Paleontological Evidence for Dinoflagellates and Ciliates as Early Eukaryotes
Abstract
:1. Introduction
2. Reassessing the Relationship between Fossil Dinoflagellates and Acritarchs
2.1. The Need for Reassessment
2.2. Limitations to the Criteria Used to Define Dinocysts
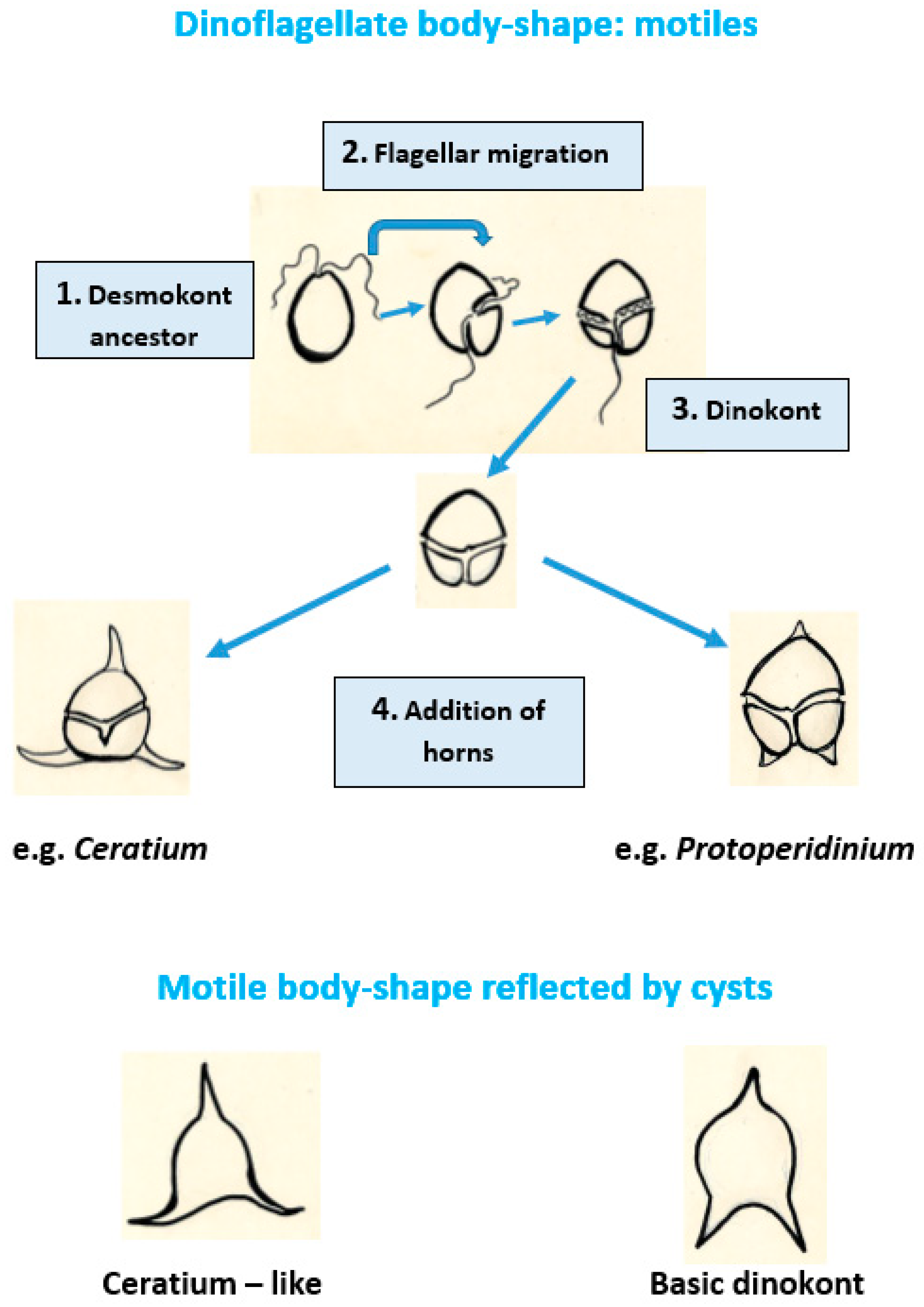
2.3. Morphology of Dinoflagellate Motile Stages Reflected by Fossil Cysts
2.4. Living Dinoflagellate Cyst Morphology
2.5. Reassessing The Archeopyle in Fossil Cysts
2.6. The Acritarchs
3. The Fossil Record of Early Life
3.1. Bacteria and Cyanobacteria
3.2. The First Appearance of the Acritarchs—A New Group and a New Strategy
3.3. The Role of Sporopollenin-Like Walls in Dinoflagellate Cysts and Acritarchs
4. Discussion
4.1. The Belief That Some Acritarchs May Be Dinoflagellate Cysts Is Not New
4.2. A New Approach
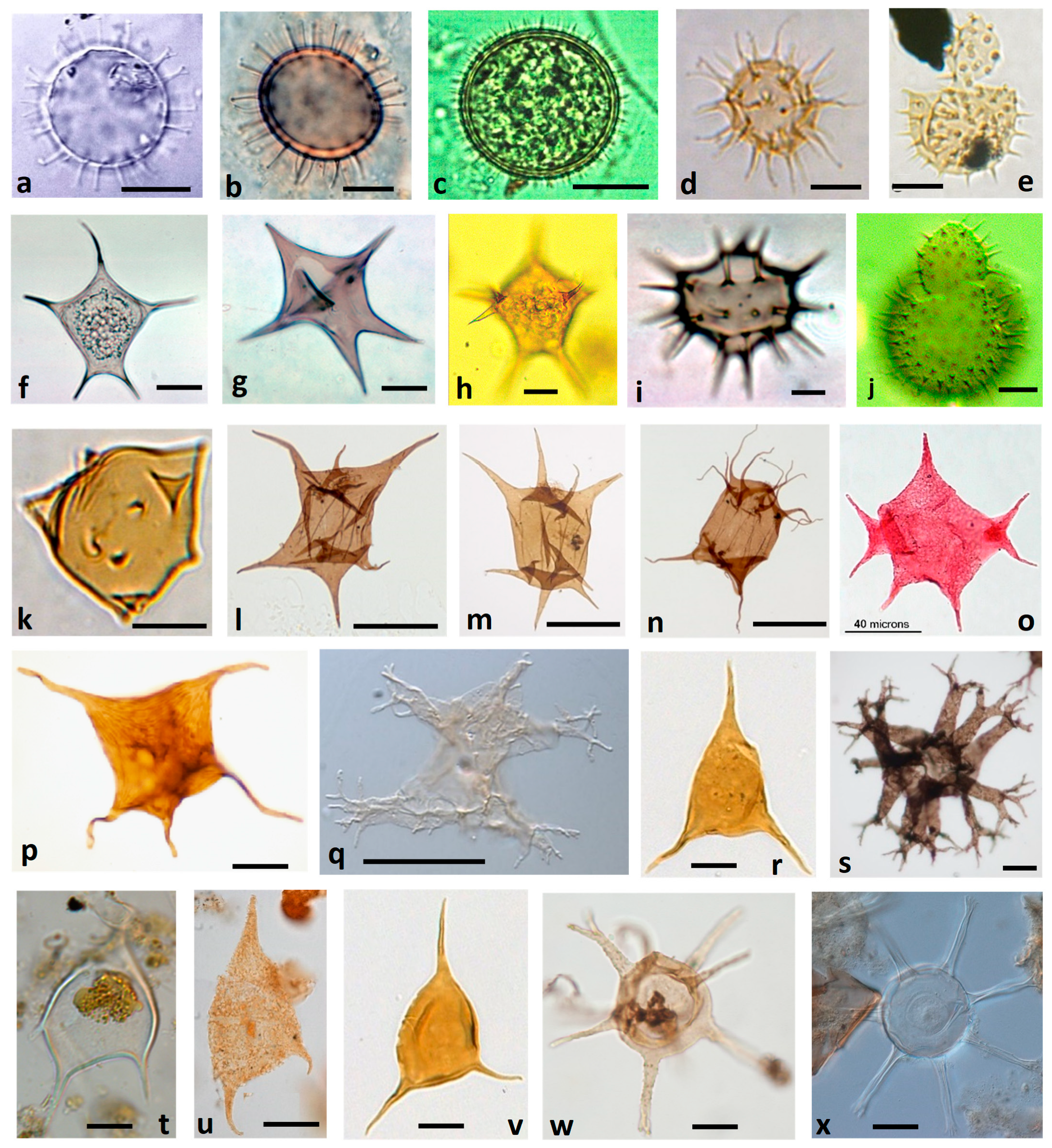
4.3. Examples of Early Dinoflagellate Affinities in Acritarchs
4.3.1. Micrhystridium: A Basic Cyst Morphotype Persisting from Early Dinoflagellate Evolution
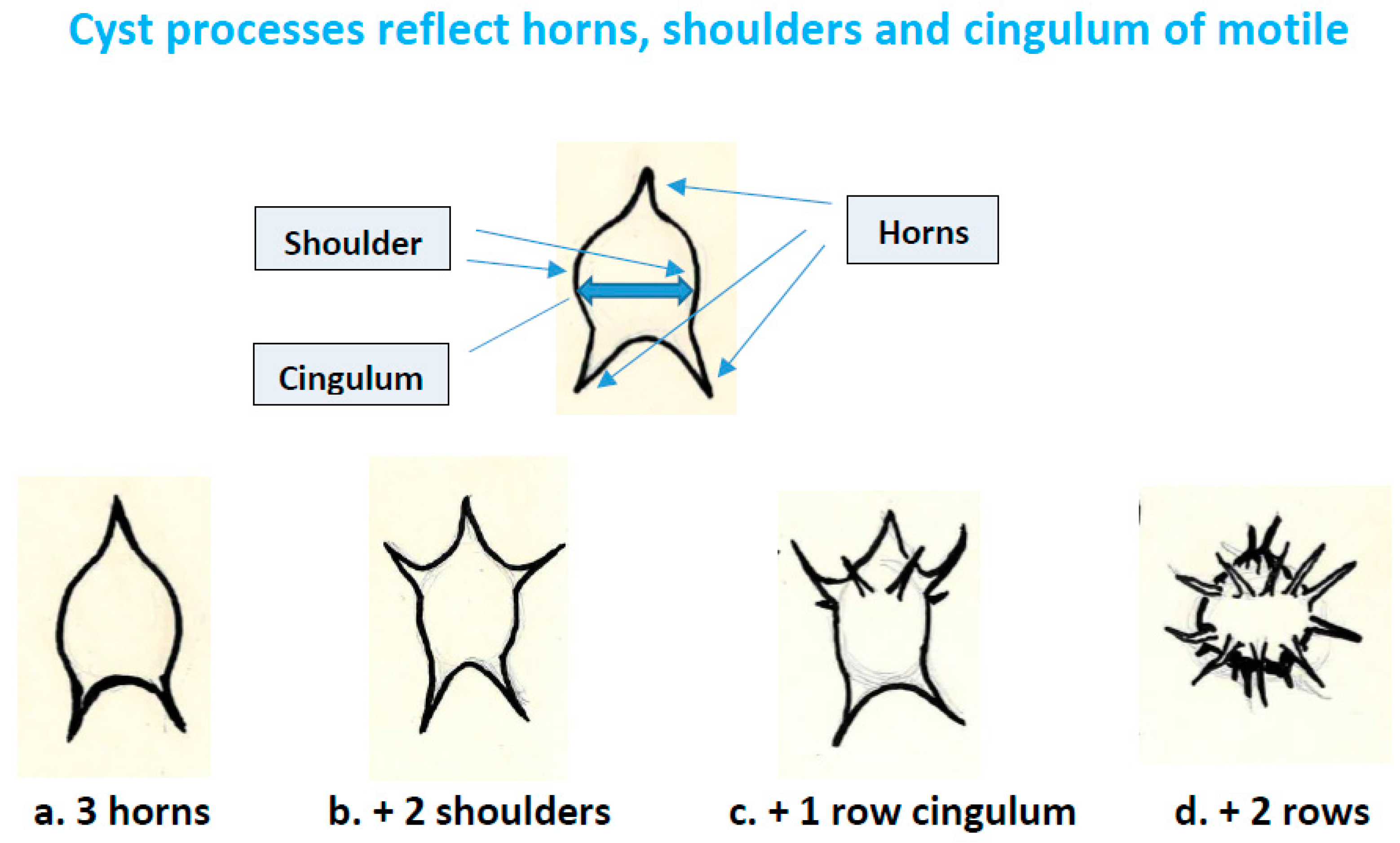
4.3.2. Processes on Cysts and Acritarchs Reflecting Horns and Cingulum from Motile Dinoflagellates
4.3.3. Paleontological Evidence for Early Ciliates
4.4. Suggestions for Future Work
- Freshly excysted cysts of living dinoflagellates in incubation experiments should be carefully examined for archeopyles. Pentapharsodinium dalei is a particular example where questions raised here of rare or absent archeopyles in some species could be investigated.
- The acritarch record of the late Paleozoic/Early Triassic prior to the first traditional dinocysts in the Middle Triassic is particularly interesting. Acritarchs should be examined for evidence of transition to thecate dinoflagellates, from the perspective suggested here that dinoflagellates were present before the traditional record starts.
- Many previous palynological studies of Mesozoic and Cenozoic samples were concentrated on traditional dinocysts useful for biostratigraphy. Acritarchs were often seen and sometimes recorded, but seldom studied in detail. The research here suggests the value of a new approach that investigates the whole acritarch record for evidence complementing the traditional dinocyst record. The Late Cretaceous to Late Eocene is an interesting interval where acritarchs identified as Micrhystridium occur together with Micrhystridium-like dinoflagellate cysts identified as Impletosphaeridium [54]. More research is needed to clarify the morphologic differences within these groups.
- Spinose acritarchs (acanthomorphs) should be examined for evidence of processes reflecting horns and cingulum. Horns may be represented by more prominent processes, and by their critical positions (1 for apex, usually 2 but possibly more for antapex, and shoulders); the cingulum by rows of processes separated towards the poles (Figure 4). The acritarch genus Dorsennidium Wicander 1974 may prove to be an example [55].
- The research presented here has obvious implications for the application of the traditional dinoflagellate record in other studies. For example, previous interpretations of pre-Mesozoic dinosteranes were considered unlikely to originate from dinoflagellates partly because they predate “unambiguous fossils” [56], but the research here suggests that could be circular reasoning. The research here also challenges studies of phytoplankton evolution that treat acritarchs and dinoflagellate cysts in the traditional record as separate groups. For example, one line of research recognizes two basic groups of plankton (referred to as “red”, including dinoflagellates, or “green”, including acritarchs) and links a switch between relative amounts of these groups to paleo nutrients [57] (and references therein). There is still much that we do not know about how morphology persists or is repeated within dinoflagellate cysts, but the research here proposes significant overlap between the two traditionally separated groups as an alternative perspective to be considered in future studies such as these.
- Gilan Attaran-Fariman et al. [58] described the living athecate Gymnodinium trapeziforme with a trapezoidal-shaped cyst with a micro reticulate wall reflecting alveolae, cingulum and sulcus, and with a split archeopyle in the sulcal region. This most likely represents a living cyst form with morphological linkage to trapezoidal acritarchs. The closely related group of micro reticulate Gymnodinium species should be closely examined for similar morphologies comparable with dinocysts and acritarchs.
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, D.; Yoon, H.S.; Hedges, S.B.; Hackett, J.D. Eukaryotes (Eukaryota). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 116–120. [Google Scholar]
- Butterfield, N.J. Early evolution of the eukaryota. Palaeontology 2015, 58, 5–17. [Google Scholar] [CrossRef]
- Porter, S.M. Insights into eukaryogenesis from the fossil record. Interface Focus 2020, 10, 20190105. [Google Scholar] [CrossRef] [PubMed]
- Riding, J.B.; Fensome, R.A.; Soyer-Gobillard, M.-O.; Medlin, L.K. A review of the dinoflagellates and their evolution from fossils to modern. J. Mar. Sci. Eng. 2022, 11, 1. [Google Scholar] [CrossRef]
- Taylor, F.J.R. General group characteristics; special features of interest; short history of dinoflagellates study. In The Biology of Dinoflagellates; Taylor, F.J.R., Ed.; Botanical Monographs; Wiley-Blackwell: Hoboken, NJ, USA, 1987; Volume 21, pp. 1–23. [Google Scholar]
- Talyzina, N.M.; Moldowan, J.M.; Johannisson, A.; Fago, F.J. Affinities of Early Cambrian acritarchs studied by using microscopy, fluorescence flow cytometry and biomarkers. Rev. Palaeobot. Palynol. 2000, 108, 37–53. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Dahl, J.; Jacobson, S.R.; Huizinga, B.J.; Fago, F.J.; Shetty, R.; Watt, D.S.; Peters, K.E. Chemostratographic reconstruction of biofacies: Molecular evidence linking cyst-forming dinoflagellates with pre-Triassic ancestors. Geology 1996, 24, 159–162. [Google Scholar] [CrossRef]
- Strother, P.K. Acritarchs. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 1, pp. 81–106. [Google Scholar]
- Fensome, R.A.; Saldarriaga, J.F.; Taylor, F.J.R. Dinoflagellate phylogeny revisited: Reconciling morphological and molecular based phylogenies. Grana 1999, 38, 66–80. [Google Scholar] [CrossRef]
- Dale, B. New observations on Peridinium faeroense Paulsen (1905), and classification of small orthoperidinioid dinoflagellates. Br. Phycol. J. 1977, 12, 241–253. [Google Scholar] [CrossRef]
- Dale, B. Acritarchous cysts of Peridinium faeroense Paulsen: Implications for dinoflagellate systematics. Palynology 1978, 2, 187–193. [Google Scholar] [CrossRef]
- Evitt, W.R. Observations on the morphology of fossil dinoflagellates. Micropaleontology 1961, 7, 385–420. [Google Scholar] [CrossRef]
- Evitt, W.R. A discussion and proposals concerning fossil dinoflagellates, hystrichospheres, and acritarchs. Nat. Acad. Sci. Proc. USA 1963, 49, 158–164 and 298–302. [Google Scholar] [CrossRef] [Green Version]
- Dale, B. From hystrichospheres to dinoflagellate cysts: Scandinavian contributions to E’vitt’s pivotal recognition of fossil dinoflagellate cysts. Palynology 2021, 45, 165–170. [Google Scholar] [CrossRef]
- Jansonius, J.; McGregor, D.C. (Eds.) Palynology: Principles and Applications; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 1, pp. 1–443. [Google Scholar]
- Dale, B. Dinoflagellate resting cysts: «benthic plankton». In Survival Strategies of the Algae; Fryxel, G.A., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 69–136. [Google Scholar]
- Evitt, W.R. Sporopollenin Dinoflagellate Cysts–Their Morphology and Interpretation; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1985; p. 333. [Google Scholar]
- Fensome, R.A.; Riding, J.B.; Taylor, F.J.R. Dinoflagellates. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 1, pp. 107–169. [Google Scholar]
- Taylor, F.J.R. Dinoflagellate morphology. In The Biology of Dinoflagellates; Taylor, F.J.R., Ed.; Botanical Monographs; Blakwell Scientific Publications: Hoboken, NJ, USA, 1987; Volume 2, pp. 24–91. [Google Scholar]
- Wall, D.; Dale, B. Modern dinoflagellate cysts and evolution of the Peridiniales. Micropaleontology 1968, 14, 265–304. [Google Scholar] [CrossRef]
- Head, M.J. Modern dinoflagellate cysts and their biological affinities. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 3, pp. 1197–1248. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. Annu. Rev. 2019, 57, 1–88. [Google Scholar]
- Persson, A.; Smith, B.C. Preservation of Dinoflagellate Cysts in Different Oxygen Regimes: Differences in Cyst Survival between Oxic and Anoxic Natural Environments. Phycology 2022, 2, 384–418. [Google Scholar] [CrossRef]
- Zonneveld, K.A.F.; Versteegh, G.J.M.; de Lange, G.J. Preservation of organic-walled dinoflagellate cysts in different oxygen regimes: A 10,000 year natural experiment. Mar. Micropaleontol. 1997, 29, 393–405. [Google Scholar] [CrossRef]
- Evitt, W.R. Dinoflagellate Studies II. In The Archeopyle; Stanford University Publications: Stanford, CA, USA, 1967; Volume 10, p. 88. [Google Scholar]
- Zonneveld, K.A.F.; Pospelova, V. A determination key for modern dinoflagellate cysts. Palynology 2015, 39, 387–409. [Google Scholar] [CrossRef]
- Anderson, D.M.; Taylor, C.D.; Armbrust, E.V. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 1987, 32, 340–351. [Google Scholar] [CrossRef]
- Genovesi-Giunti, B.; Laabir, A.; Vaquer, A. The benthic resting cyst: A key actor in harmful dinoflagellate blooms—A review. Vie. Milieu. 2006, 56, 327–337. [Google Scholar]
- Matsuoka, K. Archeopyle structures in modern gymnodinialean dinoflagellate cysts. Rev. Palaeobot. Palynol. 1985, 44, 217–231. [Google Scholar] [CrossRef]
- Dale, B.; Montresore, M.; Zingone, A.; Zonneveld, K. The cyst-motile stage relationships of the dinoflagellates Diplopelta simmetrica and Diplopsalopsis latipeltata. Eur. J. Phycol. 1993, 28, 129–137. [Google Scholar] [CrossRef]
- Ellegaard, M.; Dale, B.; Amorim, A. The acritarchous cyst of the athecate dinoflagellate Warnowia cf. rosea (Dinophyceae). Phycologia 2001, 40, 542–546. [Google Scholar] [CrossRef]
- Dale, B. Dinoflagellate cyst ecology: Modeling and geological application. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 3, pp. 1249–1275. [Google Scholar]
- Radi, T.; Bonnet, S.; Cormier, M.A.; de Vernal, A.; Durantou, L.; Faubert, É.; Head, M.J.; Henry, M.; Pospelova, V.; Rochon, A.; et al. Operational taxonomy and (paleo-)autecology of round, brown, spiny dinoflagellate cysts from the Quaternary of high northern latitudes. Mar. Micropaleontol. 2013, 98, 41–57. [Google Scholar] [CrossRef]
- Ribeiro, S.; Berge, T.; Lundholm, N.; Andersen, T.J.; Abrantes, F.; Ellegaard, M. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat. Commun. 2011, 2, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, T.R. The acritarchs and chitinozoa from the Wenlock and Ludlow Series of the Ludlow and Millichope areas, Shropshire. Monogr. Palaeontogr. Soc. 1970, 124, 1–100. [Google Scholar] [CrossRef]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic Biol Med. 2019, 140, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Precambrian paleobiology: Precedents, progress, and prospects. Front. Ecol. Evol. 2021, 9, 707072. [Google Scholar] [CrossRef]
- Tiwari, M. Organic-walled microfossils from the Chert-phosphate Member, Tal Formation, Precambrian-Cambrian Boundary, India. Precambrian Res. 1999, 97, 99–113. [Google Scholar] [CrossRef]
- Dodd, M.S.; Papineau, D.; Grenne, T.; Slack, J.F.; Rittner, M.; Pirajno, F.; O’Neil, J.; Little, C.T.S. Evidence for early life in Earth’s oldest hydrothermal vent precipitates. Nature 2017, 543, 60–64. [Google Scholar] [CrossRef]
- Knoll, A. Archean and Proterozoic paleontology. In Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Dallas, TX, USA, 1996; Volume 1, pp. 51–81. [Google Scholar]
- Suh, D.Y.; Ashton, N.W. A sporopollenin definition for the genomic age. New Phytol. 2022, 236, 2009–2013. [Google Scholar] [CrossRef]
- Butterfield, N.J.; Rainbird, R.H. Diverse organic-walled fossils, including “possible dinoflagellates” from the early Neoproterozoic of Arctic Canada. Geology 1998, 26, 963–966. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, C.; Yin, L.; Chen, Z.; Yuan, X. The oldest known dinoflagellates: Morphological and molecular evidence from Mesoproterozoic rocks at Yongji, Shanxi Province. Chin. Sci. Bull. 2005, 50, 1231–1234. [Google Scholar] [CrossRef]
- Sarjeant, W.A.S.; Stancliffe, R.P.W. The Michystridium and Veryhachium complexes (Acritarcha: Acanthomorphitae and Polygonomorphitae): A taxonomic reconsideration. Micropaleontology 1994, 40, 1–77. [Google Scholar] [CrossRef]
- Van Soelen, E.E.; Kürschner, W.M. Late Permian to Early Triassic changes in acritarch assemblages and morphology in the Boreal Arctic: New data from the Finnmark Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 505, 120–127. [Google Scholar] [CrossRef]
- Montresor, M.; Zingone, A.; Marino, D. The calcareous resting cyst of Pentapharsodinium Tyrrhenicum comb.nov. (Dinophyceae). J. Phycol. 1993, 29, 223–230. [Google Scholar] [CrossRef]
- Mertens, K.N.; Carbonell-Moore, C. Introduction to Spiniferites Mantell 1850. Palynology 2018, 42, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lundholm, N.; dos Santos Ribeiro, S.I.; Andersen, T.J.; Koch, T.A.; Godhe, A.; Ekelund, F.; Ellegaard, M. Buried alive–germination of up to a century-old marine protist resting stages. Phycologia 2011, 50, 629–640. [Google Scholar] [CrossRef]
- Wall, D.; Evitt, W.R. A Comparison of the Modern Genus Ceratium Schrank, 1733, with Certain Cretaceous Marine Dinoflagellates. Micropaleontology 1975, 21, 14–44. [Google Scholar] [CrossRef]
- Yan, K.; Li, J.; Molyneux, S.G.; Raevskaya, E.G.; Servais, T. A review of the Ordovician acritarch genus Barakella Cramer & Díez 1977. Palynology 2017, 41, 80–94. [Google Scholar]
- Gurdebeke, P.R.; Mertens, K.N.; Takano, Y.; Yamaguchi, A.; Bogus, K.; Dunthorn, M.; Matsuoka, K.; Vrielinck, H.; Louwye, S. The affiliation of Hexasterias problematica and Halodinium verrucatum sp. Nov. to ciliat cysts based on molecular phylogeny and cyst wall composition. Eur. J. Protistol. 2018, 66, 115–135. [Google Scholar] [CrossRef] [Green Version]
- Servais, T.; Eiserhardt, K.H. A discussion and proposals concerning the Lower Paleozoic « galeate» acritarch plexus. Palynology 1995, 19, 191–210. [Google Scholar] [CrossRef]
- Roncaglia, L. New acritarch species from Holocene sediments in central West Greenland. Grana 2004, 43, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Bowman, V.C.; Riding, J.B.; Francis, J.E.; Crame, J.A.; Hannah, M.J. The taxonomy and palaeobiogeography of small chorate dinoflagellate cysts from the Late Cretaceous to Quaternary of Antarctica. Palynology 2013, 37, 151–169. [Google Scholar] [CrossRef] [Green Version]
- Stancliffe, R.P.W.; Sarjeant, W.A.S. The acritarch genus Dorsennidium Wicander 1974, emend. Sarjeant, W.A.S.; Stancliffe 1994: A reassessment of its constituent species. Micropaleontology 1996, 42, 151–166. [Google Scholar] [CrossRef]
- Janouškovec, J.; Gavellis, G.S.; Burki, F.; Donna, D.; Bachvaroff, S.R.; Gomik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. USA 2016, 114, E171–E180.56. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Servais, T. Did the evolution of the phytoplankton fuel the diversification of the marine biosphere. Lethaia 2020, 53, 5–31. [Google Scholar] [CrossRef]
- Attaran-Fariman, G.; de Salas, M.F.; Negri, A.P.; Bolch, C.J.S. Morphology and phylogeny of Gymnodinium trapeziforme sp. nov. (Dinophyceae): A new dinoflagellate from the southeast coast of Iran that forms microreticulate resting cysts. Phycologia 2007, 46, 644–656. [Google Scholar] [CrossRef]
- Colbath, G.K.; Grenfell, H.R. Review of biological affinities of Paleozoic acid-resistant, organic-walled eukaryotic algal microfossils (including “acritarchs”). Rev. Palaeobot. Palynol. 1995, 86, 287–314. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.; Luo, K. Cryogenian magmatic activity and early life evolution. Sci. Rep. 2019, 9, 6586. [Google Scholar] [CrossRef] [Green Version]
- Hamm, C.; Smetacek, V. Armor: Why, When, and How. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 311–332. [Google Scholar]
- Hallam, A. Mass extinctions in Phanerozoic Time. Geol. Soc. Lond. Spec. Publ. 2007, 140, 259. [Google Scholar] [CrossRef]
- Dale, B. The sedimentary record of dinoflagellate cysts: Looking back into the future of phytoplankton blooms. Sci. Mar. 2001, 65 (Suppl. S2), 257–272. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dale, B. Paleontological Evidence for Dinoflagellates and Ciliates as Early Eukaryotes. J. Mar. Sci. Eng. 2023, 11, 533. https://doi.org/10.3390/jmse11030533
Dale B. Paleontological Evidence for Dinoflagellates and Ciliates as Early Eukaryotes. Journal of Marine Science and Engineering. 2023; 11(3):533. https://doi.org/10.3390/jmse11030533
Chicago/Turabian StyleDale, Barrie. 2023. "Paleontological Evidence for Dinoflagellates and Ciliates as Early Eukaryotes" Journal of Marine Science and Engineering 11, no. 3: 533. https://doi.org/10.3390/jmse11030533
APA StyleDale, B. (2023). Paleontological Evidence for Dinoflagellates and Ciliates as Early Eukaryotes. Journal of Marine Science and Engineering, 11(3), 533. https://doi.org/10.3390/jmse11030533




