Effects of UVR on Photosynthesis in Sargassum horneri (Turner) C. Agardh Adapted to Different Nitrogen Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Determination of Fv/Fm and NPQ
2.4. Acquisition of Rapid Light Response Curves
2.5. Determination of Rapid Chlorophyll Fluorescence Induction and JIP Test
2.6. Determination of Photosynthetic Pigments
2.7. Determination of Nitrate Reductase Activity (NRA)
2.8. Statistical Analysis of Data
3. Results
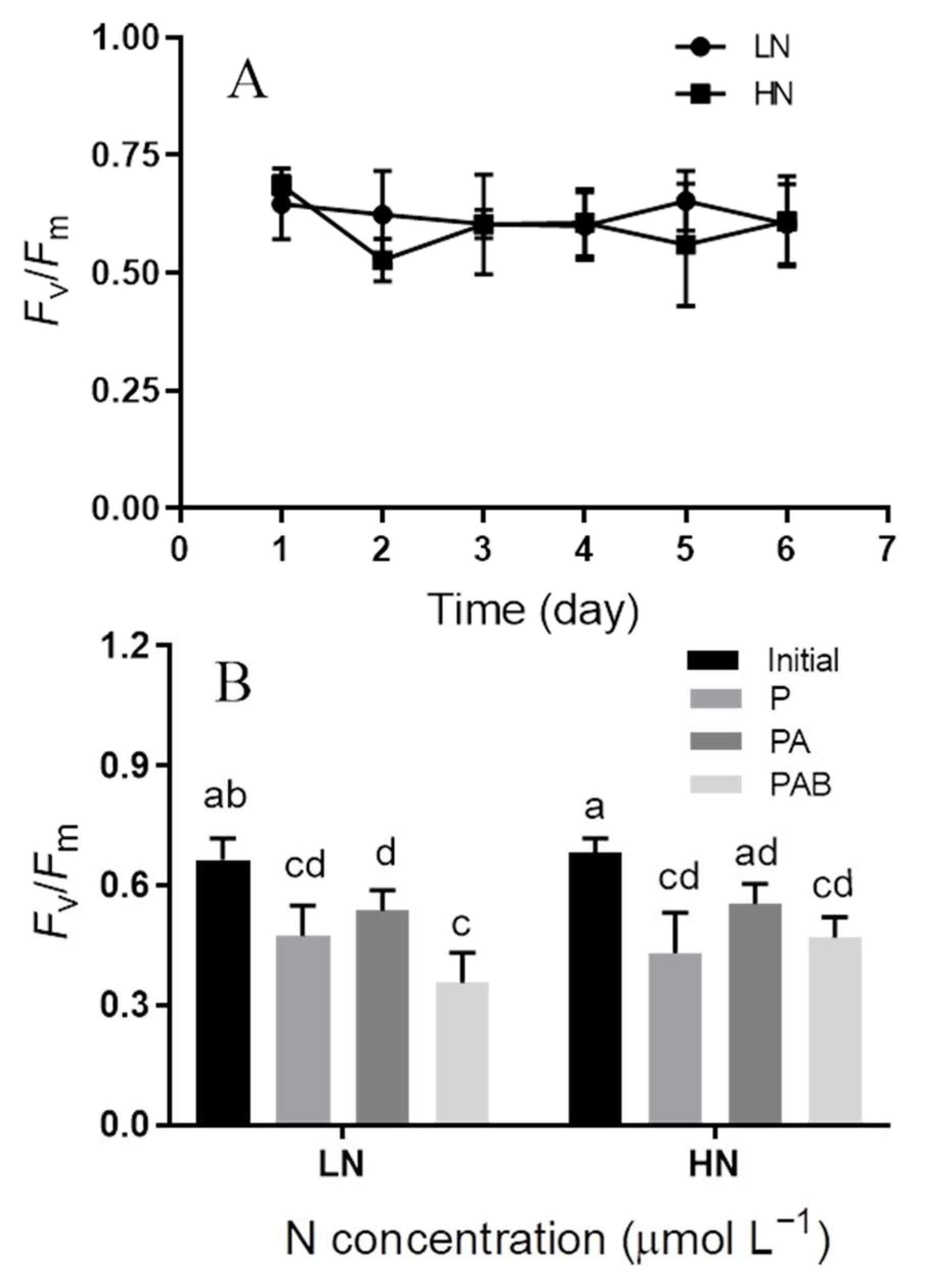
3.1. Maximum Photochemical Quantum Yield (Fv/Fm)
3.2. Rapid Light Curve and Non-Photochemical Quenching
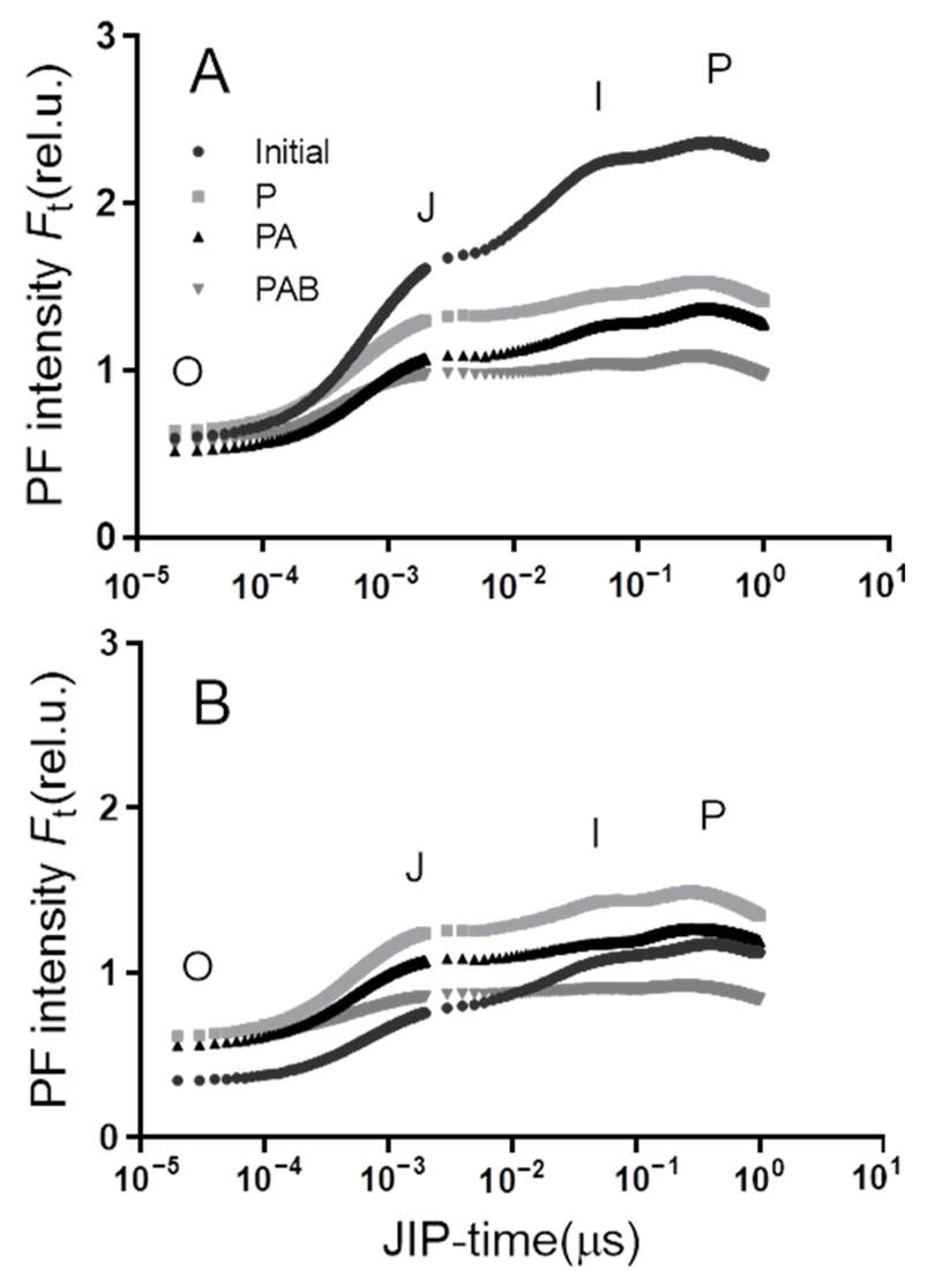
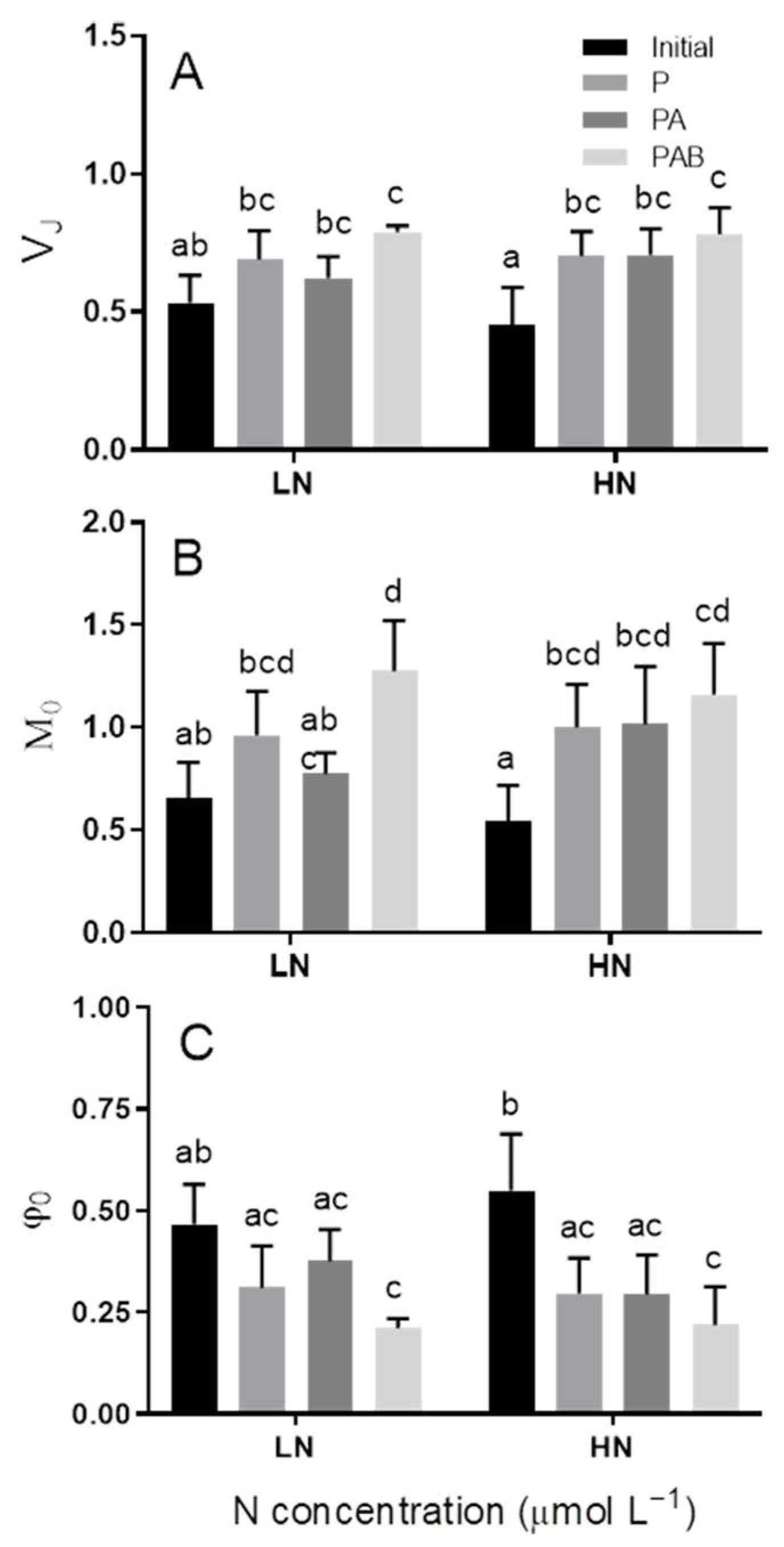
3.3. OJIP Curve
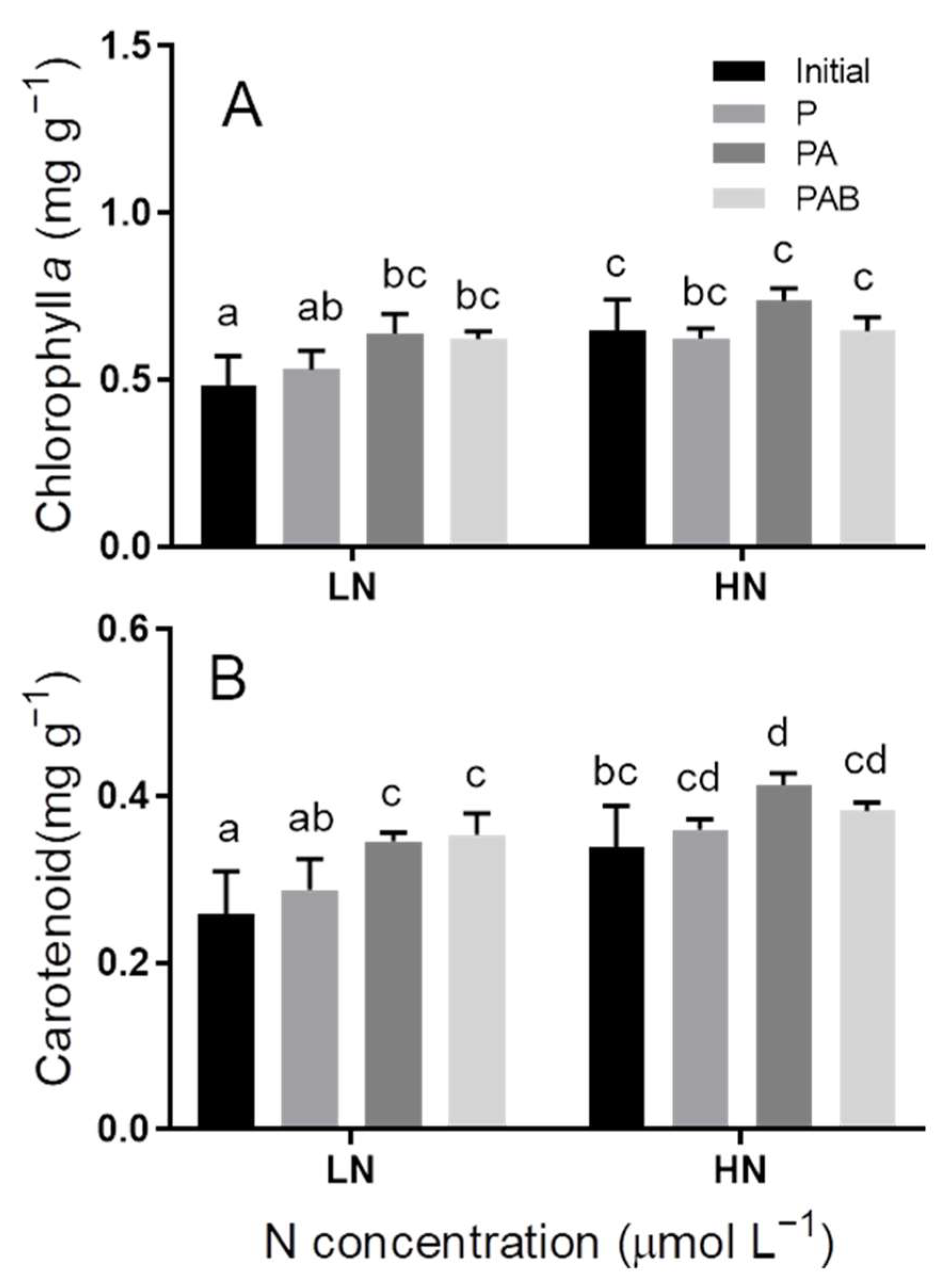
3.4. Pigment Contents
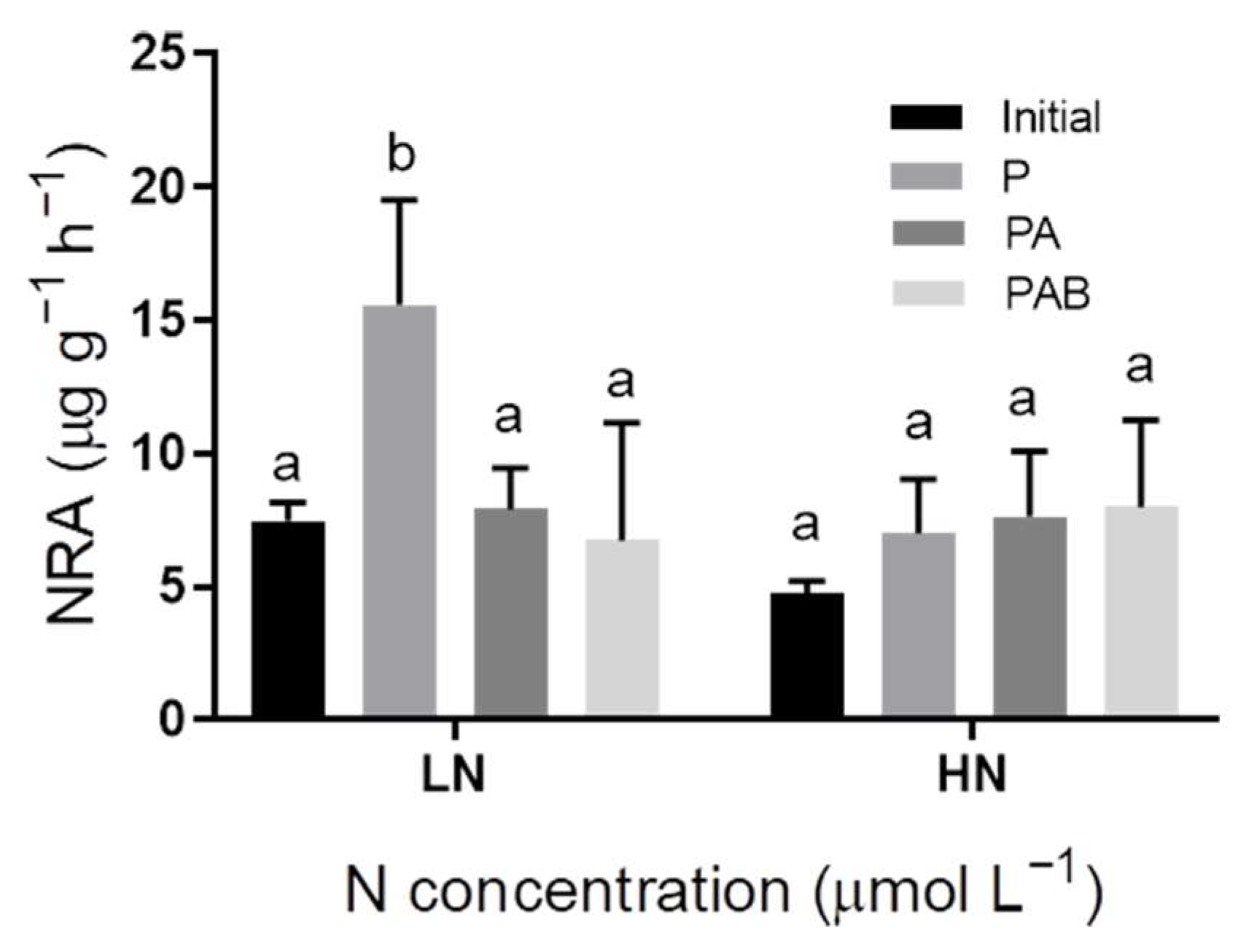
3.5. The Nitrate Reductase Activity (NRA)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Nelson, T.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.A.V.; Corbisier, T.N.; Soto-Jimenez, M.F.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Change Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shi, J.T.; Gao, S.; Huo, Y.Z.; Cui, J.J.; Shen, H.; Liu, G.Y.; He, P.M. Annual patterns of macroalgal blooms in the Yellow Sea during 2007–2017. PLoS ONE 2019, 14, e0210460. [Google Scholar] [CrossRef]
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Norkko, J.; Bonsdorff, E.; Norkko, A. Drifting algal mats as an alternative habitat for benthic invertebrates: Species specific responses to a transient resource. J. Exp. Mar. Biol. Ecol. 2000, 248, 79–104. [Google Scholar] [CrossRef]
- Xing, Q.G.; Guo, R.H.; Wu, L.L.; An, D.Y.; Cong, M.; Qin, S.; Li, X.R. High-Resolution Satellite Observations of a New Hazard of Golden Tides Caused by Floating Sargassum in Winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.L.; Liu, D.Y.; Fu, M.Z.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef]
- Wu, H.L.; Feng, J.C.; Li, X.S.; Zhao, C.Y.; Liu, Y.H.; Yu, J.T.; Xu, J.T. Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. Mar. Pollut. Bull. 2019, 146, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, I.; Pizarro, M.; Ramos, M.; Thiel, M. Spatial and temporal distribution of floating kelp in the channels and fjords of southern Chile. Estuar. Coast. Shelf Sci. 2010, 87, 367–377. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Wang, Y.; Jin, Z.; Moejes, F.W.; Sun, S. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol. Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.L.; Song, H.J.; Fan, S.L.; Yuan, C.; Fu, M.Z.; Miao, X.X.; Zhang, X.L.; Su, R.G.; Hu, C.M. An anomalous bi-macroalgal bloom caused by Ulva and Sargassum seaweeds during spring to summer of 2017 in the western Yellow Sea, China. Harmful Algae 2020, 93, 101760. [Google Scholar] [CrossRef]
- Bao, M.; Park, J.-S.; Wu, H.; Lee, H.J.; Park, S.R.; Kim, T.-H.; Son, Y.B.; Lee, T.H.; Yarish, C.; Kim, J.K. A comparison of physiological responses between attached and pelagic populations of Sargassum horneri under nutrient and light limitation. Mar. Environ. Res. 2022, 173, 105544. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, C.M.; Wang, M.Q.; Shang, S.L.; Wilson, C. Floating Algae Blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11501–11509. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Cordi, B.; Donkin, M.E.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Comparison of ultraviolet-induced genotoxicity detected by random amplified polymorphic DNA with chlorophyll fluorescence and growth in a marine macroalgae, Palmariapalmata. Aquat. Toxicol. 2000, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2001, 33, 179–197. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.Y.; Wang, Q.H.; Liu, Y.; Gong, Q.L. Growth and Resource Accumulation of Drifting Sargassum horneri (Fucales, Phaeophyta) in Response to Temperature and Nitrogen Supply. J. Ocean Univ. China 2019, 18, 1216–1226. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.-F. Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. J. Exp. Mar. Biol. Ecol. 2008, 367, 142–148. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, X.; Liu, Y.; Wang, C.; Ji, C.; Xu, J. Increased Temperature and Nitrogen Enrichment Inhibit the Growth of the Golden Tide Blooming Macroalgae Sargassum horneri in the Yellow Sea, China. J. Mar. Sci. Eng. 2022, 10, 1692. [Google Scholar] [CrossRef]
- Ding, G.; Yu, X.Q.; Zhan, D.M.; Zhang, S.C.; Lu, X.; Li, M.Z.; Wu, H.Y. Effects of nitrogen and phosphorus concentrations and ratio on growth in sea weed Sargassum thunbergii seedlings. Fish. Sci. 2014, 33, 219–222. [Google Scholar] [CrossRef]
- Rumyantseva, A.S.; Henson, S.A.; Martin, A.P.; Thompson, A.F.; Damerell, G.M.; Kaiser, J.; Heywood, K.J. Phytoplankton spring bloom initiation: The impact of atmospheric forcing and light in the temperate North Atlantic Ocean. Prog. Oceanogr. 2019, 178, 102202. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Henley, W.J. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 1993, 29, 729–739. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, E., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Corzo, A.; Niell, F.X. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J. Exp. Mar. Biol. Ecol. 1991, 146, 181–191. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Koch, K.; Thiel, M.; Hagen, W.; Graeve, M.; Gómez, I.; Jofre, D.; Hofmann, L.C.; Tala, F.; Bischof, K. Short- and long-term acclimation patterns of the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) along a depth gradient. J. Phycol. 2016, 52, 260–273. [Google Scholar] [CrossRef]
- Nan, G.N.; Zhang, Q.S.; Sheng, Z.T.; Zhang, D. Coordination between xanthophyll cycle and antioxidant system in Sargassum thunbergii (Sargassaceae, Phaeophyta) in response to high light and dehydration stresses. J. Appl. Phycol. 2016, 28, 2587–2596. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Differential response of photosynthetic pigments, rubisco activity and rubisco protein of Arabidopsis thaliana EXPOSED TO UVB and OZONE. Photochem. Photobiol. 1995, 62, 727–735. [Google Scholar] [CrossRef]
- Yakovleva, I.M.; Titlyanov, E.A. Effect of high visible and UV irradiance on subtidal Chondrus crispus: Stress, photoinhibition and protective mechanisms. Aquat. Bot. 2001, 71, 47–61. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Y.-G.; Yan, Z.-L.; Zeng, G.-M.; Liu, S.-B.; Wang, W.-J.; Tan, X.-F.; Deng, J.-Q.; Tang, X.; Wang, Q.-P. Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Wang, G.J.; Zeng, F.L.; Song, P.; Sun, B.; Wang, Q.; Wang, J.Y. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Nowicka, B.; Jakubowska, A.; Strzalka, W.; Burda, K.; Strzalka, K. The xanthophyll cycle in diatom Phaeodactylum tricornutum in response to light stress. Plant Physiol. Biochem. 2020, 152, 125–137. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Strasserf, R.J.; Srivastava, A. Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria*. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Govindje, E. Sixty-Three Years Since Kautsky: Chlorophyll a Fluorescence. Funct. Plant Biol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Ma, Z.L.; Wang, C.X.; Qin, W.L.; Wang, M.; Chen, B.B.; Jia, Y.; Qin, Z.X.; Dai, C.J.; Yu, H.G.; Li, G.; et al. Inhibitory effects of Prorocentrum donghaiense allelochemicals on Sargassum fusiformis zygotes probed by JIP-test based on fast chlorophyll fluorescence kinetics. Mar. Environ. Res. 2021, 170, 105453. [Google Scholar] [CrossRef]
- Kruger, G.H.J.; Tsimilli-Michael, M.; Strasser, R.J. Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camellia leaves. Physiol. Plant. 1997, 101, 265–277. [Google Scholar] [CrossRef]




| α | rETRmax μmol e− m−2 s−1 | Ek | ||
|---|---|---|---|---|
| Initial | LN | 0.24 ± 0.05 a | 57.11 ± 18.36 ab | 232.15 ± 42.65 ab |
| HN | 0.24 ± 0.06 a | 45.41 ± 13.67 a | 188.88 ± 36.07 a | |
| P | LN | 0.21 ± 0.05 a | 52.89 ± 18.59 a | 248.03 ± 49.10 ab |
| HN | 0.30 ± 0.06 a | 85.41 ± 25.07 b | 277.58 ± 33.44 ab | |
| PA | LN | 0.25 ± 0.09 a | 55.24 ± 8.75 ab | 232.52 ± 59.54 ab |
| HN | 0.21 ± 0.07 a | 67.46 ± 4.94 ab | 344.44 ± 99.36 b | |
| PAB | LN | 0.24 ± 0.06 a | 63.55 ± 20.15 ab | 277.77 ± 105.03 ab |
| HN | 0.26 ± 0.05 a | 57.98 ± 13.60 ab | 227.24 ± 62.02 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Jiang, X.; Li, B.; Lv, Z.; Wu, H.; Zang, S.; Yan, F.; Bao, M. Effects of UVR on Photosynthesis in Sargassum horneri (Turner) C. Agardh Adapted to Different Nitrogen Levels. J. Mar. Sci. Eng. 2023, 11, 498. https://doi.org/10.3390/jmse11030498
Xu Z, Jiang X, Li B, Lv Z, Wu H, Zang S, Yan F, Bao M. Effects of UVR on Photosynthesis in Sargassum horneri (Turner) C. Agardh Adapted to Different Nitrogen Levels. Journal of Marine Science and Engineering. 2023; 11(3):498. https://doi.org/10.3390/jmse11030498
Chicago/Turabian StyleXu, Zhiguang, Xiaotong Jiang, Baoqi Li, Zhengzheng Lv, Hongyan Wu, Shasha Zang, Fang Yan, and Menglin Bao. 2023. "Effects of UVR on Photosynthesis in Sargassum horneri (Turner) C. Agardh Adapted to Different Nitrogen Levels" Journal of Marine Science and Engineering 11, no. 3: 498. https://doi.org/10.3390/jmse11030498
APA StyleXu, Z., Jiang, X., Li, B., Lv, Z., Wu, H., Zang, S., Yan, F., & Bao, M. (2023). Effects of UVR on Photosynthesis in Sargassum horneri (Turner) C. Agardh Adapted to Different Nitrogen Levels. Journal of Marine Science and Engineering, 11(3), 498. https://doi.org/10.3390/jmse11030498






