Adsorptive Treatment of Residues on Macroporous Adsorbent for Marine Fuel Production Scheme on Refinery
Abstract
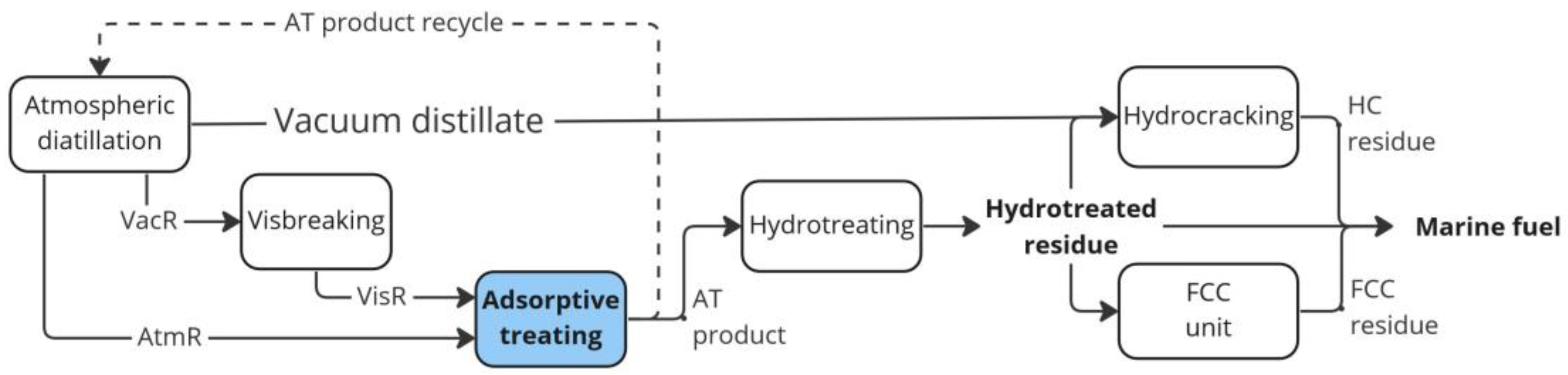
:1. Introduction
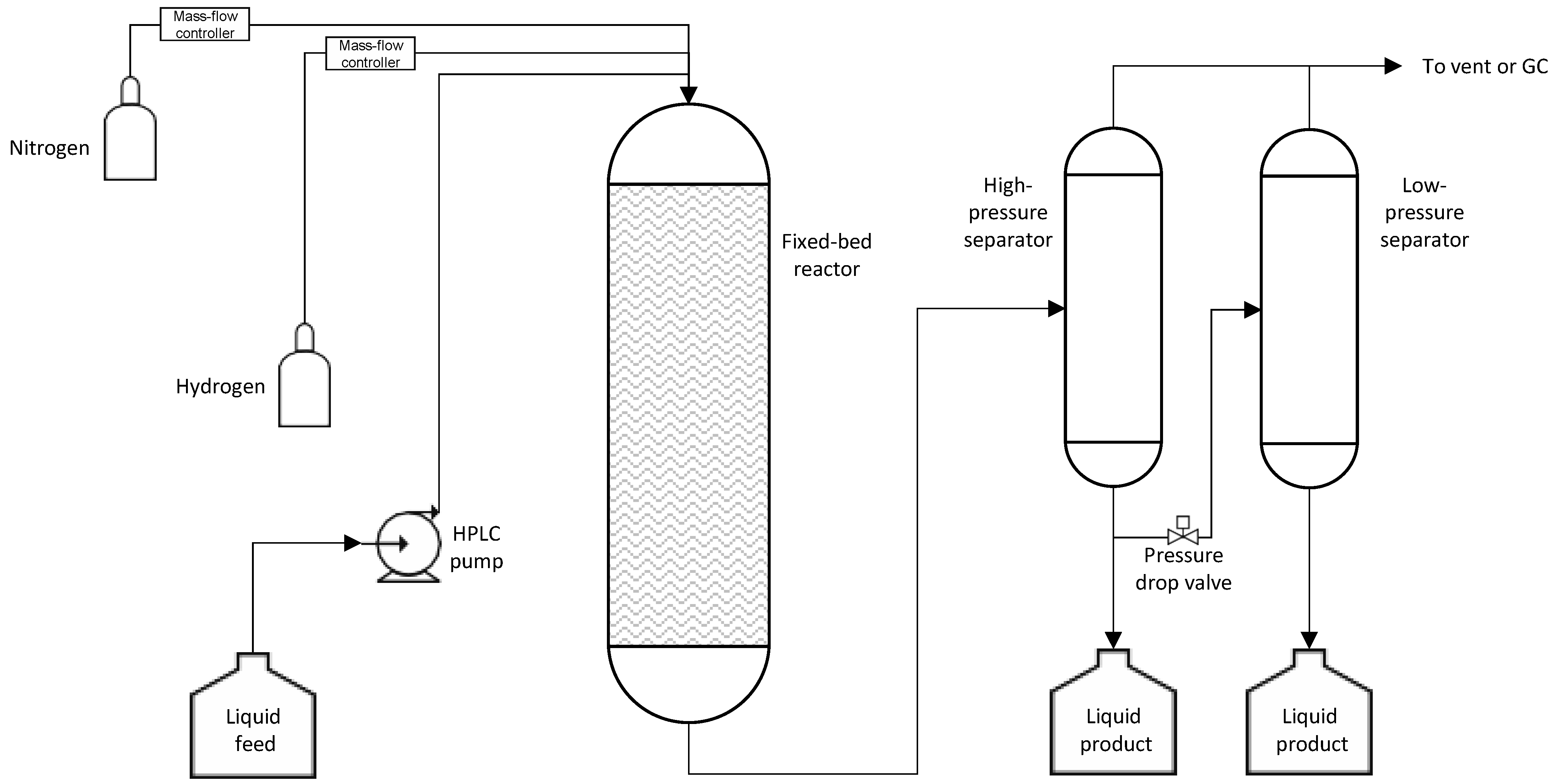
2. Materials and Methods
3. Results and Discussion
3.1. Adsorptive Treatment of Different Feedstock
3.2. Temperature and Feed Rate Influence on Products Yield and Quality
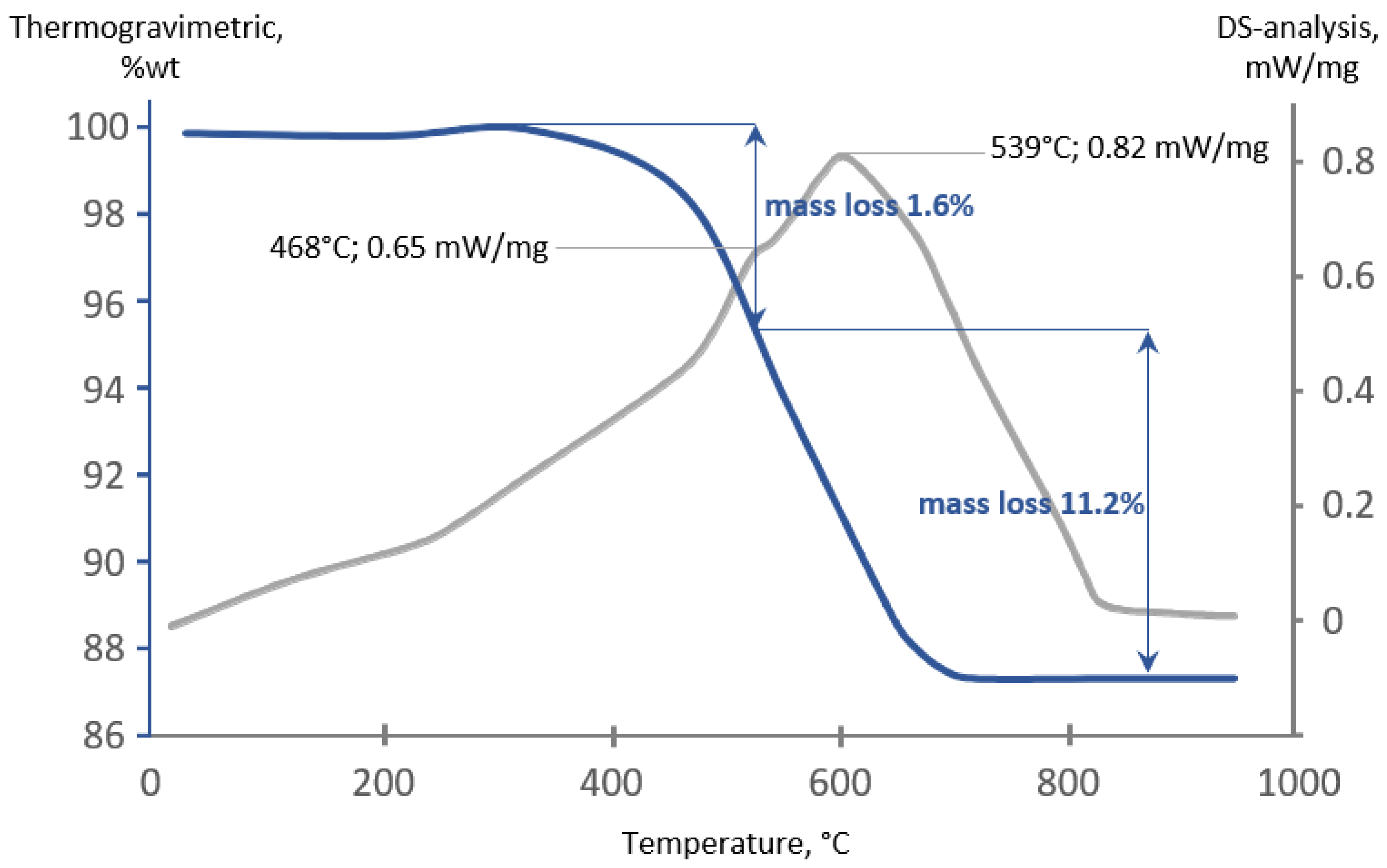
3.3. Spent Adsorbent Analysis and Regeneration
3.4. Products of Adsorptive Treatment Characterization
3.5. Hydrotreating of Adsorptive Treatment Residue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- From the Particular to the General. 2021. Available online: https://www.vedomosti.ru/opinion/articles/2021/11/22/896881-chastnogo-obschemu (accessed on 28 May 2022).
- International Maritime Organization. Sulfur 2020—Cutting Sulfur Oxide Emissions. 2019. Available online: http://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulfur-2020.aspx (accessed on 28 May 2022).
- Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Rekhletskaya, E.S.; Makhova, U.A.; Kapustin, V.M.; Mukhina, D.Y.; Abdellatief, T.M.M. Technological Potential Analysis and Vacant Technology Forecasting in Properties and Composition of Low-Sulfur Marine Fuel Oil (VLSFO and ULSFO) Bunkered in Key World Ports. J. Mar. Sci. Eng. 2022, 10, 1828. [Google Scholar] [CrossRef]
- Vedachalam, S.; Baquerizo, N.; Dalai, A.K. Review on impacts of low sulfur regulations on marine fuels and compliance options. Fuel 2022, 310, 122243. [Google Scholar] [CrossRef]
- Marafi, A.; Albazzaz, H.; Rana, M.S. Hydroprocessing of heavy residual oil: Opportunities and challenges. Cat. Tod. 2019, 329, 125–134. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Lu, N.; Xu, T.; Zhu, G.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- Speight, J.G. Heavy and Extra-Heavy Oil Upgrading Technologies; Gulf Professional Publishing: Oxford, UK, 2013; pp. 1–13. [Google Scholar] [CrossRef]
- Sahu, R.; Song, B.; Im, J.; Jeon, Y.; Lee, C. A review of recent advances in catalytic hydrocracking of heavy residues. J. Ind. Eng. Chem. 2015, 27, 12–24. [Google Scholar] [CrossRef]
- Magomedov, R.N.; Popova, A.Z.; Maryutina, T.A. Current status and prospects of demetallization of heavy petroleum feedstock (Review). Pet. Chem. 2015, 55, 423–443. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 10, 1017. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Denisov, K.; Zhurkevich, A.; Islamov, S. Reduction of Sulphur in Marine Residual Fuels by Deasphalting to Produce VLSFO. J. Mar. Sci. Eng. 2022, 10, 1765. [Google Scholar] [CrossRef]
- Akhmetov, S.; Serikov, T. Technology and Equipment of Oil and Gas Refining Processes; Akhmetov, S., Ed.; Nedra: Saint Petersburg, Russia, 2006; p. 868. [Google Scholar]
- Al-Obaidi, S.H.; Guliaeva, N.I. Thermal Adsorption Processing Of Hydrocarbon Residues. Int. J. Sci. Technol. Res. 2017, 6, 137–140. [Google Scholar]
- Oil Residual Feed Upgrading Method. Patent SU1799029A1, 10 December 2001.
- Contact Adsorbent for Thermocontact Processing of Oil Residues. Patent RU2176546C1, 10 December 2001.
- Ancheyta, J.; Rana, M.; Furimsky, E. Hydroprocessing of heavy petroleum feeds: Tutorial. Cat. Tod. 2005, 109, 3–15. [Google Scholar] [CrossRef]
- Ancheyta, J.; Speight, J.G. Hydroprocessing of Heavy Oils and Residua, 1st ed.; CRC Press: Boca Raton, FL, USA. [CrossRef]
- Pine, D.; Imhof, A. Ordered macroporous materials by emulsion templating. Nature 1997, 389, 948–951. [Google Scholar]
- Parkhomchuk, E.V.; Sashkina, K.A.; Rudina, N.A.; Kulikovskaya, N.A.; Parmon, V.N. Template synthesis of 3D-structured macroporous oxides and hierarchical zeolites. Catal. Ind. 2013, 5, 80–89. [Google Scholar] [CrossRef]
- Method for Using Hydrodemetallization Catalyst in the Process of Petroleum Feed Hydrotreating. Patent RU2737374C1, 27 November 2020.
- Fuel Oil Deasphalting. Patent RU 2522745C2, 20 July 2014.
- Yuzmukhametova, R.F.; Shmelkova, O.I.; Boldushevsky, R.E.; Khamzin, Y.A.; Shipiltsyna, A.A.; Antonov, S.A.; Nikulshin, P.A. Coarsely Granulated Macroporous Adsorbent for Adsorption Cleaning of Heavy Oil Stock. Chem. Technol. Fuels Oils 2022, 58, 582–589. [Google Scholar] [CrossRef]
- Tomina, N.N.; Pimerzin, A.A.; Zhilkina, E.O.; Eremina, Y.V. Comprehensive analysis of diesel hydrotreating feedstock. Pet. Chem. 2009, 49, 114–119. [Google Scholar] [CrossRef]
- Sawarkar, A.N.; Pandit, A.B.; Samant, S.D.; Joshi, J.B. Petroleum Residue Upgrading Via Delayed Coking: A Review. Can. J. Chem. Eng. 2007, 85, 1–24. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Huy, C.; Shin, E.W. Hierarchical macro–mesoporous Al2O3-supported NiK catalyst for steam catalytic cracking of vacuum residue. Fuel 2016, 169, 1–6. [Google Scholar] [CrossRef]
- Chen, K.; Liu, H.; Guo, A.; Ge, D.; Wang, Z. Study of the Thermal Performance and Interaction of Petroleum Residue Fractions during the Coking Process. Energy Fuels 2012, 26, 6343–6351. [Google Scholar] [CrossRef]
- Kondrasheva, N.; Rudko, V.; Kondrashev, D.; Konoplin, R.; Smyshlyaeva, K.; Shakleina, V. Functional influence of depressor and depressor-dispersant additives on marine fuels and their distillates components. Pet. Sci. Technol. 2018, 36, 2099–2105. [Google Scholar] [CrossRef]

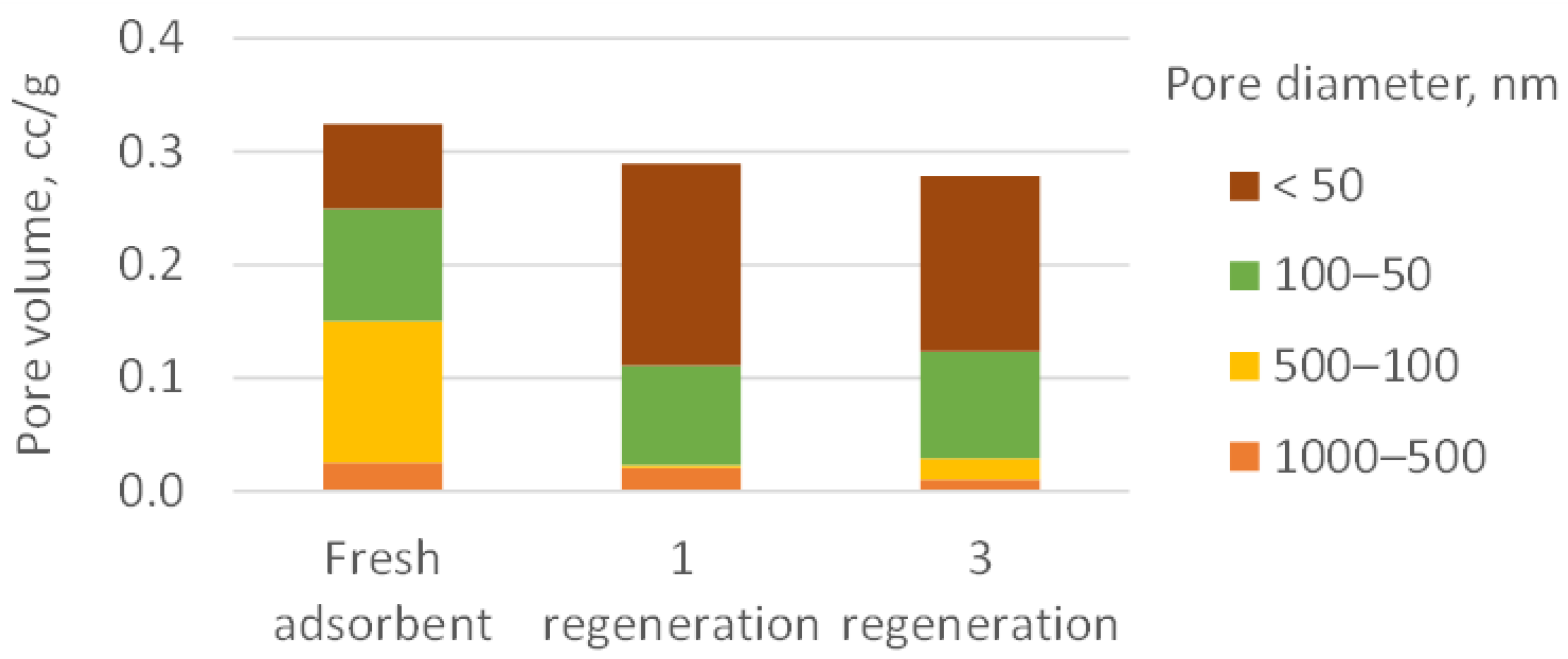
| Properties | Values |
|---|---|
| Average pellet diameter, mm | 3.0–6.0 |
| Loss on ignition at 800 ± 10 °C, % | ≤5.0 |
| Bulk density, g/cc | 0.85–0.95 |
| Specific surface area, m2/g | ≥25.0 |
| Pore volume (including macropore) 1, cc/g | 0.32 |
| Mechanical strength, kg/mm | 3.42 |
| Macropore volume 2 (diameter > 50 cc/g) | 0.25 |
| Properties | Values | |||||
|---|---|---|---|---|---|---|
| AtmR | VisR | AtmR (70 vol%) + SRGO (30 vol%) | VisR (70 vol%) + SRGO (30 vol%) | AtmR (70 vol%) + LCG (30 vol%) | VisR (70 vol%) + LCG (30 vol%) | |
| Density at 20 °C, kg/m3 | 992 | 1010 | 955 | 980 | 970 | 996 |
| Kinematic viscosity at 100 °C, mm2/s | 52.50 | 112.90 | 17.20 | 20.11 | 18.13 | 20.24 |
| Carbon residue, wt% | 16.9 | 18.9 | 12.3 | 13.2 | 12.6 | 15.4 |
| Asphaltenes, wt% | 10.98 | 12.9 | 7.69 | 9.05 | 8.01 | 9.80 |
| Sulfur content, wt% | 2.30 | 2.64 | 1.68 | 1.85 | 1.71 | 2.04 |
| Metals content (Ni+V+Fe), ppm | 257 | 281 | 180 | 197 | 217 | 217 |
| Fraction yield1, wt%: IBP-195 °C 195–350 °C >350 °C, including: 350–540 °C >540 °C | 2.0 12.0 86.0 21.9 64.1 | 1.8 7.2 91.0 19.1 71.9 | 2.6 32.8 64.6 20.6 44.0 | 2.5 32.6 64.9 17.9 47.0 | 1.6 28.6 69.8 25.5 44.3 | 1.3 25.9 72.8 23.4 49.4 |
| Properties | Values for Feed | ||||
|---|---|---|---|---|---|
| AtmR | AtmR (70 vol%) + SRGO (30 vol%) | VisR (70 vol%) + SRGO (30 vol%) | AtmR (70 vol%) + LCG (30 vol%) | VisR (70 vol%) + LCG (30 vol%) | |
| Total liquid product | |||||
| Density at 20 °C, kg/m3 | 903 | 884 | 919 | 889 | 920 |
| Sulfur content, wt% | 1.86 | 1.32 | 1.50 | 1.41 | 1.72 |
| Carbon residue, wt% | 4.25 | 3.79 | 3.19 | 4.58 | 3.52 |
| Metals content (Ni+V+Fe), ppm | 19 | 14 | 4 | 4 | 4 |
| Fraction yield 1, wt%: IBP-195 °C 195–350 °C >350 °C, including: 350–540 °C >540 °C | 11.6 36.2 52.2 37.1 15.1 | 11.7 51.3 37.0 28.9 8.1 | 10.1 50.2 39.7 28.2 11.5 | 4.8 45.3 49.9 38.6 11.3 | 5.0 42.2 52.8 40.5 12.3 |
| Fractions yield calculated to residue without diluent, wt % | |||||
| Gas Liquid product, including: IBP-195 °C 195–350 °C >350 °C, including: 350–540 °C >540 °C Coke | 12.2 79.5 9.2 28.8 41.5 29.5 12.0 8.3 | 16.6 76.0 13.9 31.0 31.1 21.5 9.6 7.4 | 11.1 72.9 11.7 28.1 33.1 19.8 13.3 16.0 | 13.0 74.4 5.6 23.1 45.7 32.4 13.3 12.6 | 8.0 73.7 5.8 19.2 48.7 34.4 14.3 18.3 |
| Feed conversion and purification | |||||
| Conversion, %, of: fraction > 350 °C fraction > 540 °C | 39.3 76.4 | 42.7 81.6 | 38.8 75.5 | 28.5 74.5 | 27.5 75.1 |
| Demetallization, % | 92.6 | 92.2 | 98.0 | 98.2 | 98.2 |
| Desulfurization, % | 19.2 | 21.4 | 18.9 | 17.5 | 15.7 |
| Properties | Values for Feed | ||
|---|---|---|---|
| Feed Space Velocity, h−1 | |||
| 1.0 | 1.5 | 1.7 | |
| Total liquid product | |||
| Density at 20°C, kg/m3 | 884 | 892 | 894 |
| Sulfur content, wt% | 1.32 | 1.44 | 1.50 |
| Carbon residue, wt% | 3.79 | 3.98 | 4.10 |
| Metals content (Ni+V+Fe), ppm | 14 | 13 | 2 |
| Fractions yield calculated to residue without diluent, wt % | |||
| Gas Liquid product, including: IBP-195 °C 195–350 °C >350 °C, including: 350–540 °C >540 °C Coke | 16.6 76.0 13.9 31.0 31.1 21.5 9.6 7.4 | 14.1 77.9 12.7 28.4 36.8 26.4 10.4 8.0 | 13.6 79.7 12.4 28.5 38.9 27.5 11.4 6.7 |
| Feed conversion and purification | |||
| Conversion, %, of: fraction > 350 °C fraction > 540 °C | 42.7 81.6 | 36.4 80.5 | 34.7 78.9 |
| Demetallization, % | 92.2 | 92.8 | 93.0 |
| Desulfurization, % | 21.4 | 14.3 | 10.7 |
| Properties | Values | |
|---|---|---|
| AR (70 vol%) + LGO (30 vol%) | VisR (70 vol%) + LGO (30 vol%) | |
| Naphta (IBP-195 °C) | ||
| Density at 20 °C, kg/m3 | 764 | 770 |
| Sulfur content, wt% | 0.65 | 0.60 |
| Hydrocarbon content, wt%. Paraffins Isoparaffins Aromatics Naphthenes Olefins | 18.3 20.5 27.4 13.6 20.3 | 16.0 18.9 19.1 13.5 32.5 |
| Iodine number, g iodine/100 g of sample | 48.5 | 79.9 |
| Light gasoil (195–350 °C) | ||
| Density at 20 °C, kg m−3 | 863 | 879 |
| Sulfur content, wt% | 0.92 | 1.08 |
| Aromatics content, wt%, including: Monoaromatics Diaromatics Polyaromatics | 33.5 19.0 11.3 3.2 | 41.1 19.7 18.4 3.0 |
| Iodine number, g iodine/ 100 g of sample | 9.8 | 36.6 |
| Olefins content, wt%. | 8.0 | 33.1 |
| Pour point, °C | −23 | −24 |
| Atmospheric residue (350 °C+) | ||
| Metals content (Ni+V+Fe), ppm | 2 | 2 |
| Kinematic viscosity at 100 °C, mm2 s−1 | 5.65 | 6.27 |
| Density at 20 °C, kg m−3 | 953 | 961 |
| Sulfur content, wt% | 1.79 | 2.00 |
| Carbon residue, wt% | 3.65 | 4.25 |
| Saturates, wt%: Aromatics, wt%: Polar (I), wt%: Polar (II), wt%: | 31.2 55.3 12.4 1.1 | 33.0 50.7 14.6 1.7 |
| Property | Requirements for Marine Fuel Type | Obtained Sample | |
|---|---|---|---|
| RMA 10 | RMB 30 | ||
| Kinematic viscosity at 50 °C, mm²/s, max | 10.00 | 30.00 | 20.17 |
| Density at 15 °C, kg/m³, max | 920.0 | 960.0 | 899.6 |
| Calculation aromatization index SSAI, max | 850.0 | 860.0 | 800.0 |
| Mass fraction of sulfur %, max. | Depends on region (less than 0.5 wt% or 0.1wt% ) | 0.097 | |
| Flash point in a closed crucible, °C, min | 61.0 | 61.0 | 72.0 |
| Acid number, mg KHO/g, max | 2.5 | 2.5 | 0.09 |
| Total sediment after aging, wt%, max | 0.10 | 0.10 | 0.01 |
| Carbon residue (micromethod), wt%, max | 2.50 | 10.00 | 2.0 |
| Flow temperature, °C, not higher: | – | – | minus 6 |
| winter | 0 | 0 | |
| summer | 6 | 6 | |
| Water content, % vol, max | 0.30 | 0.50 | absence |
| As content, %, max | 0.040 | 0.070 | absence |
| Vanadium content, mg/kg, max | 50 | 150 | 0.5 |
| Sodium content, mg/kg, max | 50 | 100 | absence |
| Aluminum and silicon (total) ∑, mg/kg, max | 25 | 40 | absence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuzmukhametova, R.; Boldushevskii, R.; Shmelkova, O.; Khamzin, Y.; Minaev, A.; Nikulshin, P. Adsorptive Treatment of Residues on Macroporous Adsorbent for Marine Fuel Production Scheme on Refinery. J. Mar. Sci. Eng. 2023, 11, 525. https://doi.org/10.3390/jmse11030525
Iuzmukhametova R, Boldushevskii R, Shmelkova O, Khamzin Y, Minaev A, Nikulshin P. Adsorptive Treatment of Residues on Macroporous Adsorbent for Marine Fuel Production Scheme on Refinery. Journal of Marine Science and Engineering. 2023; 11(3):525. https://doi.org/10.3390/jmse11030525
Chicago/Turabian StyleIuzmukhametova, Renata, Roman Boldushevskii, Olga Shmelkova, Yunir Khamzin, Artem Minaev, and Pavel Nikulshin. 2023. "Adsorptive Treatment of Residues on Macroporous Adsorbent for Marine Fuel Production Scheme on Refinery" Journal of Marine Science and Engineering 11, no. 3: 525. https://doi.org/10.3390/jmse11030525






