; Antunes, I.C.; Teixeira, M.A.L.; Borges, L.M.S.; Sousa, R.; Gomes, P.A.; Costa, M.H.; Cunha, M.R.; Costa, F.O. Contrasting morphological and DNA barcode-suggested species boundaries among shallow-water amphipod fauna from the southern European Atlantic coast. Genome 2017, 60, 147–157. [Google Scholar] [CrossRef]
- Dewaard, J.R.; Hebert, P.D.N.; Humble, L.M. A Comprehensive DNA Barcode Library for the Looper Moths (Lepidoptera: Geometridae) of British Columbia, Canada. PLoS ONE 2011, 6, e18290. [Google Scholar] [CrossRef]
- Panova, M.; Nygren, A.; Jonsson, P.R.; Leidenberger, S. A molecular phylogeny of the north-east Atlantic species of the genus Idotea (Isopoda) with focus on the Baltic Sea. Zool. Scr. 2017, 46, 188–199. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hong, S.S.; Conlan, K.E.; Lee, K.S. The genus Peramphithoe Conlan & Bousfield, 1982 from Korean waters (Crustacea: Amphipoda: Ampithoidae). Zootaxa 2012, 3400, 1–19. [Google Scholar] [CrossRef]
- Kim, K.; Shin, J.; Kim, K.Y.; Ryu, J.H. Long-Term Trend of Green and Golden Tides in the Eastern Yellow Sea. J. Coastal Res. 2019, 90, 317–323. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.L.; Song, H.J.; Fan, S.L.; Yuan, C.; Fu, M.Z.; Miao, X.X.; Zhang, X.L.; Su, R.G.; Hu, C.M. An anomalous bi-macroalgal bloom caused by Ulva and Sargassum seaweeds during spring to summer of 2017 in the western Yellow Sea, China. Harmful Algae 2020, 93, 101760. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.M.; Wang, M.Q.; Shang, S.L.; Wilson, C. Floating algae blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11501–11509. [Google Scholar] [CrossRef]
- Huang, B.X.; Ding, L.P.; Qin, S.; Fu, W.T.; Lu, Q.Q.; Liu, Z.Y.; Pang, Y.L.; Li, X.L.; Sun, Z.M. The taxonomical status and biogeographical distribution of Sargassum horneri with the origin analysis of its drifting population in the end of 2016 at the Western Yellow Sea. Oceanol. Et Limnol. Sin. 2018, 49, 214–223. [Google Scholar] [CrossRef]
- Ding, X.W.; Zhang, J.H.; Zhuang, M.M.; Kang, X.Y.; Zhao, X.H.; He, P.M.; Liu, S.R.; Liu, J.F.; Wen, Y.; Shen, H.; et al. Growth of Sargassum horneri distribution properties of golden tides in the Yangtze Estuary and adjacent waters. Mar. Fish. 2019, 41, 188–196. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, P.; Qiu, R.; Ma, Y.Y.; Wu, C.H.; Fu, H.H.; Chen, H.X.; Li, F.C. The Yellow Sea green tide: A risk of macroalgae invasion. Harmful Algae 2018, 77, 11–17. [Google Scholar] [CrossRef]
- Thiel, M. Rafting of benthic macrofauna: Important factors determining the temporal succession of the assemblage on detached macroalgae. Hydrobiologia 2003, 503, 49–57. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr. Mar. Biol. 2005, 43, 279–418. [Google Scholar] [CrossRef]
- Riera, R. Are marine drifting species cosmopolitan? the example of the isopod Idotea metallica. Thalassas 2014, 30, 75–78. [Google Scholar]
- Gutow, L.; Strahl, J.; Wiencke, C.; Franke, H.-D.; Saborowski, R. Behavioural and metabolic adaptations of marine isopods to the rafting life style. Mar. Biol. 2006, 149, 821–828. [Google Scholar] [CrossRef]
- Franke, H.D.; Gutow, L.; Janke, M. The recent arrival of the oceanic isopod Idotea metallica Bosc off Helgoland (German Eight, North Sea): An indication of a warming trend in the North Sea? Helgol. Mar. Res. 1998, 52, 347–357. [Google Scholar] [CrossRef]
- Duffy, J.E. Amphipods on seaweeds: Partners or pests? Oecologia 1990, 83, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Liess, A.; Kahlert, M. Gastropod grazers affect periphyton nutrient stoichiometry by changing benthic algal taxonomy and through differential nutrient uptake. J. N. Am. Benthol. Soc. 2009, 28, 283–293. [Google Scholar] [CrossRef]
- Edgar, G.J.; Shaw, C. The production and trophic ecology of shallow-water fish assemblages in southern Australia III. General relationships between sediments, seagrasses, invertebrates and fishes. J. Exp. Mar. Biol. Ecol. 1995, 194, 107–131. [Google Scholar] [CrossRef]
- Zheng, X.Q. A Preliminary Study on the Impacts of Amphipods’ Grazing on the Macroalgal Community in Yundang Lagoon; Xiamen University: Xiamen, China, 2008; 106p. [Google Scholar]
- Sano, M.; Omori, M.; Taniguchi, K. Predator-prey systems of drifting seaweed communities off the Tohoku coast, northern Japan, as determined by feeding habit analysis of phytal animals. Fish. Sci. 2003, 69, 260–268. [Google Scholar] [CrossRef]
- Geertz-Hansen, O.; Sand-Jensen, K.; Hansen, D.F.; Christiansen, A. Growth and grazing control of abundance of the marine macroalga, Ulva lactuca L. in a eutrophic Danish estuary. Aquat. Bot. 1993, 46, 101–109. [Google Scholar] [CrossRef]
- Hauxwell, J.; McClelland, J.; Behr, P.J.; Valiela, I. Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 1998, 21, 347–360. [Google Scholar] [CrossRef]
- Yu, J.; Kuang, L.L.; Wang, Q.H.; Liu, Y.; Gong, Q.L.; Li, J.Y. Differences in photosynthesis, growth and resource accumulation bet ween drif-ting alga Sargassum horneri and cultured alga Undaria pinnatifida and their roles in interspecies competition. Period. Ocean Univ. China 2021, 51, 1–10. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, S.Y.; Xu, W.D.; Li, H.L.; He, P. Effects of temperature, light intensity and ratio of nitrogen to phosphorus on growth of seedlings in sea weed Sargassum horneri. J. Dalian Ocean Univ. 2020, 35, 376–381. [Google Scholar] [CrossRef]
- Lavaut, E.; Guillemin, M.L.; Colin, S.; Faure, A.; Coudret, J.; Destombe, C.; Valero, M. Pollinators of the sea: A discovery of animal-mediated fertilization in seaweed. Science 2022, 377, 528–530. [Google Scholar] [CrossRef]
- Li, H.M.; Zhang, C.S.; Han, X.R.; Shi, X.Y. Changes in concentrations of oxygen, dissolved nitrogen, phosphate, and silicate in the southern Yellow Sea, 1980–2012: Sources and seaward gradients. Estuar. Coast. Shelf Sci. 2015, 164, 44–45. [Google Scholar] [CrossRef]
- Zhao, S.B.; Xu, B.C.; Yao, Q.Z.; Burnette, W.C.; Charettef, M.A.; Su, R.G.; Lian, E.; Yu, Z. Nutrient-rich submarine groundwater discharge fuels the largest green tide in the world. Sci. Total Environ. 2021, 770, 144845. [Google Scholar] [CrossRef]



| Species Number | Authority | Collection Locality | Latitude, Longitude | GenBank Information | Source | Specimen Location | Reference |
|---|---|---|---|---|---|---|---|
| 2021SYSA1, 2021SYSA2, 2021SYSA3 | This study | Southern Yellow Sea, China | 33.4283° N, 121.4723° E | OM444204, OM444205, OM444206 | This study | Shanghai Ocean University | This study |
| 2021SYSB1, 2021SYSB2, 2021SYSB3 | This study | Southern Yellow Sea, China | 33.0997° N, 121.6630° E | OM444207, OM444208, OM444209 | This study | Shanghai Ocean University | This study |
| 2021SYSC1, 2021SYSC2, 2021SYSC3 | This study | Southern Yellow Sea, China | 32.7661° N, 121.7887° E | OM444210, OM444211, OM444212 | This study | Shanghai Ocean University | This study |
| 2021SYSD1, 2021SYSD2, 2021SYSD3 | This study | Southern Yellow Sea, China | 32.8477° N, 121.8477° E | OM444213, OM444214, OM444215 | This study | Shanghai Ocean University | This study |
| dl-3 | Sargassum horneri | Qingdao, China | N.A. | KC782896 | NCBI | Chinese Academy of Sciences | [62] |
| HY20130629.201 | Sargassum horneri | Yantai, China | 36.7900° N, 120.9700° E | KY047195 | NCBI | Ocean University of China | [56] |
| FJ20130427.193 | Sargassum fusiforme | Fujian, China | 25.1800° N, 119.2700° E | KY047223 | NCBI | Ocean University of China | [56] |
| RZ20121016.86 | Sargassum confusum | Rizhao, China | 35.3900° N, 119.5700° E | KY047204 | NCBI | Ocean University of China | [56] |
| TJS0168 | Sargassum fallax | Tasmania, Australia | N.A. | GQ368266 | NCBI | Muséum National d’Histoire Naturelle of Paris | [63] |
| SAM539 | Sargassum muticum | Celestia, Italy | 45.4386° N, 12.3496° E | KY682972 | NCBI | University of Messina | [64] |
| QD20120918.77 | Sargassum thunbergii | Qingdao, China | 36.09° N, 120.49° E | KY047198 | NCBI | Ocean University of China | [56] |
| mbccc20 | Pyropia yezoensis | Qingdao, China | N.A. | JQ619144 | NCBI | Chinese Academy of Sciences | [62] |
| Species Number | Authority | Collection Locality | Latitude, Longitude | GenBank Information | Source | Specimen Location | Reference |
|---|---|---|---|---|---|---|---|
| 2021SYSAe1, 2021SYSAe2, 2021SYSAe3, 2021SYSAe4, 2021SYSAe5, 2021SYSAe6, 2021SYSAe7, 2021SYSAe8 | This study | Southern Yellow Sea, China | 33.4283° N, 121.4723° E | OM714522, OM714523, OM723195, OM714524, OM714525, OM723196, OM723197, OM723198 | This study | Shanghai Ocean University | This study |
| 2021SYSBe1, 2021SYSBe2, 2021SYSBe3 | This study | Southern Yellow Sea, China | 33.0997° N, 121.6630° E | OM665403, OM723205, OM714526 | This study | Shanghai Ocean University | This study |
| 2021SYSCe1, 2021SYSCe2, 2021SYSCe3, 2021SYSCe4, 2021SYSCe5, 2021SYSCe6, 2021SYSCe7 | This study | Southern Yellow Sea, China | 32.7661° N, 121.7887° E | OM723199, OM723200, OM723201, OM714527, OM701800, OM701801, OM701802 | This study | Shanghai Ocean University | This study |
| 2021SYSDe1, 2021SYSDe2, 2021SYSDe3, 2021SYSDe4, 2021SYSDe5, 2021SYSDe6, 2021SYSDe7, 2021SYSDe8, 2021SYSDe9, 2021SYSDe10 | This study | Southern Yellow Sea, China | 32.8477° N, 121.8477° E | OM723206, OM723207, OM714528, OM723202, OM723203, OM714529, OM723204, OM701797, OM701798, OM701799 | This study | Shanghai Ocean University | This study |
| 17 | Ampithoe lacertosa | Zhoushan, China | 30.7400° N, 122.8300° E | OK480914 | NCBI | Shanghai Ocean University | [39] |
| SFAM13-003 | Ampithoe helleri | Minho, Portugal | 41.6940° N, 8.8510° W | KX223985 | NCBI | University of Minho | [71] |
| YSGT2021 | Apohyale sp. | Southern Yellow Sea, China | 36.1369° N, 121.4222° E | OK180438 | NCBI | Ministry of Natural Resources | [68] |
| BCAMP0132 | Apohyale cf. pugettensis | British Columbia, Canada | 48.8580° N, 125.1600° W | MG315613 | NCBI | University of Guelph | [72] |
| NRM-CGI-000108 | Idotea metallica | Helgoland Island, Germany | 54.1726° N, 7.8791° E | KU530515 | NCBI | Swedish Museum of Natural History | [73] |
| NRM-CGI-000072 | Idotea emarginata | Helgoland Island, Germany | 54.1726° N, 7.8791° E | KU530492 | NCBI | Swedish Museum of Natural History | [73] |
| AP312 | Peramphithoe tea | Pado-ri, South Korea | 36.705° N, 126.13° E | JN575608 | NCBI | Inha University | [74] |
| H38 | Oratosquilla oratoria | Lianyungang, China | N.A. | JX522527 | NCBI | Yancheng Teachers University | [70] |
| Species | Taxonomic Status | Major Morphological Features | Distribution |
|---|---|---|---|
| Ampithoe lacertosa Spence Bate, 1858 | Animalia; Arthropoda; Malacostraca; Amphipoda; Ampithoidae; Ampithoe Leach, 1814 | The first and second antennae are almost equal in length. The gnathopods are sub-chelate, the second is larger than the first. The third pereiopod is shorter. The exopodite of the third uropod has hooked terminal spines and the tip of the endopodite has setae [66]. | USA, Canada, Japan, South Korea, and China |
| Apohyale sp. | Animalia; Arthropoda; Malacostraca; Amphipoda; Hyalidae; Apohyale Bousfield & Hendrycks, 2002 | The second antennae is slightly longer than the first, and accessory flagellum is absent. The first gnathopod is smaller than the second. The third uropod is not segmented, and the telson is cleft [68]. | China [68] |
| Idotea metallica Bosc, 1802 | Animalia; Arthropoda; Malacostraca; Isopoda; Idoteidae; Idotea Fabricius, 1798 | The body is wide and flat. The thoracic segments are almost the same length. The number of segments of the second antennae is more. The abdomen is generally composed of 3 sections, of which each side of the base of the last section has a side suture [65]. | USA, Japan, Mexico, Canada, Australia, Germany, France, U.K., Brazil, New Zealand, Spain, and Sweden |
| Peramphithoe tea J.L.Barnard, 1965 | Animalia; Arthropoda; Malacostraca; Amphipoda; Ampithoidae; Sunamphitoe Spence Bate, 1857 | The second gnathopod is larger than the first. Pereiopods 5~7 are similar to each other. The tip of the exopodite of the third uropod has 2 hooked spines, the endopodite has small spines and terminal setae, and the telson is complete [67]. | South Korea, Canada, and the USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Li, C.; Tang, Y.; Li, J.; Wu, T.; Liu, J.; Zhang, J. Epizoans on Floating Golden Tide Macroalgae in the Southern Yellow Sea. J. Mar. Sci. Eng. 2023, 11, 479. https://doi.org/10.3390/jmse11030479
Xia J, Li C, Tang Y, Li J, Wu T, Liu J, Zhang J. Epizoans on Floating Golden Tide Macroalgae in the Southern Yellow Sea. Journal of Marine Science and Engineering. 2023; 11(3):479. https://doi.org/10.3390/jmse11030479
Chicago/Turabian StyleXia, Jing, Chongxiang Li, Yiyuan Tang, Ji Li, Tingjian Wu, Jinlin Liu, and Jianheng Zhang. 2023. "Epizoans on Floating Golden Tide Macroalgae in the Southern Yellow Sea" Journal of Marine Science and Engineering 11, no. 3: 479. https://doi.org/10.3390/jmse11030479
APA StyleXia, J., Li, C., Tang, Y., Li, J., Wu, T., Liu, J., & Zhang, J. (2023). Epizoans on Floating Golden Tide Macroalgae in the Southern Yellow Sea. Journal of Marine Science and Engineering, 11(3), 479. https://doi.org/10.3390/jmse11030479








