Phytoplankton Seasonal Dynamics under Conditions of Climate Change and Anthropogenic Pollution in the Western Coastal Waters of the Black Sea (Sevastopol Region)
Abstract
1. Introduction
2. Materials and Methods
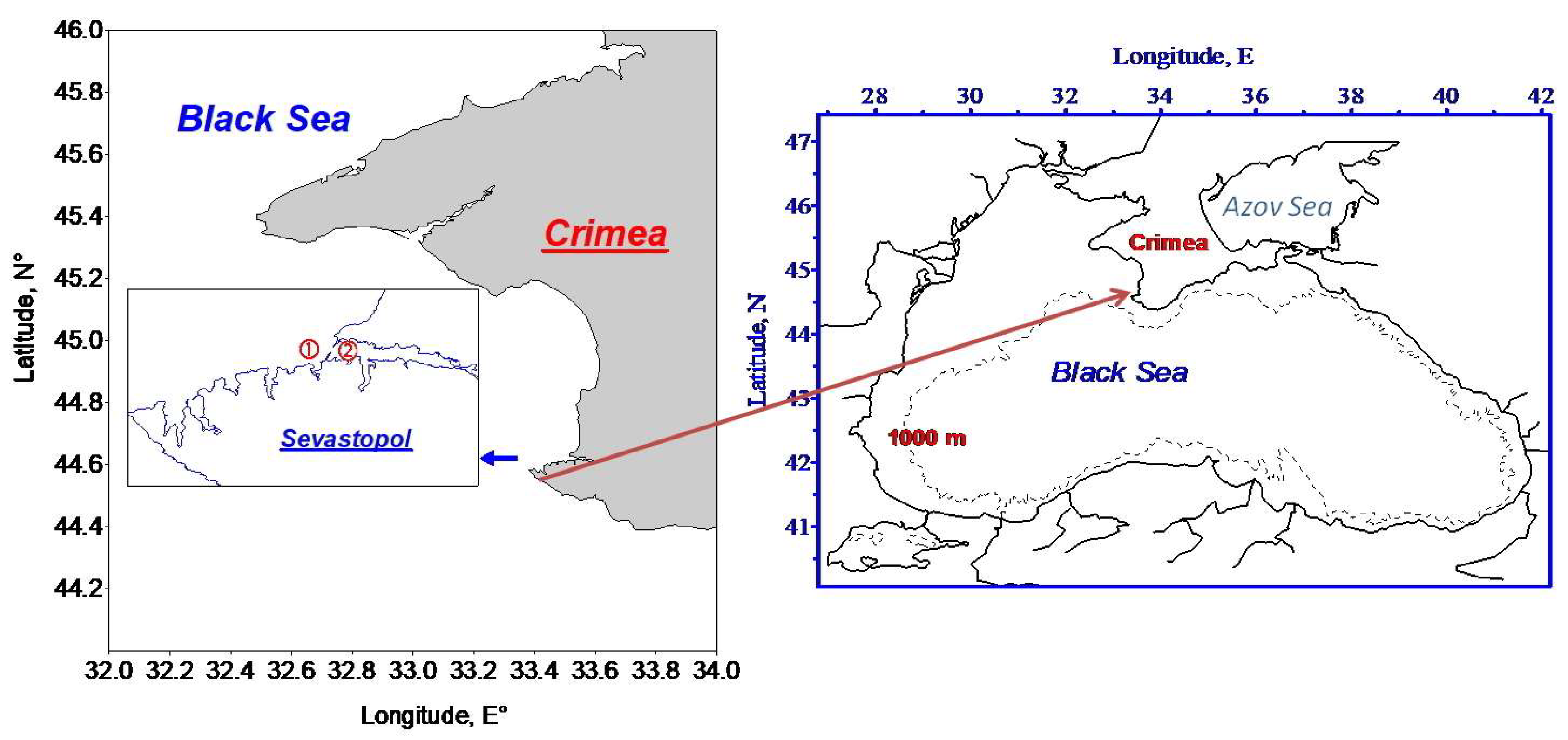
2.1. Study Area and Processing
2.2. Data Analysis
3. Results
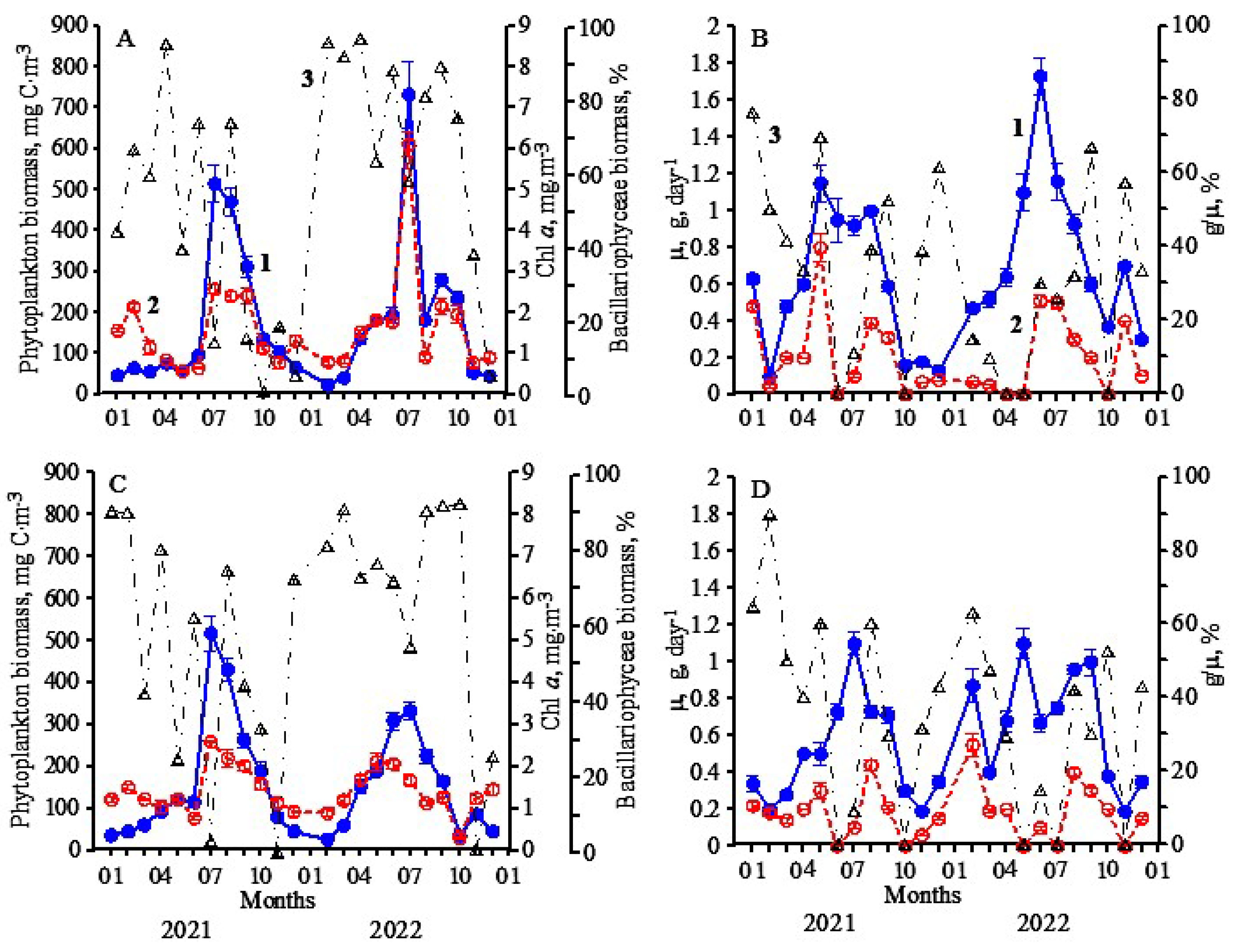
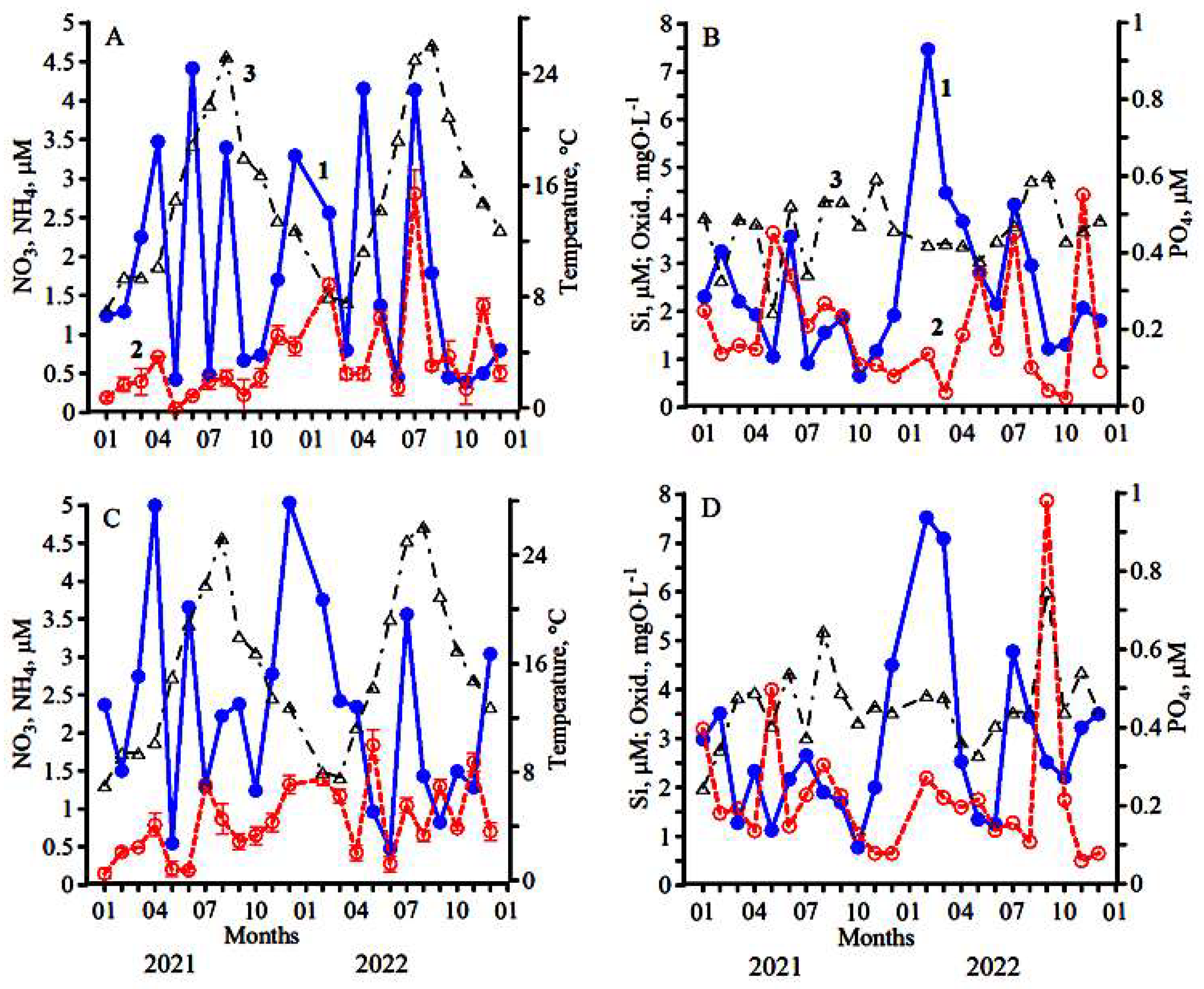
3.1. Research Conducted during 2021
3.2. Research Conducted during 2022
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P.G. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Cebrián, J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996, 41, 1758–1766. [Google Scholar] [CrossRef]
- Bologa, A.S.; Frangopol, P.T.; Vedernikov, V.I.; Stelmakh, L.V.; Yunev, O.A.; Yilmaz, A.; Oguz, T. Distribution of Planktonic Primary Production in the Black Sea. In Environmental Degradation of the Black Sea: Challenges and Remedies; Besiktepe, S.T., Unluata, U., Bologa, A.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 131–145. [Google Scholar]
- Senicheva, M.I. Species Diversity, Seasonal and Interannual Variability of Microalgae in Plankton off the Coast of Crimea. In the Black Sea microalgae: Problems of Biodiversity Preservation and Biotechnological Usage; Tokarev, Y.N., Finenko, Z.Z., Shadrin, N.V., Eds.; ECOSI-Gidrofizika: Sevastopol, Russia, 2008; pp. 5–17. [Google Scholar]
- Stelmakh, L.V.; Georgieva, E.Y. Microzooplankton: The trophic role and involvement in the phytoplankton loss and bloom formation in the Black Sea. Turk. J. Fish. Aquat. Sci. 2014, 14, 955–964. [Google Scholar] [CrossRef]
- Finenko, Z.Z.; Stelmakh, L.V.; Mansurova, I.M.; Georgieva, E.Y.; Tsilinsky, V.S. Seasonal dynamics of structural and functional indicators of phytoplankton community in Sevastopol Bay. Environ. Control. Syst. 2017, 9, 73–82. [Google Scholar]
- Stelmakh, L.; Kovrigina, N. Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region). Water 2021, 13, 3230. [Google Scholar] [CrossRef]
- Repetin, L.N. Spatial and temporal variability of the temperature regime of the Black Sea coastal zone. Ecol. Saf. Coast. Shelf Zones Integr. Use Shelf Resour. 2012, 26, 99–116. [Google Scholar]
- Stelmakh, L.; Kovrigina, N.; Gorbunova, T. Phytoplankton adaptation strategies under the influence of climatic changes and anthropogenic pressure on the Black Sea coastal ecosystems on the example Sevastopol Bay. Ecol. Montenegrina 2020, 37, 34–42. [Google Scholar] [CrossRef]
- Orekhova, N.A.; Varenik, A.V. Current Hydrochemical Regime of the Sevastopol Bay. Phys. Oceanogr. 2018, 25, 124–135. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Academic Press: New York, NY, USA, 1997; p. 858. [Google Scholar]
- Landry, M.R.; Hassett, R.P. Estimating the Grazing Impact of Marine Micro-Zooplankton. Mar. Biol. 1982, 67, 283–288. [Google Scholar] [CrossRef]
- JGOFS Protocols. Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurement; Manual and Guides; UNESCO: Paris, France, 1994; p. 170. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany, 1976; p. 317. [Google Scholar]
- Skopintsev, B.A. Formation of the Modern Chemical Composition of the Black Sea Waters; Gidrometeoizdat: Leningrad, Russia, 1975; p. 33. [Google Scholar]
- Sarno, D.; Kooistra, W.H.C.F.; Balzano, S.; Hargraves, P.E.; Zignone, A. Diversity in the genus Skeletonema (Bacillariophyceae): III. Phylogenetic position and morphological variability of Skeletonema costatum and Skeletonema grevillei, with the description of Skeletonema ardens sp. Nov. J. Phycol. 2007, 43, 156–170. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, X.; Sun, J.; Luo, Z. Diversity and Seasonal Occurrence of Skeletonema(Bacillariophyta) Species in Xiamen Harbour and Surrounding Seas, China. Cryptogam. Algologie. 2012, 33, 245–263. [Google Scholar] [CrossRef]
- Shevchenko, O.G.; Ponomareva, A.A.; Shulgina, M.A.; Orlova, T.Y. Phytoplankton in the Coastal Waters of Russky Island, Peter the Great Bay, Sea of Japan. Bot. Pac. A J. Plant Sci. Conserv. 2019, 8, 133–141. [Google Scholar] [CrossRef]
- Yunev, O.A.; Carstensen, J.; Stelmakh, L.V.; Belokopytov, V.N.; Suslin, V.V. Reconsideration of the phytoplankton seasonality in the open Black Sea. Limnol. Oceanogr. Lett. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Kubryakov, A.A.; Silkin, V.A.; Pautova, L.A.; Chasovnikov, V.K. Regional climate and patterns of phytoplankton annual succession in the open waters of the Black Sea. Deep Sea Res. Part I 2018, 142, 44–57. [Google Scholar] [CrossRef]
- Oguz, T.; Gilbert, D. Abrupt transitions of the top-down controlled Black Sea pelagic ecosystem during 1960–2000: Evidence for regime-shifts under strong fishery exploitation and nutrient enrichment modulated by climate-induced variations. Deep Sea Res. Part I 2007, 54, 220–242. [Google Scholar] [CrossRef]
- Hã Der, D.-P.; Gao, K. Interactions of anthropogenic stress factors on marine phytoplankton. Front. Environ. Sci. 2015, 3, 1–14. [Google Scholar] [CrossRef]
- Finenko, Z.Z.; Krupatkina, D.K. Primary production in the Black Sea in the winter-spring period. Oceanology 1993, 33, 94–104. [Google Scholar]
- Yunev, O.; Carstensen, J.; Stelmakh, L.; Belokopytov, V.; Suslin, V. Temporal changes of phytoplankton biomass in the western Black Sea shelf waters: Evaluation by satellite data (1998–2018). Estuarine. Coast. Shelf Sci. 2022, 271, 107865. [Google Scholar] [CrossRef]
- Sovga, E.E.; Mezenceva, I.V.; Hmara, T.V.; Slepchuk, K.A. On the prospects and possibilities of assessing the self-cleaning ability of the water area of Sevastopol Bay. Environ. Saf. Coast. Shelf Zones Integr. Use Shelf Resour. 2014, 28, 153–164. (In Russian) [Google Scholar]
- Kuftarkova, E.A.; Kovrigina, N.P.; Rodionova, N.Y.; Bobko, N.I. Hydrochemical Characteristics of the Coastal Waters of the Crimean Peninsula. In Mariculture of Mussels in the Black Sea; Ivanov, V.A., Ed.; ECOSI-Hydrophysica: Sevastopol, Russia, 2007; pp. 74–93. [Google Scholar]
- Stelmakh, L.; Kovrigina, N.; Gorbunova, T. Response of marine microalgae Phaeodactylum tricornutum, Prorocentrum cordatum and Gyrodinium fissum to complex pollution of Sevastopol bays (Black Sea). Ecol. Montenegrina 2021, 48, 109–116. [Google Scholar] [CrossRef]
- Stelmakh, L.V.; Mansurova, I.M. Functional state of cultures of marine microalgae as an indicator of the level of pollution of the waters of the Sevastopol Bay. Monit. Syst. Environ. 2021, 4, 83–90. [Google Scholar] [CrossRef]
- Akimov, A.I.; Solomonova, E.S. Characteristics of Growth and Fluorescence of Certain Types of Algae during Acclimation to Different Temperatures under Culture Conditions. Oceanology 2019, 59, 316–326. [Google Scholar] [CrossRef]
- Silkin, V.; Fedorov, A.; Flynn, K.J.; Paramonov, L.; Pautova, L. Protoplasmic streaming of chloroplasts enables rapid photoacclimation in large diatoms. J. Plankton Res. 2021, 43, 831–845. [Google Scholar] [CrossRef]
- Finkel, Z.V. Light absorption and size scaling of light-limited metabolism in marine diatoms. Limnol. Oceanogr. 2001, 46, 86–94. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Irwin, A.J. Modeling Size-dependent Photosynthesis: Light Absorption and the Allometric Rule. J. Theor. Biol. 2000, 204, 361–369. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Bouman, H.A.; Platt, T.; Doblin, M.; Figueiras, F.G.; Gudmundsson, K.; Gudfinnsson, H.G.; Huang, B.; Hickman, A. Photosynthesis–irradiance parameters of marine phytoplankton: Synthesis of a global data set. Earth Syst. Sci. Data 2018, 10, 251–266. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Heterotrophic dinoflagellates: A significant part of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Progr. Ser. 2007, 352, 187–197. [Google Scholar] [CrossRef]
- Putland, J.N.; Iverson, R.L. Microzooplankton: Major herbivores in an estuarine planktonic food web. Mar. Ecol. Prog. Ser. 2007, 345, 63–73. [Google Scholar] [CrossRef]
- Goldenberg, S.U.; Taucher, J.; Fernandez-Méndez, M.; Ludwig, A.; Aristegui, J.; Baumann, M.; Ortiz, J.; Stuhr, A.; Riebesell, U. Nutrient composition (Si: N) as driver of plankton communities during artificial upwelling. Front. Mar. Sci. 2022, 9, 1015188. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Giordano, M.; Chasovnikov, V.K.; Vostokov, S.V.; Podymov, O.I.; Pakhomova, S.V.; Moskalenko, L.V. Drivers of phytoplankton blooms in the northeastern Black Sea. Mar. Pollut. Bull. 2018, 138, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, M.A. The Si:C:N ratio of marine diatoms: Interspecific variability and the effect of some environmental variables. J. Phycol. 1985, 21, 347–357. [Google Scholar] [CrossRef]
- Choudhury, A.K.; Bhadury, P. Relationship between N:P: Si ratio and phytoplankton community composition in a tropical estuarine mangrove ecosystem. Biogeosciences Discuss. 2015, 12, 2307–2355. [Google Scholar] [CrossRef]
- Stelmakh, L.V. Features of the Structural and Functional Characteristics of the Diatom Alga Pseudosolenia calcar-avis. Inland Water Biol. 2022, 15, 315–323. [Google Scholar] [CrossRef]
- Raven, J.A. The role of vacuoles. New Phytol. 1987, 106, 357–422. [Google Scholar] [CrossRef]
- Richardson, T.L.; Ciotti, A.M.; Cullen, J.J. Physiological and optical properties of rhizosolenia formosa (bacillariophyceae) in the context of open-ocean vertical migration. J. Phycol. 1996, 32, 741–757. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Han, M.-S.; Kikuchi, T. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of nutrients. Harmful Algae 2008, 7, 729–739. [Google Scholar] [CrossRef]
- Barton, A.D.; Ward, B.A.; Williams, R.G.; Follows, M.J. The impact of fine-scale turbulence on phytoplankton community structure. Limnol. Oceanogr. Fluids Environ. 2014, 4, 34–49. [Google Scholar] [CrossRef]
- Anderson, E.E.; Wilson, C.; Knap, A.H.; Villareal, T.A. Summer diatom blooms in the eastern North Pacific gyre investigated with a long-endurance autonomous surface vehicle. PeerJ 2018, 6, e5387. [Google Scholar] [CrossRef]
- Villareal, T.A.; Woods, S.; Moore, J.K.; Culver-Rymsza, K. Vertical migration of Rhizosolenia mats and their significance to NO3 −fluxes in the central North Pacific gyre. J. Plankton Res. 1996, 18, 1103–1121. [Google Scholar] [CrossRef]
- Gemmell, B.J.; Oh, G.; Buskey, E.J.; Villareal, T.A. Dynamic sinking behaviour in marine phytoplankton: Rapid changes in buoyancy may aid in nutrient uptake. Proc. R. Soc. B. 2016, 283, 20161126. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Skibinski, D.O.F.; Lindemann, C. Effects of growth rate, cell size, motion, and elemental stoichiometry on nutrient transport kinetics. PLoS Comput. Biol. 2018, 14, e1006118. [Google Scholar] [CrossRef] [PubMed]
- Kiørboe, T.A. Mechanistic Approach to Plankton Ecology; Princeton University Press: Princeton, NJ, USA, 2008; p. 224. [Google Scholar]
- Kiørboe, T. How zooplankton feed: Mechanisms, traits and tradeoffs. Biol. Rev. Cam. Phil. Soc. 2011, 86, 311–339. [Google Scholar] [CrossRef]
- Ward, B.A.; Dutkiewicz, S.; Jahn, O.; Follows, M.J. A size-structured food-web model for the global ocean. Limnol. Oceanogr. 2012, 57, 1877–1891. [Google Scholar] [CrossRef]
- Wirtz, K.W. Who is eating whom? Morphology and feeding type determine the size relation between planktonic predators and their ideal prey. Mar. Ecol. Prog. Ser. 2012, 445, 1–12. [Google Scholar] [CrossRef]
- Wirtz, K.W.; Sommer, U. Mechanistic origins of variability in phytoplankton dynamics. Part II: Analysis of mesocosm blooms under climate change scenarios. Mar. Biol. 2013, 160, 2503–2516. [Google Scholar] [CrossRef]
- Saito, H.; Ota, T.; Suzuki, K.; Nishioka, J.; Tsuda, A. Role of heterotrophic dinoflagellate Gyrodinium sp. in the fate of an iron induced diatom bloom. Geophys. Res. Lett. 2006, 33, 1–4. [Google Scholar] [CrossRef]
- McBeain, K.H.; Halsey, K.A. Altering phytoplankton growth rates changes their value as food for microzooplankton grazers. Aquat. Microb. Ecol. 2018, 82, 19–29. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Halsey, K.H.; Boss, E.; Karp-Boss, L.; Milligan, A.J.; Peers, G. Thoughts on the evolution and ecological niche of diatoms. Ecol. Monogr. 2021, 91, e01457. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Halsey, K.H. Phytoplankton community structuring and succession in a competition-neutral resource landscape. ISME Commun. 2021, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Kimmance, S.A.; Clark, D.R.; Mitra, A.; Polimene, L.; Wilson, W.H. Modelling the Effects of Traits and Abiotic Factors on Viral Lysis in Phytoplankton. Front. Mar. Sci. 2021, 8, 667184. [Google Scholar] [CrossRef]
- Schmoker, C.; Hernandes-Leon, S.; Calbet, A. Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 2013, 35, 691–706. [Google Scholar] [CrossRef]
- Landry, M.R.; Karen, E.; Selph, M.D.; Décima, M.; Gutiérrez-Rodríguez, A.; Stukel, M.R.; Taylor, A.G.; Pasulka, A.L. Phytoplankton production and grazing balances in the Costa Rica Dome. J. Plankton Res. 2015, 38, 366–379. [Google Scholar] [CrossRef] [PubMed]
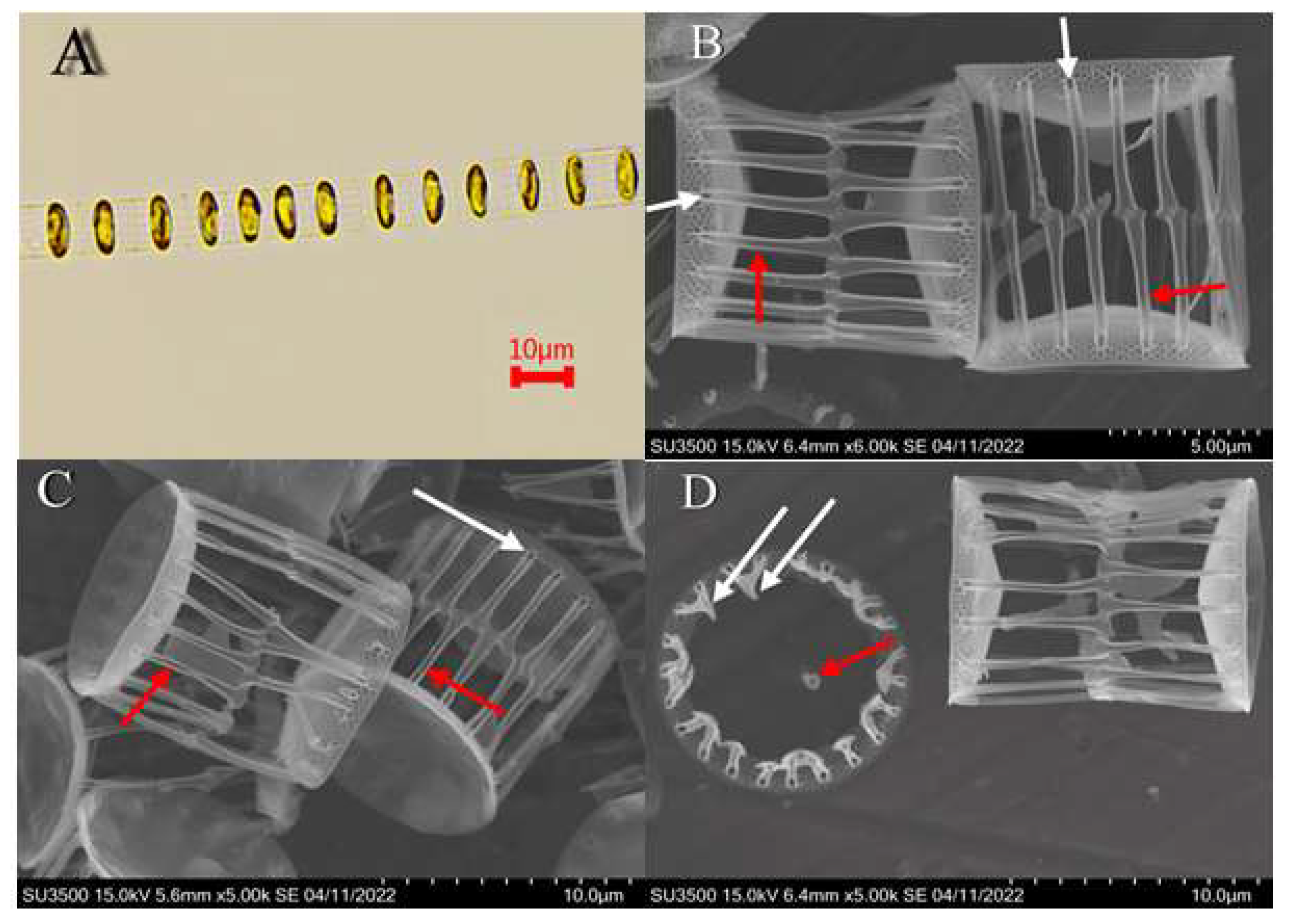
| Month | Bacillariophyceae | Dinophyceae |
|---|---|---|
| January | Skeletonema costatum (Greville) Cleve, Chaetoceros subtilis Cleve, Dactyliosolen fragilissimus (Bergon) Hasle, Coscinodiscus granii Gough | Scrippsiella trochoidea (F. Stein) A.R. Loeblich III, Gymnodinium wulffii J. Schiller, Prorocentrum micans Ehrenberg |
| February | S. costatum, Proboscia alata (Brightwell) Sundström, Pseudo-nitzschia sp. | Prorocentrum cordatum (Ostenfeld) J.D. Dodge, P. micans, Gymnodinium sp., S. trochoidea |
| March | P. alata, S. costatum, Pseudo-nitzchia sp., | P. micans, Tripos furca (Ehrenberg) F. Gómez, Diplopsalis lenticula Bergh |
| April | P. alata, Pseudo-nitzschia sp., Chaetoceros affinis Lauder, Chaetoceros curvisetus Cleve, D. fragilissimus | P. micans, P. cordatum, Prorocentrum compressum (Bailey) T.H.Abé ex J.D.Dodge |
| May | Pseudosolenia calcar-avis (Schultze) B.G. Sundström, P. alata, C. affinis | P. micans, P. cordatum, D. lenticula, Gymnodinium variabile E.C.Herdman, Gymnodinium sp., Gyrodiniym pingue (F.Schütt) Kofoid & Swezy |
| June | C. affinis, Chaetoceros peruvianus Brightwell, C. curvisetus, C. pelagica | Gymnodinium wulffii J.Schiller, Gymnodinium sp., P. micans |
| July | D. fragilissimus, Cyclotella caspia Grunow, C. affinis, Thalassionema nitzschioides (Grunow) | Gymnodinium simplex (Lohmann) Kofoid & Swezy, P. micans, P. cordatum, P. compressum, G.variabile, Akashiwo sanquinea (K.Hirasaka) Gert Hansen & Moestrup |
| August | P. calcar-avis, P. alata, C. caspia | S. trochoidea P. cordatum, T. furca, Gymnodinium variabile E.C.Herdman, G. simplex |
| September | P. calcar-avis, P. alata, Cerataulina pelagica (Cleve) Hendey | P. cordatum, P. micans, Procentrum nanum J. Schiller, D. lenticula |
| October | P. alata, D. fragilissimus, Chaetoceros compressus Lauder | P. cordatum, P. micans, T. furca |
| November | C. affinis, Cerataulina pelagica (Cleve) Hendey, Hemiaulus hauckii Grunow ex Van Heurck, P. calcar-avis | Tripos fusus (Ehrenberg) F.Gómez, P. cordatum, P. micans |
| December | S. costatum, T. nitzschioides, Cylindroteca closterium (Ehrenberg) Reimann & J.C. Lewin, C. peruvianus | P. micans, P. cordatum Procentrum lima (Ehrenberg) F.Stein |
| Month | Bacillariophyceae | Dinophyceae |
|---|---|---|
| February | S. costatum, Entomoneis paludosa (W.Smith) Reimer, P. calcar-avis, Thalassiosira parva Proschkina-Lavrenko | P. compressum, P. cordatum, S. trochoidea |
| March | S. costatum, C. curvisetus Licmophora gracilis (Ehrenberg) Grunow, Licmophora flabellata (Greville), C.Agardh, Licmophora abbreviata C.Agardh, C. closterium | S. trochoidea, D. lenticula, Gymnodinium sp., |
| April | Chaetoceros socialis H.S.Lauder, Chaetoceros simplex Ostenfeld, Chaetoceros paulsenii Ostenfeld, Chaetoceros wighamii Brightwell, L. flabellata | S. trochoidea, D. lenticula, Ceratium tripos (O.F.Müller) Nitzsch, Prorocentrum maximum (Gourret) Schiller |
| May | P. calcar-avis, P. alata, C. affinis, C. closterium | P. micans, P. cordatum, D. lenticula, Gymnodinium variabile E.C.Herdman, Gymnodinium sp., Gyrodiniym pingue (F.Schütt) Kofoid & Swezy |
| June | C. affinis, Chaetoceros peruvianus Brightwell, C. curvisetus, C. pelagica | Gymnodinium wulffii J.Schiller, Gymnodinium sp., P. micans |
| July | P. calcar-avis, P. alata, L. flabellata, Halamphora hyalina (Kützing) Rimet & R.Jahn | Glenodinium paululum Lindemann, P. micans, P. cordatum, P. compressum, G.variabile, G. simplex |
| August | P. calcar-avis, C. caspia, Thalassiosira excentrica (Ehrenberg) Cleve, Pseudo-nitzchia sp., C. affinis, C. closterium | Gyrodinium fusiforme Kofoid & Swezy, P. micans |
| September | Pseudo-nitzchia sp., P. calcar-avis, Chaetoceros insignis Proschkina-Lavrenko, H. hauckii, Chaetoceros socialis H.S.Lauder, S. costatum, C. compressus | S. trochoidea,P. cordatum, P. micans, D. lenticula |
| October | H. hauckii, P. calcar-avis, C. pelagica, S. costatum, C. affinis, | P. cordatum, P. compressum |
| November | C. compressus, C. pelagica | Ceratium tripos (O.F.Müller) Nitzsch, T. furca, T. fusus, P. cordatum, P. micans, Protoceratium reticulatum (Claparède & Lachmann) Bütschli |
| December | T. nitzschioides, C. closterium, C. pelagica | P. cordatum, P. micans, T. furca, T. fusus |
| Parameters | Winter (December–February) | Spring (March–May) | Summer (June–August) | Autumn (September–November) |
|---|---|---|---|---|
| Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | |
| B, mg C·m−3 | 45 ± 4 (36–66) | 102 ± 15 (41–190) | 343 ± 55 (96–732) | 163 ± 27 (36–312) |
| Chl a, mg· m−3 | 1.21 ± 0.11 (0.90–2.13) | 1.26 ± 0.13 (0.56–2.11) | 2.06 ± 0.42 (0.64–2.58) | 1.47 ± 0.17 (0.30–2.40) |
| µ, day−1 | 0.37 ± 0.07 (0.10–0.63) | 0.62 ± 0.10 (0.28–1.15) | 0.94 ± 0.11 (0.67–1.73) | 0.41 ± 0.08 (0.16–1.00) |
| g, day−1 | 0.20 ± 0.04 (0.05–0.48) | 0.18 ± 0.03 (0–0.80) | 0.24 ± 0.10 (0–0.50) | 0.11 ± 0.02 (0–0.40) |
| µ/g, % | 54 ± 7 (33–90) | 36 ± 9 (0–70) | 22 ± 6 (0–60) | 29 ± 7 (0–67) |
| BBacil., % | 63 ± 11 (6–96) | 70 ± 7 (40–97) | 63 ± 9 (15-91) | 42 ± 11 (2–93) |
| NO3, µM | 2.49 ± 0.29 (0.80–3.29) | 2.21 ± 0.16 (0.42–5.00) | 2.28 ± 0.43 (0.45–4.42) | 1.29 ± 0.30 (0.40–2.78) |
| NH4, µM | 0.75 ± 0.15 (0.19–1.64) | 0.69 ± 0.11 (0.05–1.84) | 0.76 ± 0.21 (0.32–2.82) | 0.81 ± 0.16 (0.22–1.62) |
| PO4, µM | 0.17 ± 0.02 (0.08–0.25) | 0.23 ± 0.03 (0.03–0.50) | 0.22 ± 0.03 (0.10–0.45) | 0.23 ± 0.02 (0.02–0.55) |
| Si, µM | 3.88 ± 0.60 (1.92–7.47) | 2.67 ± 0.50 (1.06–7.09) | 2.63 ± 0.35 (0.92–4.80) | 1.72 ± 0.30 (0.65–3.22) |
| N/P | 29 ± 9 (7–83) | 19 ± 4 (1–44) | 16 ± 3 (5–30) | 20 ± 5 (4–47) |
| Si/N | 1.25 ± 0.20 (1.00–1.84) | 1.42 ± 0.40 (0.68–4.29) | 0.68 ± 0.07 (0.39–0.92) | 0.71 ± 0.19 (0.41–1.84)) |
| Oxidizability, mg O·L−1 | 3.33 ± 0.19 (1.98–3.96) | 3.34 ± 0.19 (1.98–3.95) | 3.85 ± 0.21 (2.78–5.19) | 4.14 ± 0.24 (3.34–6.01) |
| T, °C | 10.0 ± 0.8 (7.0–12.8) | 11.3 ± 0.9 (8.0–15.1)) | 22.8 ± 1.0 (18.9–26.1) | 16.6 ± 0.8 (13.5–21.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmakh, L.; Kovrigina, N.; Gorbunova, T. Phytoplankton Seasonal Dynamics under Conditions of Climate Change and Anthropogenic Pollution in the Western Coastal Waters of the Black Sea (Sevastopol Region). J. Mar. Sci. Eng. 2023, 11, 569. https://doi.org/10.3390/jmse11030569
Stelmakh L, Kovrigina N, Gorbunova T. Phytoplankton Seasonal Dynamics under Conditions of Climate Change and Anthropogenic Pollution in the Western Coastal Waters of the Black Sea (Sevastopol Region). Journal of Marine Science and Engineering. 2023; 11(3):569. https://doi.org/10.3390/jmse11030569
Chicago/Turabian StyleStelmakh, Lyudmyla, Nelya Kovrigina, and Tatiana Gorbunova. 2023. "Phytoplankton Seasonal Dynamics under Conditions of Climate Change and Anthropogenic Pollution in the Western Coastal Waters of the Black Sea (Sevastopol Region)" Journal of Marine Science and Engineering 11, no. 3: 569. https://doi.org/10.3390/jmse11030569
APA StyleStelmakh, L., Kovrigina, N., & Gorbunova, T. (2023). Phytoplankton Seasonal Dynamics under Conditions of Climate Change and Anthropogenic Pollution in the Western Coastal Waters of the Black Sea (Sevastopol Region). Journal of Marine Science and Engineering, 11(3), 569. https://doi.org/10.3390/jmse11030569






