Biodiesel as Dispersant to Improve the Stability of Asphaltene in Marine Very-Low-Sulfur Fuel Oil
Abstract
:1. Introduction
2. Experiments Section
2.1. Materials
2.2. Characterization of Biodiesel
2.3. Asphaltene Extraction and Characterization
2.4. Model Oil Preparation
2.5. Determination of the Asphaltene Inhibition Effect
2.5.1. Asphaltene Initial Precipitation Point (IPP)
2.5.2. Asphaltene Dispersion Efficiency
2.5.3. Optical Microscope Observation
3. Simulation Section
3.1. Molecular Model of Asphaltene and Biodiesel
3.2. Simulation Procedure
4. Results and Discussion
4.1. Experimental Results
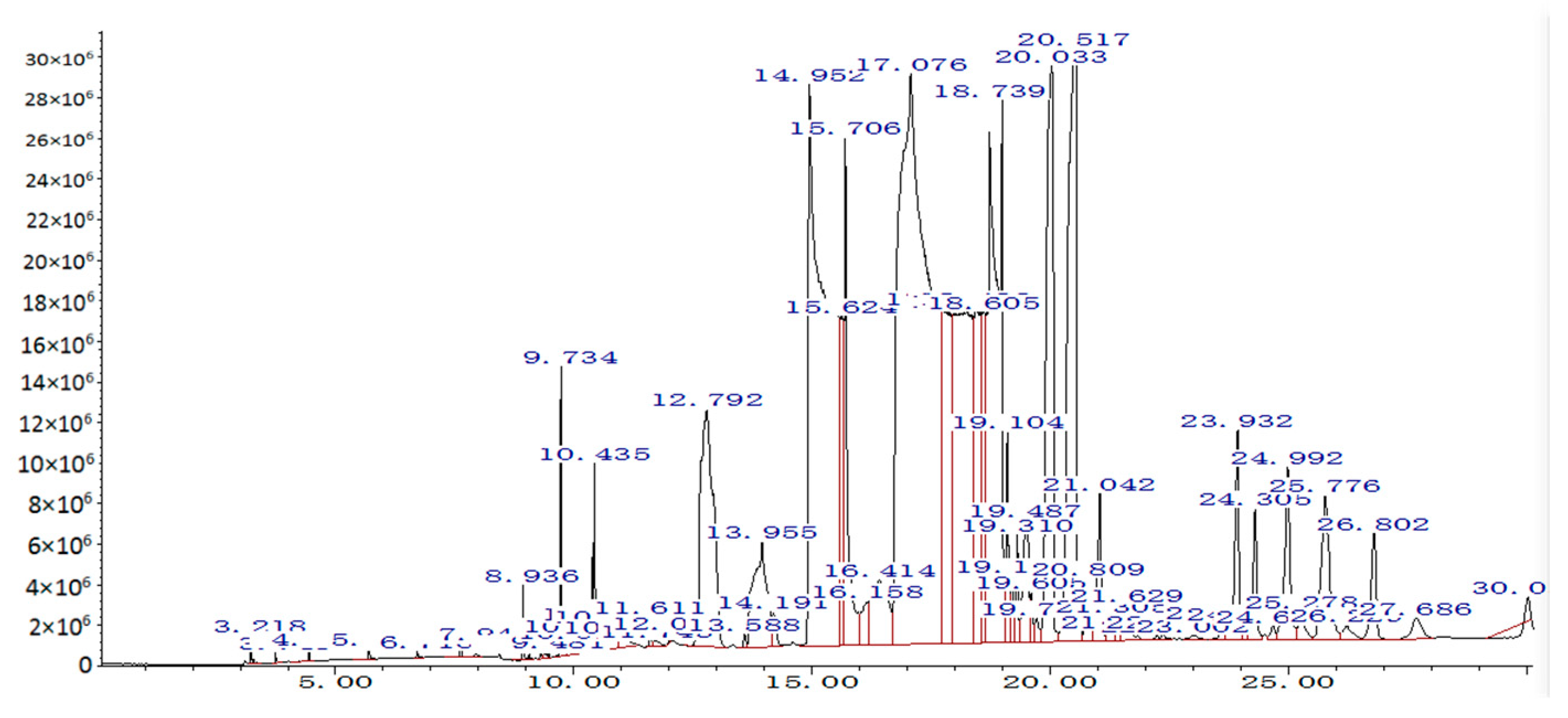
4.1.1. GC/MS Analysis
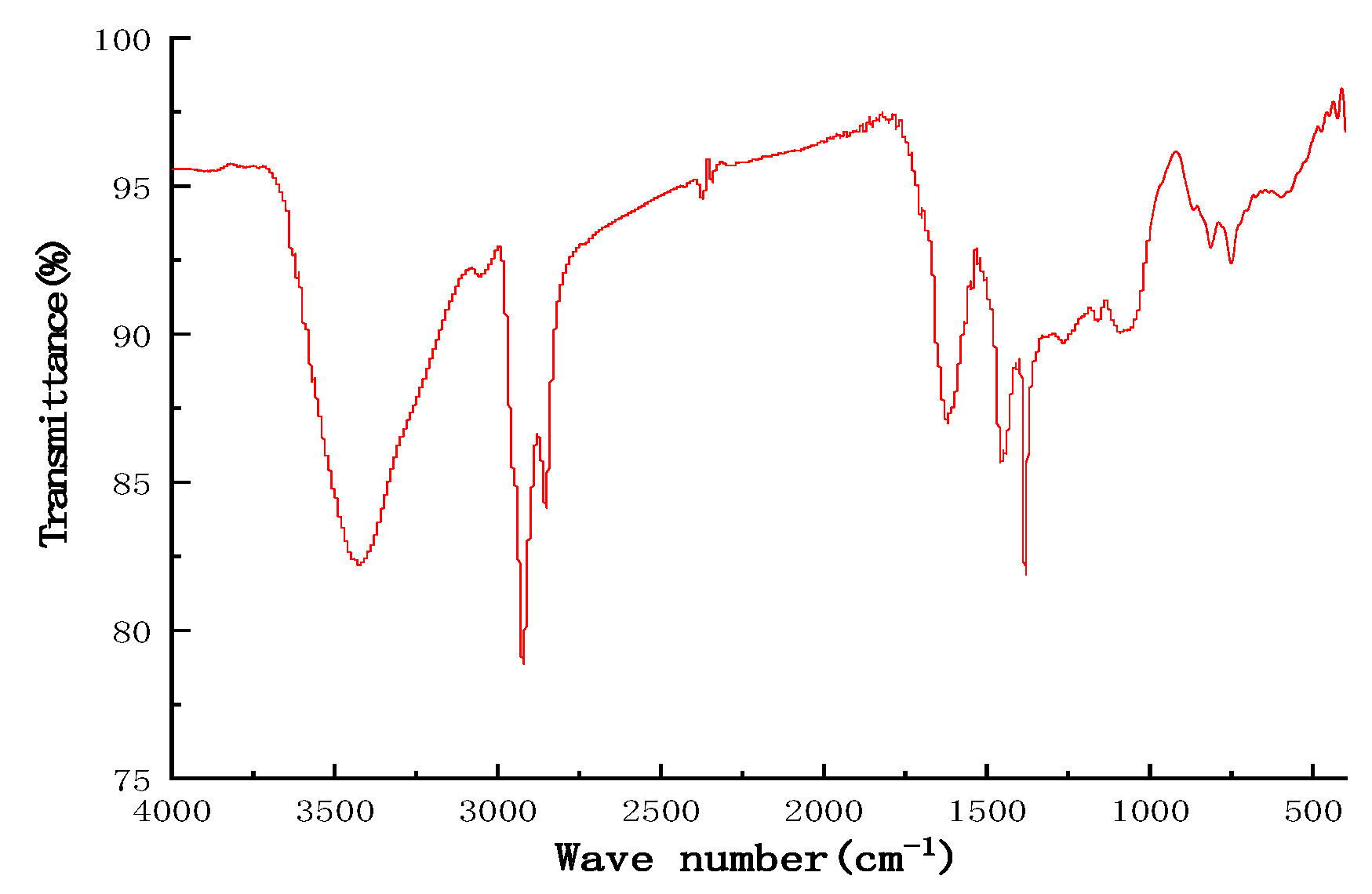
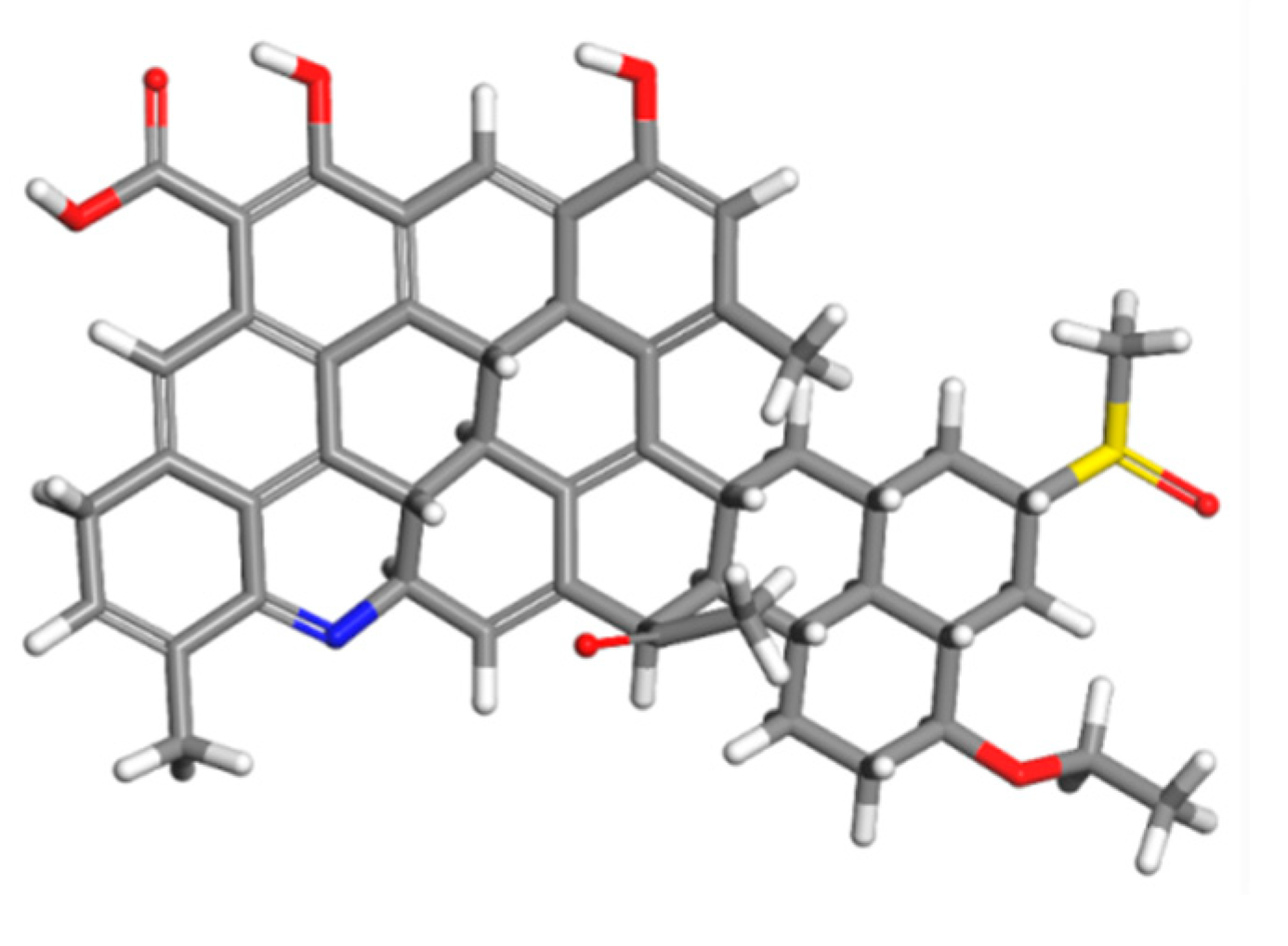
4.1.2. Chemical Structure of Asphaltene
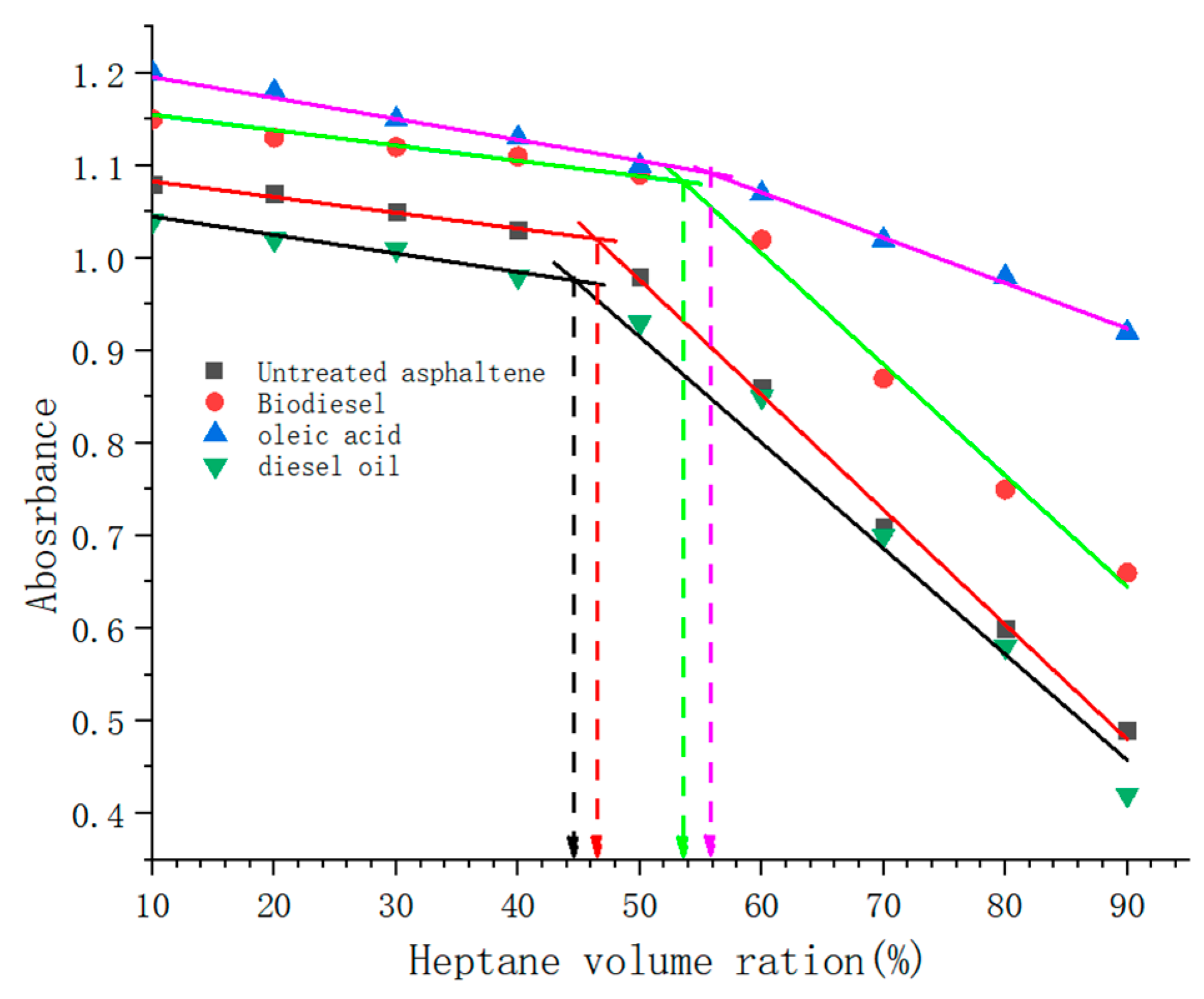
4.1.3. Asphaltene Initial Precipitation Point
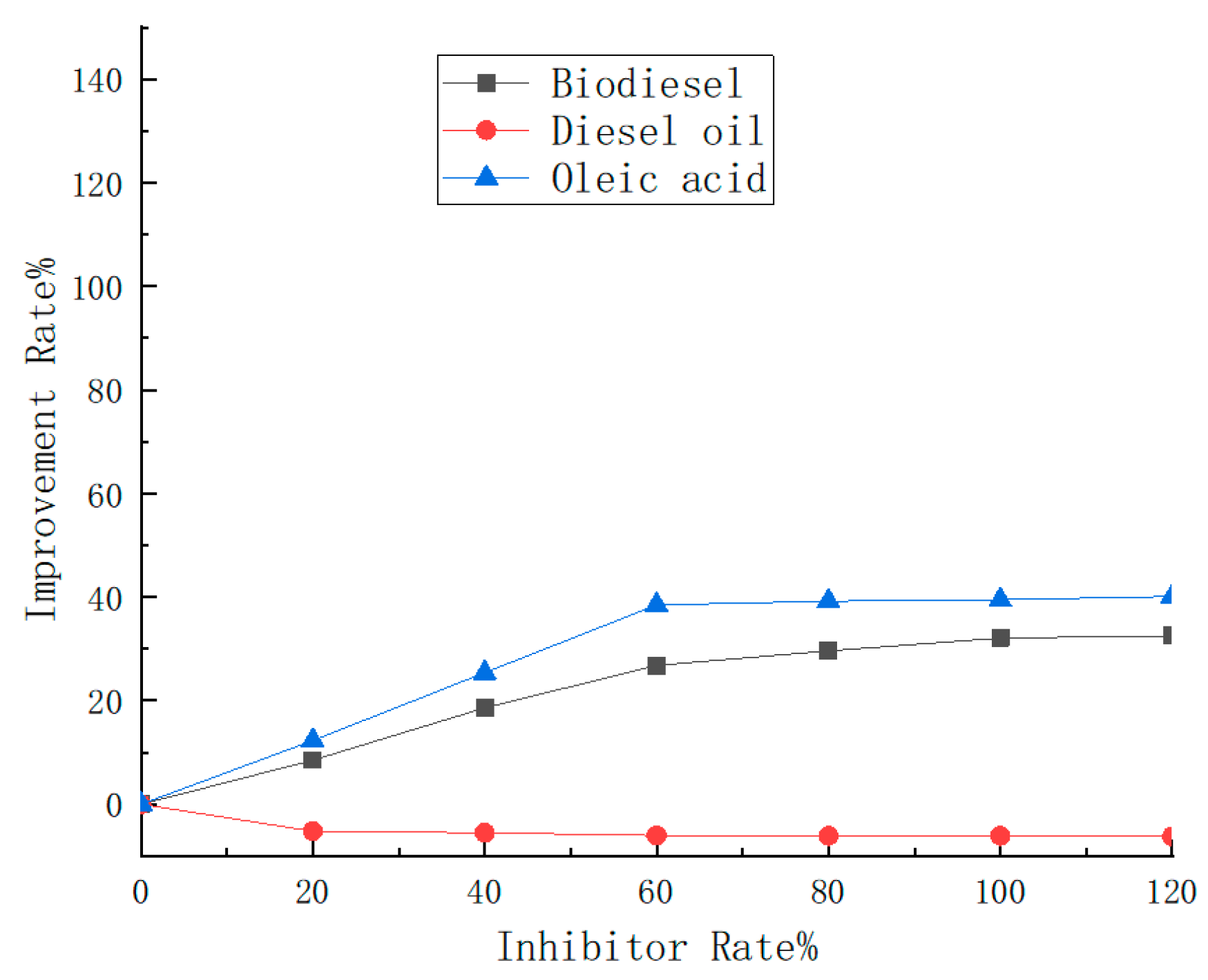
4.1.4. Asphaltene Dispersion Efficiency
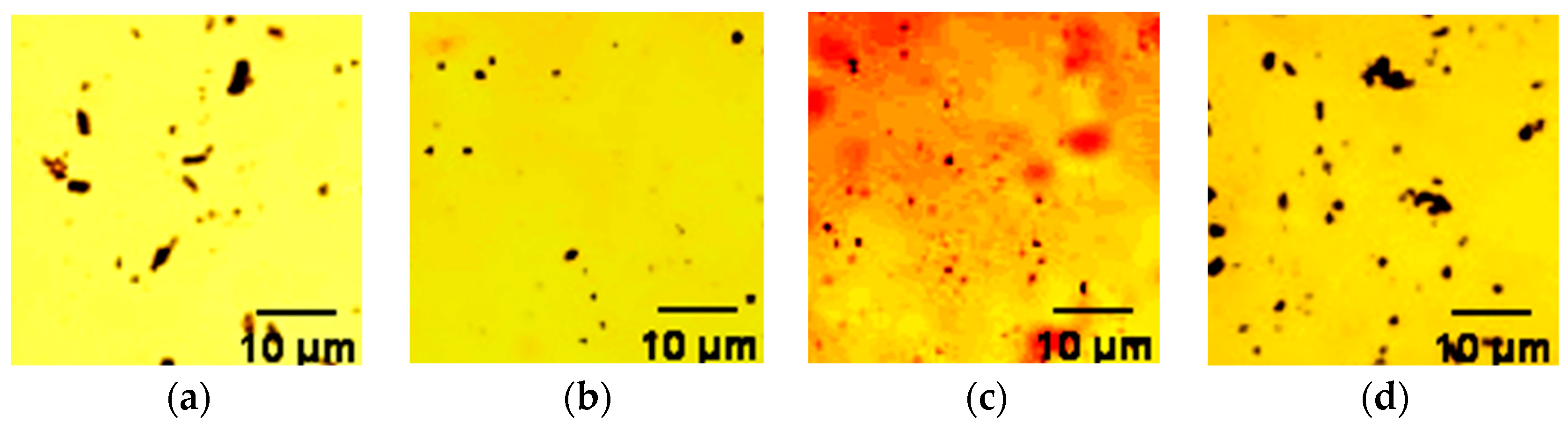
4.1.5. Morphology
4.2. Simulation Results
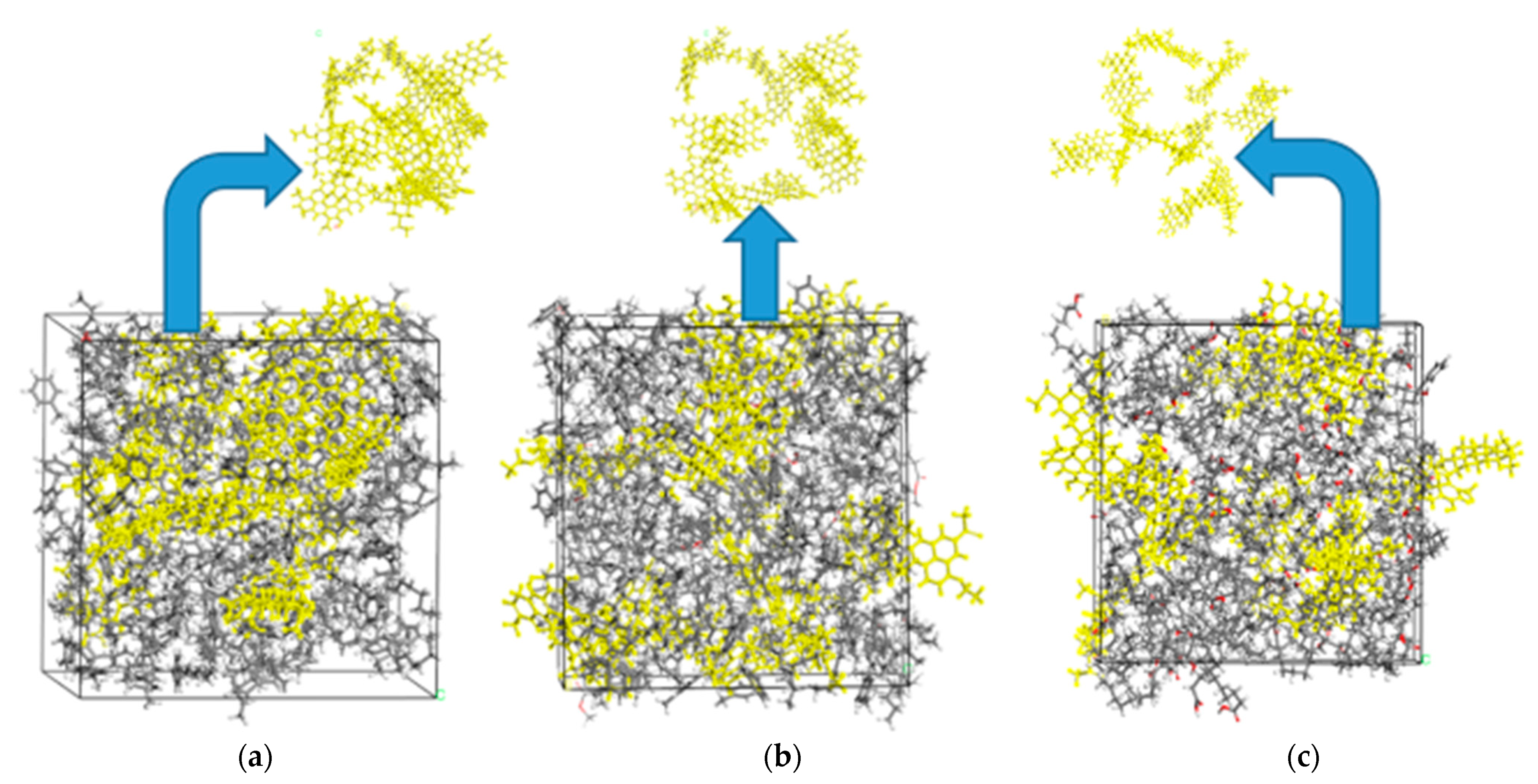
4.2.1. Interaction Energies
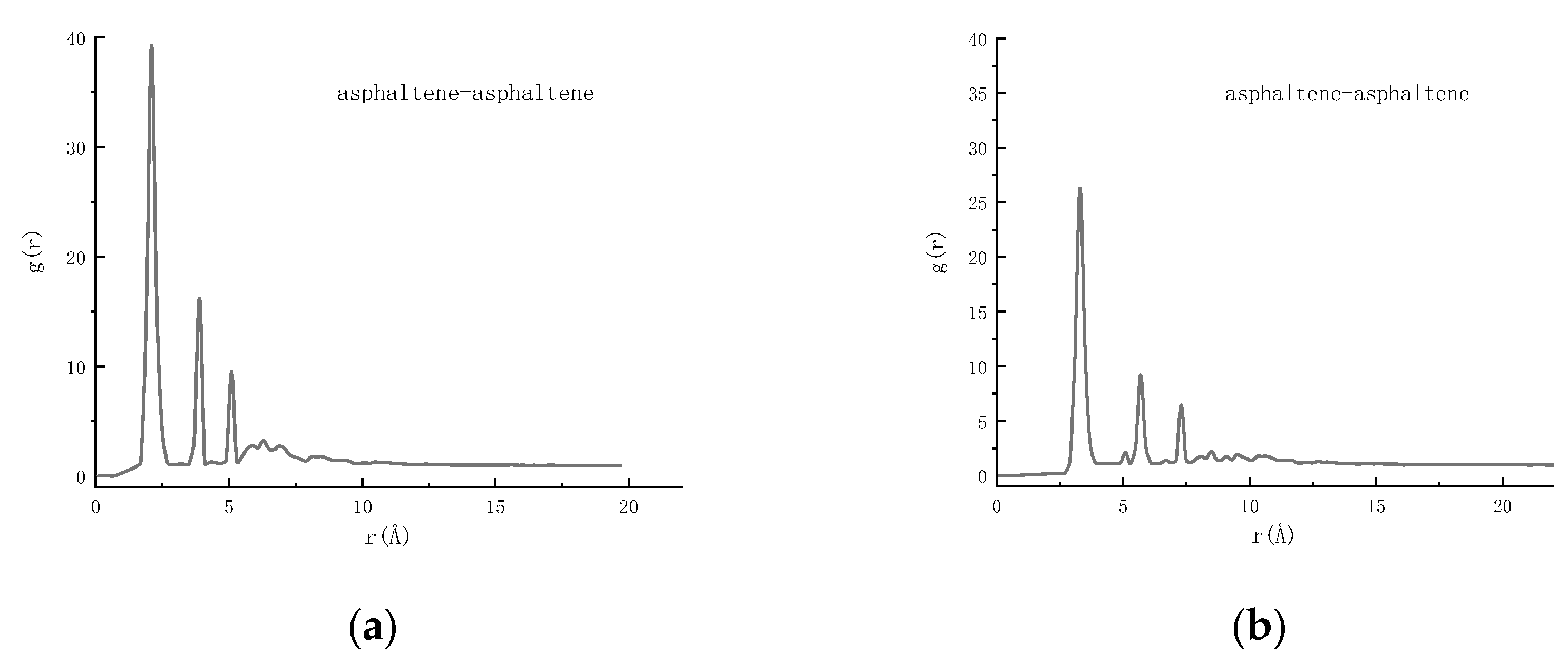
4.2.2. RDF Analysis
5. Conclusions
- The average chemical structure of asphaltene was characterized using elemental, 1HNMR, and FTIR analysis, and the results show that asphaltene was derived from marine low-sulfur fuel oil feature with a low H/C ratio, high aromaticity, and strong polarity, which made it easy to aggregate between asphaltene molecules.
- The composition of biodiesel produced with waste cooking oil is quite complex, and oleic acid and linoleic acid are the major fatty acids in WCO biodiesel, with 44.2 wt.% and 29.4 wt.%, respectively, followed by palmitic acid and linoleic acid.
- In the UV-Vis analysis, the IPP value shifted from 46% to 56% after adding biodiesel, while IPP was shifted to a low n-heptane ratio with the addition of diesel oil. Through the comparison of the IPP and improvement rate of asphaltene with oleic acid, biodiesel, and diesel oil using UV-Vis, it was found that both oleic acid and biodiesel can reduce the IPP of asphaltene and improve the asphaltene effectively. The solubility of oleic acid has a certain effect, and the analysis effect of oleic acid with the strongest polarity is better than that of biodiesel, but diesel has no dispersing effect on asphaltenes and accelerates the precipitation of asphaltenes.
- Molecular dynamics simulation results show that the interaction energy between each component in biodiesel and asphaltene is negative, and the absolute value is higher than the mutual-interaction energy of asphaltene, indicating that biodiesel molecules can be effectively adsorbed on asphaltene molecule, inhibiting the asphaltene from aggregation and precipitation.
- Both oleic acid and biodiesel can improve the stability of asphaltene in marine fuel oil, but due to the acid limit and combustion quality, biodiesel is a better choice for VLSFO production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul Jameel, A.G.; Alkhateeb, A.; Telalović, S.; Elbaz, A.M.; Roberts, W.L.; Sarathy, S.M. Environmental Challenges and Opportunities in Marine Engine Heavy Fuel Oil Combustion; Springer: Singapore, 2019; pp. 1047–1055. [Google Scholar]
- Kondrasheva, N.K.; Kondrashev, D.O.; Rudko, V.A.; Shaidulina, A.A. Effect of Hydrocarbon Composition on Quality and Operating Characteristics of Middle Distillate Fractions and Low-Viscosity Marine Fuels. Chem. Tech. Fuels Oil+ 2017, 53, 163–172. [Google Scholar] [CrossRef]
- Halff, A.; Younes, L.; Boersma, T. The likely implications of the new IMO standards on the shipping industry. Energ. Policy 2019, 126, 277–286. [Google Scholar] [CrossRef]
- Chu Van, T.; Ramirez, J.; Rainey, T.; Ristovski, Z.; Brown, R.J. Global impacts of recent IMO regulations on marine fuel oil refining processes and ship emissions. Transp. Res. Part D Transp. Environ. 2019, 70, 123–134. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Rekhletskaya, E.S.; Makhova, U.A.; Kapustin, V.M.; Mukhina, D.Y.; Abdellatief, T.M.M. Technological Potential Analysis and Vacant Technology Forecasting in Properties and Composition of Low-Sulfur Marine Fuel Oil (VLSFO and ULSFO) Bunkered in Key World Ports. J. Mar. Sci. Eng. 2022, 12, 1828. [Google Scholar] [CrossRef]
- Choi, Y.; Lim, T. Numerical Simulation and Validation in Scrubber Wash Water Discharge from Ships. J. Mar. Sci. Eng. 2020, 8, 272. [Google Scholar] [CrossRef]
- Cuong, N.M.; Hung, P.V. An analysis of available solutions for commercial vessels to comply with IMO strategy on low sulphur. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 40–47. [Google Scholar] [CrossRef]
- Vráblík, A.; Schlehöfer, D.; Dlasková Jaklová, K.; Hidalgo Herrador, J.M.; Černý, R. Comparative Study of Light Cycle Oil and Naphthalene as an Adequate Additive to Improve the Stability of Marine Fuels. ACS Omega 2022, 7, 2127–2136. [Google Scholar] [CrossRef]
- Gulyaeva, L.A.; Lobashova, M.M.; Mitusova, T.N.; Shmel Kova, O.I.; Khavkin, V.A.; Nikul Shin, P.A. Production of Low -Sulfur Marine Fuel. Chem. Tech. Fuels Oil 2020, 55, 704–711. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Denisov, K.; Zhurkevich, A.; Islamov, S. Reduction of Sulphur in Marine Residual Fuels by Deasphalting to Produce VLSFO. J. Mar. Sci. Eng. 2022, 11, 1765. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Kuzmin, K.A.; Povarov, V.G. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Schipachev, A. Manifestation of incompatibility of marine residual fuels: A method for determining compatibility, studying composition of fuels and sediment. J. Min. Inst. 2022, 257, 843–852. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 11, 1017. [Google Scholar] [CrossRef]
- Vedachalam, S.; Baquerizo, N.; Dalai, A.K. Review on impacts of low sulfur regulations on marine fuels and compliance options. Fuel 2022, 310, 122243. [Google Scholar] [CrossRef]
- Nelyubor, D.V.; Oshchenko, A.P.; Sharin, E.A. Influence of the Composition of Petroleum Residual Fuel Oils on Their Colloidal Stability. Chem. Tech. Fuels Oil+ 2018, 54, 150–157. [Google Scholar] [CrossRef]
- Cheng, R.; Zou, R.; He, L.; Liu, L.; Cao, C.; Li, X.; Guo, X.; Xu, J. Effect of Aromatic Pendants in a Maleic Anhydride-co-Octadecene Polymer on the Precipitation of Asphaltenes Extracted from Heavy Crude Oil. Energy Fuels 2021, 35, 10562–10574. [Google Scholar] [CrossRef]
- Saeedi Dehaghani, A.H.; Badizad, M.H. Inhibiting asphaltene precipitation from Iranian crude oil using various dispersants: Experimental investigation through viscometry and thermodynamic modelling. Fluid Phase Equilibr. 2017, 442, 104–118. [Google Scholar] [CrossRef]
- Chuahy, F.D.; Finney, C.E.; Kaul, B.C.; Kass, M.D. Computational exploration of bio-oil blend effects on large two-stroke marine engines. Fuel 2022, 322, 123977. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A.; et al. Current Challenge and Innovative Progress for Producing HVO and FAME Biodiesel Fuels and Their Applications. Available online: https://link.springer.com/article/10.1007/s12649-022-01880-0 (accessed on 10 December 2022).
- Kass, M.; Kaul, B.; Armstrong, B.; Szybist, J.; Lobodin, V. Stability, rheological and combustion properties of biodiesel blends with a very-low sulfur fuel oil (VLSFO). Fuel 2022, 316, 123365. [Google Scholar] [CrossRef]
- Bruun, N.; Khazraie Shoulaifar, T.; Hemming, J.; Willför, S.; Hupa, L. Characterization of waste bio-oil as an alternate source of renewable fuel for marine engines. Biofuels 2019, 13, 21–30. [Google Scholar] [CrossRef]
- Lin, C. Effects of Biodiesel Blend on Marine Fuel Characteristics for Marine Vessels. Energies 2013, 6, 4945–4955. [Google Scholar] [CrossRef]
- Cortez, L.; Franco, T.T.; Valença, G.; Rosillo-Calle, F. Perspective Use of Fast Pyrolysis Bio-Oil (FPBO) in Maritime Transport: The Case of Brazil. Energies 2021, 14, 4779. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Sędłak, P.; Seń, D. Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions. Energies 2020, 13, 5632. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.H. Evaluation of Eco Friendly Bio-Oil Dispersants on the Inhibition of Asphaltene Precipitation in a Kuwaiti Crude Oil. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar]
- Nasr-El-Din, H.A.A.F. Bio-Oil Dispersants Effectiveness on AsphalteneSludge During Carbonate Acidizing Treatment. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, Port of Spain, Trinidad and Tobago, 25–27 June 2018. [Google Scholar]
- Atiku, F.A.; Bartle, K.D.; Jones, J.M.; Lea-Langton, A.R.; Williams, A. A study of the combustion chemistry of petroleum and bio-fuel oil asphaltenes. Fuel 2016, 182, 517–524. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Bucas, M.; Laukinaite, A. Alternative fuels for marine applications:Biomethanol-biodiesel-diesel blends. Fuel 2019, 248, 161–167. [Google Scholar] [CrossRef]
- Dunn, R.O. Fuel Properties of Biodiesel/Ultra-Low Sulfur Diesel (ULSD) Blends. J. Am. Oil Chem. Soc. 2011, 88, 1977–1987. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Na, L.; Jun, L.; Yi, Z.; Zhiping, T.; Xin, G.; Lu, H.; Yumeng, C. Molecular Simulation on Aggregation of the Oxidation Products and Dispersion Mechanism of Gasoline Detergent. China Pet. Process. Petrochem. Technol. 2020, 22, 49–59. [Google Scholar]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comp. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Insight into the Interfacial Behavior of Surfactants and Asphaltenes: Molecular Dynamics Simulation Study. Energy Fuels 2020, 34, 13536–13551. [Google Scholar] [CrossRef]
- Rogel, E. Studies on asphaltene aggregation via computational chemistry. Colloids Surf. A Physicochem. Eng. Asp. 1995, 104, 85–93. [Google Scholar] [CrossRef]
- Ahmadbaygi, A.; Bayati, B.; Mansouri, M.; Rezaei, H.; Riazi, M. Chemical study of asphaltene inhibitors effects on asphaltene precipitation of an Iranian oil field. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2020, 75, 6. [Google Scholar] [CrossRef]
- Shadman, M.M.; Saeedi Dehaghani, A.H.; Badizad, M.H. How much do you know about the methods for determining onset of asphaltene precipitation? Petroleum 2017, 3, 287–291. [Google Scholar] [CrossRef]
- Hurtado, P.; Gámez, F.; Martίnez-Haya, B. One- and Two-Step Ultraviolet and Infrared Laser Desorption Ionization Mass Spectrometry of Asphaltenes. Energy Fuels 2010, 24, 6067–6073. [Google Scholar] [CrossRef]
- El-hoshoudy, A.N.; Ghanem, A.; Desouky, S.M. Imidazolium-based ionic liquids for asphaltene dispersion; experimental and computational studies. J. Mol. Liq. 2021, 324, 114698. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Lima, F.C.D.A.; Alvim, R.D.S.; Miranda, C.R. From Single Asphaltenes and Resins to Nanoaggregates: A Computational Study. Energ Fuel 2017, 31, 11743–11754. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Efimov, I.; Povarov, V.G.; Rudko, V.A. Comparison of UNIFAC and LSER Models for Calculating Partition Coefficients in the Hexane–Acetonitrile System Using Middle Distillate Petroleum Products as an Example. Ind. Eng. Chem. Res. 2022, 61, 9575–9585. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Nassar, N.N.; Acevedo, S.A.; Cortes, F.B.; Franco, C.A. Thermo-Oxidative Decomposition Behaviors of Different Sources of n-C7 Asphaltenes under High-Pressure Conditions. Energy Fuels 2020, 34, 8740–8758. [Google Scholar] [CrossRef]
- Hung, A.M.; Fini, E.H. Absorption spectroscopy to determine the extent and mechanisms of aging in bitumen and asphaltenes. Fuel 2019, 242, 408–415. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, H.; Xue, S.; Qiu, Y.; Wu, S.; Yu, H. Investigating the Compatibility of Various Components in Marine Low-Sulfur Fuel Oil by Molecular Dynamics Simulations. J. Chem. 2021, 2021, 3000079. [Google Scholar] [CrossRef]
- Rogel, E. Simulation of Interactions in Asphaltene Aggregates. Energy Fuels 2000, 14, 566–574. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Comprehensive molecular scale modeling of anionic surfactant-asphaltene interactions. Fuel 2021, 288, 119729. [Google Scholar] [CrossRef]
- Salah Yaseen, G.A.M. Molecular dynamics studies of interaction between asphaltenes and solvents. J. Petrol. Sci. Eng. 2017, 156, 118–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Siskin, M.; Gray, M.R.; Walters, C.C.; Rodgers, R.P. Mechanisms of Asphaltene Aggregation: Puzzles and a New Hypothesis. Energy Fuels 2020, 34, 9094–9107. [Google Scholar] [CrossRef]
- Rahmati, M. Effects of heteroatom and aliphatic chains of asphaltene molecules on their aggregation properties in aromatics Solvents: A molecular dynamics simulation study. Chem. Phys. Lett. 2021, 779, 138847. [Google Scholar] [CrossRef]
- Hosseini-Dastgerdi, Z.; Tabatabaei-Nejad, S.A.R.; Khodapanah, E.; Sahraei, E. A comprehensive study on mechanism of formation and techniques to diagnose asphaltene structure; molecular and aggregates: A review. Asia Pac. J. Chem. Eng. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, H.; Tang, T. Molecular dynamics simulation of model asphaltenes between surfaces of varying polarity. Fuel 2023, 331, 125842. [Google Scholar] [CrossRef]
- Kabir, S.F.; Mousavi, M.; Fini, E.H. Selective adsorption of bio-oils’ molecules onto rubber surface and its effects on stability of rubberized asphalt. J. Clean. Prod. 2020, 252, 119856. [Google Scholar] [CrossRef]
- Tirjoo, A.; Bayati, B.; Rezaei, H.; Rahmati, M. Molecular dynamics simulations of asphaltene aggregation under different conditions. J. Petrol. Sci. Eng. 2019, 177, 392–402. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hou, Q.; Wang, Y.; Chen, Z. Interfacial and molecular interactions between fractions of heavy oil and surfactants in porous media: Comprehensive review. Adv. Colloid. Interfac. 2020, 283, 102242. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; Khani, S.; Maia, J.M.; Tavares, F.W. Concentration and Solvent Effects on Structural, Dynamical, and Rheological Properties of Asphaltene Suspensions. Energy Fuels 2019, 34, 1071–1081. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H. Moisture effect on nanostructure and adhesion energy of asphalt on aggregate surface: A molecular dynamics study. Appl. Surf. Sci. 2020, 510, 145435. [Google Scholar] [CrossRef]
- Ding, H.; Wang, H.; Qu, X.; Varveri, A.; Gao, J.; You, Z. Towards an understanding of diffusion mechanism of bio-rejuvenators in aged asphalt binder through molecular dynamics simulation. J. Clean. Prod. 2021, 299, 126927. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T.; Bhattacharjee, S. Molecular Dynamics Investigation on the Aggregation of Violanthrone78-Based Model Asphaltenes in Toluene. Energy Fuels 2014, 28, 3604–3613. [Google Scholar] [CrossRef]
- Pacheco-Sánchez, J.H.; Álvarez-Ramírez, F.; Martínez-Magadán, J.M. Morphology of Aggregated Asphaltene Structural Models. Energy Fuels 2004, 18, 1676–1686. [Google Scholar] [CrossRef]
- Celia-Silva, L.G.; Vilela, P.B.; Morgado, P.; Lucas, E.F.; Martins, L.F.G.; Filipe, E.J.M. Preaggregation of Asphaltenes in the Presence of Natural Polymers by Molecular Dynamics Simulation. Energy Fuels 2020, 34, 1581–1591. [Google Scholar] [CrossRef]

| Property | VLSFO | ISO8217 |
|---|---|---|
| Density at 15 °C/(g·mL−1) | 0.972 | Max. 1010 |
| Flash point/°C | 96 | Min. 60 |
| Viscosity at 50 °C/(mm2·s −1) | 275.2 | Max. 700 |
| Sulfur content, % | 0.46 | Max. 3.5 |
| TSA (wt.%) | 0.03 | Max. 0.1 |
| Total acid number (mg KOH−1) | 0.08 | Max. 2.5 |
| Property | Biodiesel | Test Method |
|---|---|---|
| Density at 15 °C/(g·mL−1) | 0.876 | ASTM D4052 |
| Viscosity at 40 °C/(mm2·s −1) | 4.004 | ASTM D445 |
| Sulfur content, % | NA | ASTM D129 |
| Flash point (°C) | 173 | ASTM D93 |
| Pour point (°C) | −1 | ASTM D97 |
| Acid number (mg KOH−1) | 0.31 | ASTM D664 |
| Free glycerine (wt.%) | 0.01 | ASTM D6584 |
| Cetane number | 53 | ASTM D613 |
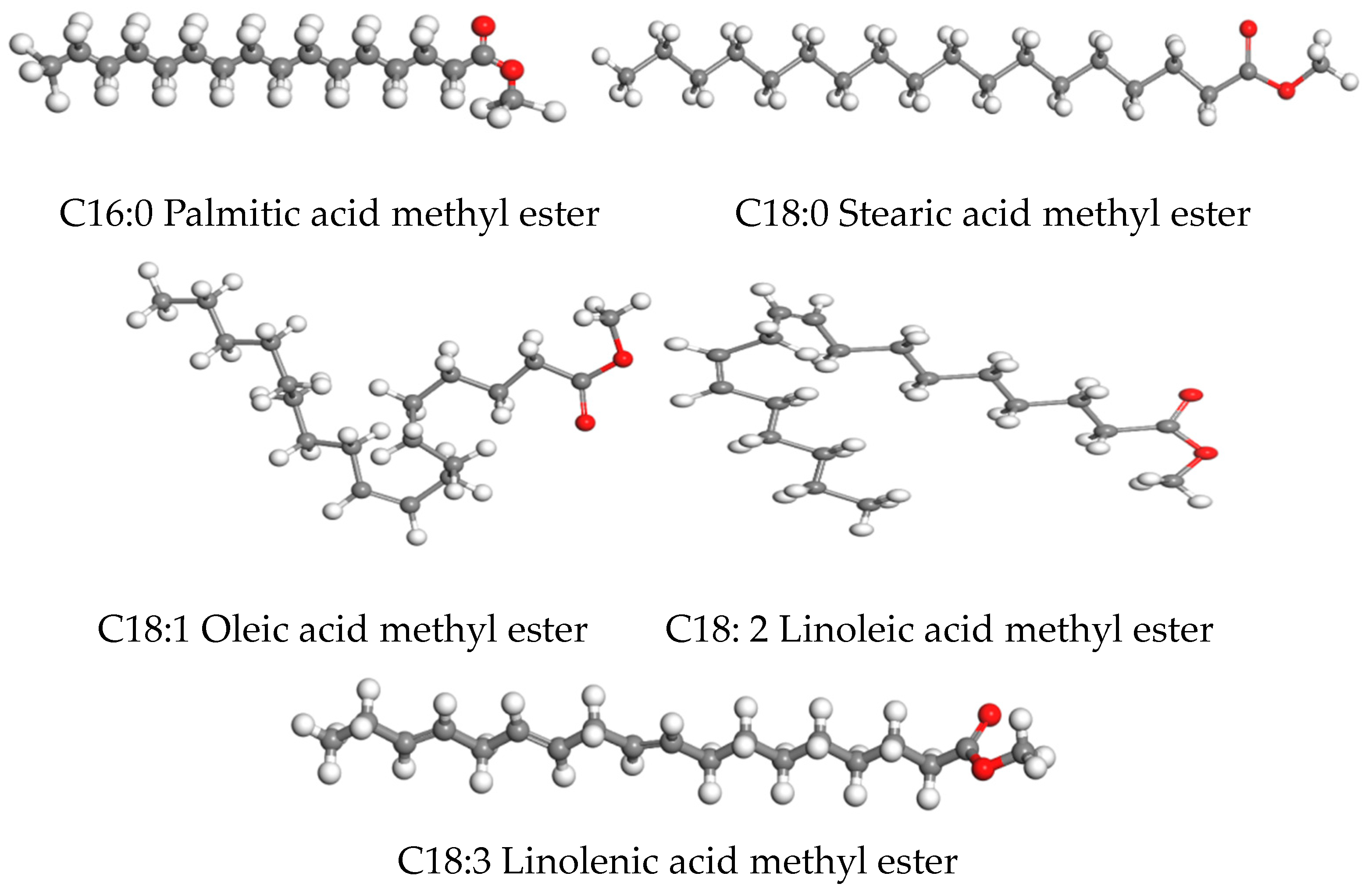
| Name | Molecule Model | Formula | Molecular Weight | Atom Number | H/C Ratio |
|---|---|---|---|---|---|
| Palmitic acid methyl ester |  | C17H34O2 | 270.457 | 53 | 2 |
| Stearic acid methyl ester | 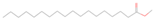 | C19H38O2 | 298.511 | 59 | 2 |
| Oleic acid methyl ester |  | C19H36O2 | 296.495 | 57 | 1.895 |
| Linoleic acid methyl ester |  | C19H34O2 | 294.479 | 55 | 1.789 |
| Linolenic acid methyl ester |  | C19H32O2 | 292.463 | 53 | 1.684 |
| Peak | Retention Time | Name | wt.% in Biodiesel |
|---|---|---|---|
| 1 | 14.95 | Palmitic acid methyl ester | 15.9 |
| 2 | 15.71 | Stearic acid methyl ester | 1.2 |
| 3 | 17.08 | Oleic acid methyl ester | 44.2 |
| 4 | 18.61 | Linoleic acid methyl ester | 29.4 |
| 5 | 20.03 | Linolenic acid methyl ester | 3.1 |
| Others | 6.2 |
| Elemental Composition (%) | Molecular Weight | |||||
|---|---|---|---|---|---|---|
| C | H | O | N | S | NH/NC | |
| 75.46 | 6.05 | 13.41 | 1.22 | 3.81 | 0.962 | 821 |
| Hydrogen Type | Hydrogen Description | Chemical Shift | Relative Amount |
|---|---|---|---|
| HA | Directly linked to aromatic carbon | 9.0–6.0 | 13.2% |
| Hα | Linked to α carbon of aromatic ring | 4.5–1.9 | 14.3% |
| Hβ | Linked to β carbon of aromatic rings and H on CH2 CH farther than β carbon | 1.9–1.0 | 55.1% |
| Hγ | Linked to γ carbon of aromatic rings and H on CH2 CH farther than β carbon | 1.0–0.5 | 17.4% |
| Symbol | Definition | Result |
|---|---|---|
| CT | Total carbon | 51.6 |
| HT | Total hydrogen | 49.6 |
| fA | Aromatic carbon weight ratio | 0.58 |
| HAU/CA | Aromatic rings condensation degree | 0.38 |
| σ | Aromatic rings substitution degree | 0.31 |
| CA | Proton aromatic carbon number | 30 |
| CS | Aromatic carbon number of lateral branches | 12 |
| CP | Peripheral Aromatic carbons | 9 |
| RA | Aromatic ring number | 8.5 |
| RT | Total rings | 12.5 |
| RN | Naphthenic rings | 4 |
| CN | Naphthenic carbon | 12 |
| L | The average length of substituted chain | 1.5 |
| Molecular Pairs | Interaction Energy/(KJ/·mol−1) |
|---|---|
| asphaltene–asphaltene | −1203.16 |
| asphaltene–palmitic acid methyl ester | −1338.31 |
| asphaltene–stearic acid methyl ester | −1397.61 |
| asphaltene–oleic acid methyl ester | −1559.24 |
| asphaltene–linoleic acid methyl ester | −1736.19 |
| asphaltene–oleic acid | −1902.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Wei, H.; Tan, Z.; Xue, S.; Qiu, Y.; Wu, S. Biodiesel as Dispersant to Improve the Stability of Asphaltene in Marine Very-Low-Sulfur Fuel Oil. J. Mar. Sci. Eng. 2023, 11, 315. https://doi.org/10.3390/jmse11020315
Zhou D, Wei H, Tan Z, Xue S, Qiu Y, Wu S. Biodiesel as Dispersant to Improve the Stability of Asphaltene in Marine Very-Low-Sulfur Fuel Oil. Journal of Marine Science and Engineering. 2023; 11(2):315. https://doi.org/10.3390/jmse11020315
Chicago/Turabian StyleZhou, Daping, Haijun Wei, Zhiwen Tan, Shuye Xue, Ye Qiu, and Shen Wu. 2023. "Biodiesel as Dispersant to Improve the Stability of Asphaltene in Marine Very-Low-Sulfur Fuel Oil" Journal of Marine Science and Engineering 11, no. 2: 315. https://doi.org/10.3390/jmse11020315
APA StyleZhou, D., Wei, H., Tan, Z., Xue, S., Qiu, Y., & Wu, S. (2023). Biodiesel as Dispersant to Improve the Stability of Asphaltene in Marine Very-Low-Sulfur Fuel Oil. Journal of Marine Science and Engineering, 11(2), 315. https://doi.org/10.3390/jmse11020315





