Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine
Abstract
:1. Introduction
2. Materials and Methods
2.1. CFD Model
- -
- Mesh 1: number of elements from 26,000 at TDC to 375,000 at BDC.
- -
- Mesh 2: number of elements from 32,000 at TDC to 450,000 at BDC.
- -
- Mesh 3: number of elements from 39,000 at TDC to 540,000 at BDC.
2.2. MCDM Model
- 1-
- Establishment of the decision matrix
- 2-
- Establishment of the weights
- 3-
- Normalization
- 4-
- Calculation of the most appropriate option
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Witkowski, J.K. Research of the effectiveness of selected methods of reducing toxic exhaust emissions of marine diesel engines. J. Mar. Sci. Eng. 2020, 8, 452. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Liang, X.; Liu, H.; Chen, Y. Research of NOx reduction on a low-speed two-stroke marine heavy-fuel oil engine. J. Energy Eng. 2021, 147, 04020088. [Google Scholar] [CrossRef]
- Lu, X.; Geng, P.; Chen, Y. NOx emission reduction technology for marine engine based on Tier-III: A review. J. Therm. Sci. 2020, 29, 1242–1268. [Google Scholar] [CrossRef]
- Varbanets, R.; Fominb, O.; Píštěk, V.; Klymenko, V.; Minchev, D.; Khrulev, A.; Zalozh, V.; Kučera, P. Acoustic method for estimation of marine low-speed engine turbocharger parameters. J. Mar. Sci. Eng. 2021, 9, 321. [Google Scholar] [CrossRef]
- Píštěk, V.; Kučera, P.; Fomin, O.; Lovska, A. Effective mistuning identification method of integrated bladed discs of marine engine turbochargers. J. Mar. Sci. Eng. 2020, 8, 379. [Google Scholar] [CrossRef]
- Napolitano, P.; Liotta, L.F.; Guido, C.; Tornatore, C.; Pantaleo, G.; Parola, V.; Beatrice, C. Insights of selective catalytic reduction technology for nitrogen oxides control in marine engine applications. Catalysts 2022, 12, 1191. [Google Scholar] [CrossRef]
- Winnes, H.; Fridell, E.; Moldanová, J. Effects of marine exhaust gas scrubbers on gas and particle emissions. J. Mar. Sci. Eng. 2020, 8, 299. [Google Scholar] [CrossRef]
- Ryu, M.; Park, P. Analysis of composite scrubber with built-in silencer for marine engines. J. Mar. Sci. Eng. 2021, 9, 962. [Google Scholar] [CrossRef]
- Puškár, M.; Kopas, M.; Sabadka, D.; Kliment, M.; Šoltésová, M. Reduction of the gaseous emissions in the marine diesel engine using biodiesel mixtures. J. Mar. Sci. Eng. 2020, 8, 330. [Google Scholar] [CrossRef]
- Lehtoranta, K.; Koponen, P.; Vesala, H.; Kallinen, K.; Maunula, T. Performance and regeneration of methane oxidation catalyst for LNG ships. J. Mar. Sci. Eng. 2021, 9, 111. [Google Scholar] [CrossRef]
- Jablonický, J.; Feriancová, P.; Tulík, J.; Hujo, L.; Tkáč, Z.; Kuchar, P.; Tomić, M.; Kaszkowiak, J. Assessment of technical and ecological parameters of a diesel engine in the application of new samples of biofuels. J. Mar. Sci. Eng. 2021, 10, 1. [Google Scholar] [CrossRef]
- dos Santos, V.A.; Pereira da Silva, P.; Serrano, L.M.V. The maritime sector and its problematic decarbonization: A systematic review of the contribution of alternative fuels. Energies 2022, 15, 3571. [Google Scholar] [CrossRef]
- Puškár, M. Advanced system determined for utilisation of sustainable biofuels in high-performance sport applications. Sustainability 2022, 14, 6713. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G.; Telmo, J.; Rodriguez, J.D. Numerical analysis of emissions from marine engines using alternative fuels. Polish Marit. Res. 2015, 22, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Chiong, M.C.; Kang, H.S.; Shaharuddin, N.M.R.; Mat, S.; Quen, L.K.; Ten, K.H.; Ong, M.C. Challenges and opportunities of marine propulsion with alternative fuels. Renew. Sustain. Energy Rev. 2021, 149, 11397. [Google Scholar] [CrossRef]
- Perčić, M.; Vladimir, N.; Fan, A.; Jovanović, I. Holistic energy efficiency and environmental friendliness model for short-sea vessels with alternative power systems considering realistic fuel pathways and workloads. J. Mar. Sci. Eng. 2022, 10, 613. [Google Scholar] [CrossRef]
- Qiu, Y.; Wei, H.; Zhou, D.; Li, J. Effect of ammonia addition on the ignition delay mechanism of methyl decanoate. J. Mar. Sci. Eng. 2022, 10, 922. [Google Scholar] [CrossRef]
- Shi, R.; Chen, X.; Qin, J.; Wu, P.; Jia, L. The state-of-the-art progress on the forms and modes of hydrogen and ammonia energy utilization in road transportation. Sustainability 2022, 14, 11904. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Wu, Y.; Wang, Y. Study on marine engine combustion and emissions characteristics under multi-parameter coupling of ammonia-diesel stratified injection mode. Int. J. Hydrogen Energy 2022, 48, 9881–9894. [Google Scholar] [CrossRef]
- Hwang, I.; Park, C.; Jeong, B. Life cycle cost analysis for Scotland short-sea ferries. J. Mar. Sci. Eng. 2023, 11, 424. [Google Scholar] [CrossRef]
- Bertagna, S.; Kouznetsov, I.; Braidotti, L.; Marinò, A.; Bucci, V. A rational approach to the ecological transition in the cruise market: Technologies and design compromises for the fuel switch. J. Mar. Sci. Eng. 2023, 11, 67. [Google Scholar] [CrossRef]
- Reusser, C.A.; Pérez Osses, J.R. Challenges for zero-emissions ship. J. Mar. Sci. Eng. 2021, 9, 1042. [Google Scholar] [CrossRef]
- Mallouppas, G.; Yfantis, E.A. Decarbonization in shipping industry: A review of research, technology development, and innovation proposals. J. Mar. Sci. Eng. 2021, 9, 415. [Google Scholar] [CrossRef]
- Kuta, K.; Przybyła, G.; Kurzydym, D.; Żmudka, A. Experimental and numerical investigation of dual-fuel CI ammonia engine emissions and after-treatment with V2O5/SiO2–TiO2 SCR. Fuel 2023, 334, 126523. [Google Scholar] [CrossRef]
- Ejder, E.; Arslanoğlu, Y. Evaluation of ammonia fueled engine for a bulk carrier in marine decarbonization pathways. J. Clean. Prod. 2022, 379, 134688. [Google Scholar] [CrossRef]
- Lanni, D.; Galloni, E.; Fontana, G.; D’Antuono, G. Assessment of the operation of an SI engine fueled with ammonia. Energies 2022, 15, 8583. [Google Scholar] [CrossRef]
- Seddiek, I.S.; Ammar, N.R. Technical and eco-environmental analysis of blue/green ammonia-fueled RO/RO ships. Transp. Res. Part D Transp. Environ. 2023, 114, 103547. [Google Scholar] [CrossRef]
- Wu, S.; Miao, B.; Chan, S.H. Feasibility assessment of a container ship applying ammonia cracker-integrated solid oxide fuel cell technology. Int. J. Hydrogen Energy 2022, 47, 27166–27176. [Google Scholar] [CrossRef]
- Karvounis, P.; Dantas, J.L.D.; Tsoumpris, C.; Theotokatos, G. Ship power plant decarbonisation using hybrid systems and ammonia fuel—A techno-economic–environmental analysis. J. Mar. Sci. Eng. 2022, 10, 1675. [Google Scholar] [CrossRef]
- Toneatti, L.; Deluca, C.; Fraleoni Morgera, A.; Piller, M.; Pozzetto, D. Waste to energy onboard cruise ships: A new paradigm for sustainable cruising. J. Mar. Sci. Eng. 2022, 10, 480. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A preliminary study on an alternative ship propulsion system fueled by ammonia: Environmental and economic assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Fan, H.; Xu, X.; Liu, Z. Life cycle greenhouse gas emission assessment for using alternative marine fuels: A very large crude carrier (VLCC) case study. J. Mar. Sci. Eng. 2022, 10, 1969. [Google Scholar] [CrossRef]
- Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.D.; Abbas, A. Possibilities of ammonia as both fuel and NOx reductant in marine engines: A numerical study. J. Mar. Sci. Eng. 2022, 10, 43. [Google Scholar] [CrossRef]
- Rodriguez, C.G.; Lamas, M.I.; Rodriguez, J.D.; Abbas, A. Analysis of the pre-injection system of a marine diesel engine through multiple-criteria decision making and artificial neural networks. Polish Marit. Res. 2021, 28, 88–96. [Google Scholar] [CrossRef]
- Lamas Galdo, M.I.; Castro-Santos, L.; Rodríguez Vidal, C.G. Selection of an appropriate pre-injection pattern in a marine diesel engine through a multiple-criteria decision making approach. Appl. Sci. 2020, 10, 2482. [Google Scholar] [CrossRef] [Green Version]
- di Sarli, V.; Cammarota, F.; Salzano, E.; di Benedetto, A. Explosion behavior of ammonia and ammonia/methane in oxygen-enriched air. Process Saf. Prog. 2017, 36, 368–371. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G. NOx reduction in diesel-hydrogen engines using different strategies of ammonia injection. Energies 2019, 12, 1255. [Google Scholar] [CrossRef] [Green Version]
- Lamas, M.I.; Rodriguez, C.G. Numerical model to analyze NOx reduction by ammonia injection in diesel-hydrogen engines. Int. J. Hydrogen Energy 2017, 42, 26132–26141. [Google Scholar] [CrossRef]
- Lamas Galdo, M.I.; Castro-Santos, L.; Rodriguez Vidal, C.G. Numerical analysis of NOx reduction using ammonia injection and comparison with water injection. J. Mar. Sci. Eng. 2020, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Ra, Y.; Reitz, R.D. A reduced chemical kinetic model for IC engine combustion simulations with primary reference fuels. Combust. Flame 2008, 155, 713–738. [Google Scholar] [CrossRef]
- Yang, H.; Krishnan, S.R.; Srinivasan, K.K.; Midkiff, K.C. Modeling of NOx emissions using a super-extended Zel’dovich mechanism. In Proceedings of the ICEF03 2003 Fall Technical Conference of the ASME Internal Combustion Engine Division, Erie, PA, USA, 7–10 September 2003. [Google Scholar] [CrossRef]
- Miller, J.A.; Glarborg, P. Modeling the formation of N2O and NO2 in the thermal DeNOx process. Springer Ser. Chem. Phys. 1996, 61, 318–333. [Google Scholar]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef] [Green Version]
- Hulgaard, T.; Dam-Johansen, K. Homogeneous nitrous oxide formation and destruction under combustion conditions. React. Kinet. Catal. 1993, 39, 1342–1354. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, A.; Guo, Y.; Zhang, L.; Zhang, M.; Deng, X.; Li, J.; Ying, W. Auto-ignition delay characteristics of ammonia substitution on methane. Processes 2022, 10, 2214. [Google Scholar] [CrossRef]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.M.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Vinogradova, I.; Podvezko, V.; Zavadskas, E. The recalculation of the weights of criteria in MCDM methods using the Bayes approach. Symmetry 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Lamas, M.I.; Castro-Santos, L.; Rodriguez, C.G. Optimization of a multiple injection system in a marine diesel engine through a multiple-criteria decision-making approach. J. Mar. Sci. Eng. 2020, 8, 946. [Google Scholar] [CrossRef]
- Gürbüz, H.; Demirtürk, S.; Akçay, I.H.; Akçay, H. Effect of port injection of ethanol on engine performance, exhaust emissions and environmental factors in a dual-fuel diesel engine. Energy Environ. 2021, 32, 784–802. [Google Scholar] [CrossRef]
- Gürbüz, H.; Akçay, H.; Aldemir, M.; Akçay, I.H.; Topalcı, U. The effect of euro diesel-hydrogen dual fuel combustion on performance and environmental-economic indicators in a small UAV turbojet engine. Fuel 2021, 306, 121735. [Google Scholar] [CrossRef]
- Methodenhandbuch Zum Bundesverkehrswegeplan 2030; FE-Projekt-Nr.: 97.358/2015; Bundesministerium für Verkehr und Digitale Infrastruktur: Berlin, Germany, 2016; Available online: https://www.bi-nordzulauf-ko.de/wp-content/uploads/2019/09/bvwp-2030-methodenhandbuch.pdf (accessed on 10 February 2023).
- AEA Technology Environment. Damages per Tonne Emission of PM2.5, NH3, SO2, NOx and VOCs from Each EU25 Member State (excluding Cyprus) and Surrounding Seas; AEA Technology Environment: Didcot, UK.
- Rodriguez, C.G.; Lamas, M.I.; Rodriguez, J.D.; Caccia, C. Analysis of the pre-injection configuration in a marine engine through several MCDM techniques. Brodogradnja 2021, 72, 1–17. [Google Scholar] [CrossRef]
- Krishnan, A.R. Past efforts in determining suitable normalization methods for multi-criteria decision-making: A short survey. Front. Big Data 2022, 5, 990699. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Valeria-Medina, A.; Hatem, F.; Azad, A.; Dedoussi, I.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on ammonia as a potential fuel: From synthesis to economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]



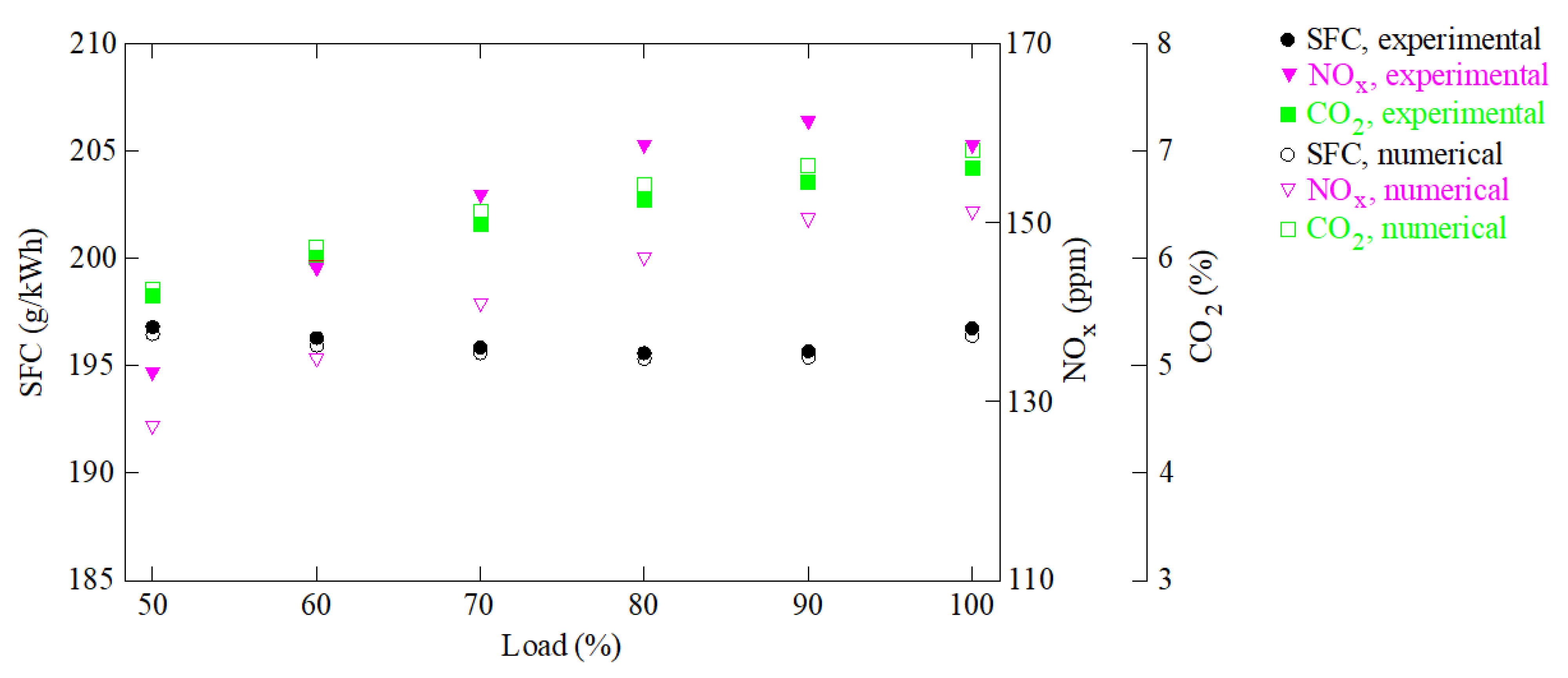

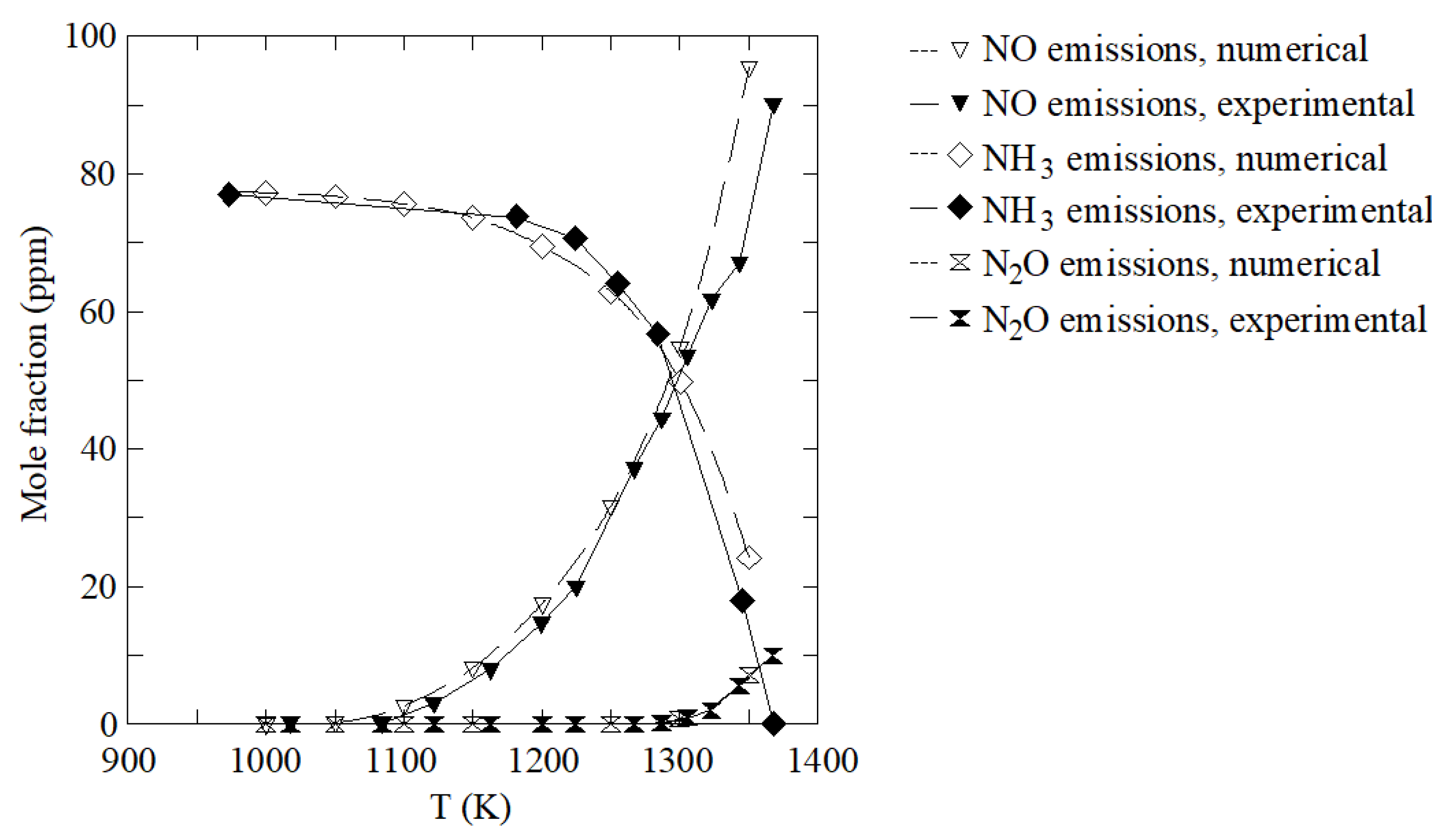
| Mesh | Time Step (s) | SFC Error (%) | CO2 Error (%) | NOx Error (%) |
|---|---|---|---|---|
| Mesh 1 | 1.1 × 10−5 | 3.9 | 4.3 | 6.1 |
| Mesh 2 | 1.5 × 10−5 | 3.8 | 4.2 | 5.9 |
| Mesh 2 | 1.1 × 10−5 | 3.8 | 4.1 | 5.8 |
| Mesh 2 | 0.8 × 10−5 | 3.8 | 4.1 | 5.8 |
| Mesh 3 | 1.1 × 10−5 | 3.8 | 4.1 | 5.7 |
| Case (i) | (%) | Criterion (j) | |||
|---|---|---|---|---|---|
| j = 1 CO2 (%) | j = 2 NOx (ppm) | j = 3 NH3 (ppm) | j = 4 N2O (ppm) | ||
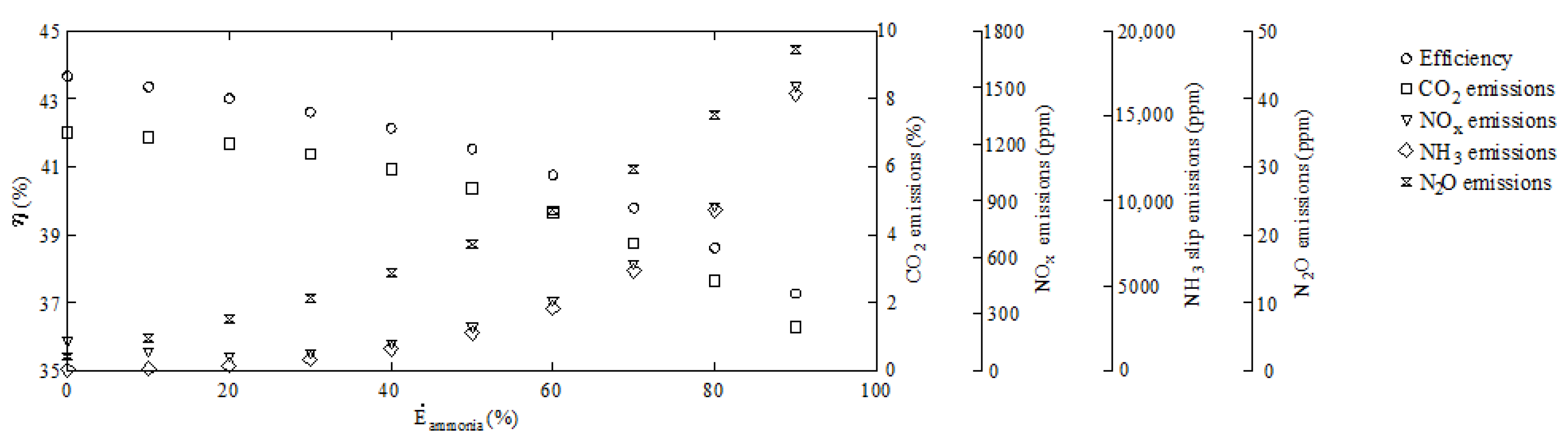
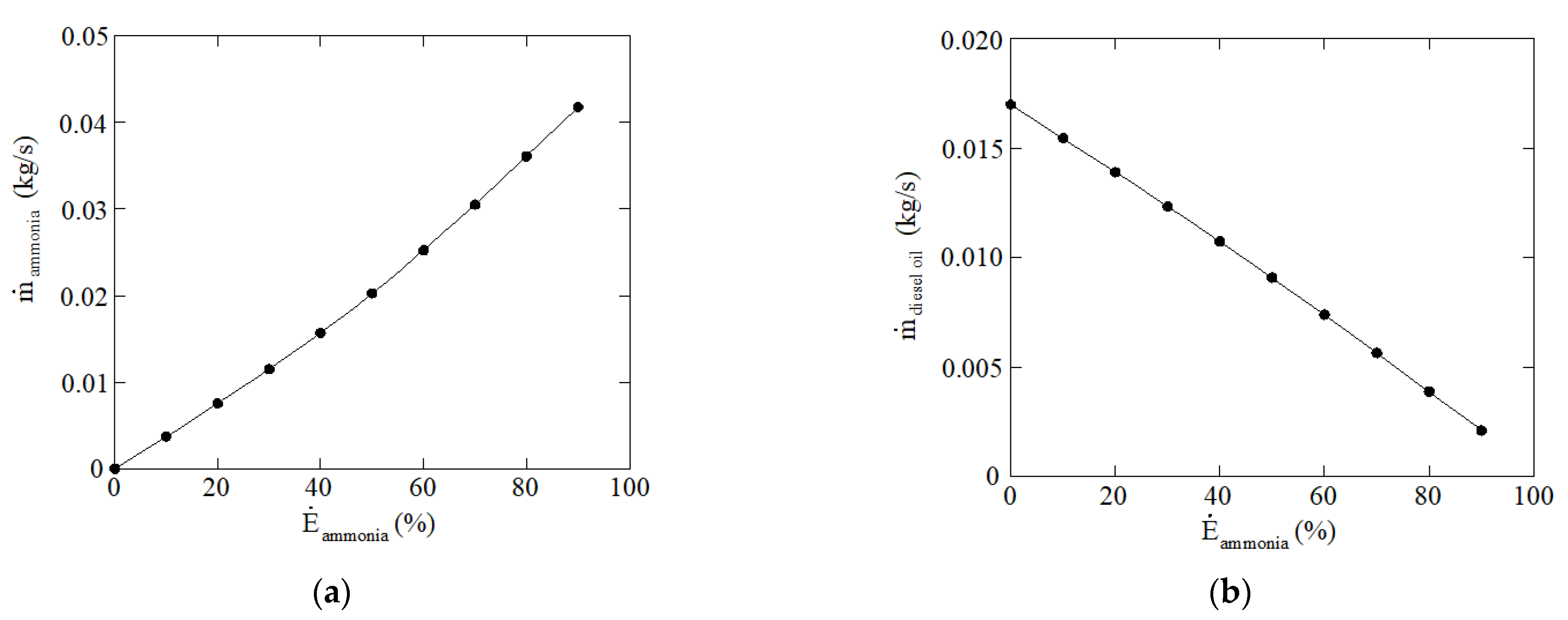
| 1 | 0 | 6.99 | 157.31 | 7.14 | 1.72 |
| 2 | 10 | 6.87 | 101.71 | 32.14 | 4.38 |
| 3 | 20 | 6.67 | 77.70 | 214.26 | 7.19 |
| 4 | 30 | 6.37 | 93.22 | 648.84 | 10.33 |
| 5 | 40 | 5.93 | 144.92 | 1262.68 | 13.95 |
| 6 | 50 | 5.37 | 234.44 | 2217.18 | 18.18 |
| 7 | 60 | 4.64 | 369.08 | 3677.33 | 23.30 |
| 8 | 70 | 3.74 | 564.71 | 5883.80 | 29.34 |
| 9 | 80 | 2.63 | 866.79 | 9426.51 | 37.28 |
| 10 | 90 | 1.28 | 1498.85 | 16,288.74 | 46.95 |
| Case (i) | (%) | Criterion (j) | |||
|---|---|---|---|---|---|
| j = 1 CO2 | j = 2 NOx | j = 3 NH3 | j = 4 N2O | ||
| 1 | 0 | 0.00 | 0.90 | 1.00 | 0.96 |
| 2 | 10 | 0.02 | 0.93 | 1.00 | 0.91 |
| 3 | 20 | 0.05 | 0.95 | 0.99 | 0.85 |
| 4 | 30 | 0.09 | 0.94 | 0.96 | 0.78 |
| 5 | 40 | 0.15 | 0.90 | 0.92 | 0.70 |
| 6 | 50 | 0.23 | 0.84 | 0.86 | 0.61 |
| 7 | 60 | 0.34 | 0.75 | 0.77 | 0.50 |
| 8 | 70 | 0.47 | 0.62 | 0.64 | 0.38 |
| 9 | 80 | 0.62 | 0.42 | 0.42 | 0.21 |
| 10 | 90 | 0.82 | 0.00 | 0.00 | 0.00 |
| Weights | Most Appropriate Alternative | |||
|---|---|---|---|---|
| CO2 | NOx | NH3 | N2O | (%) |
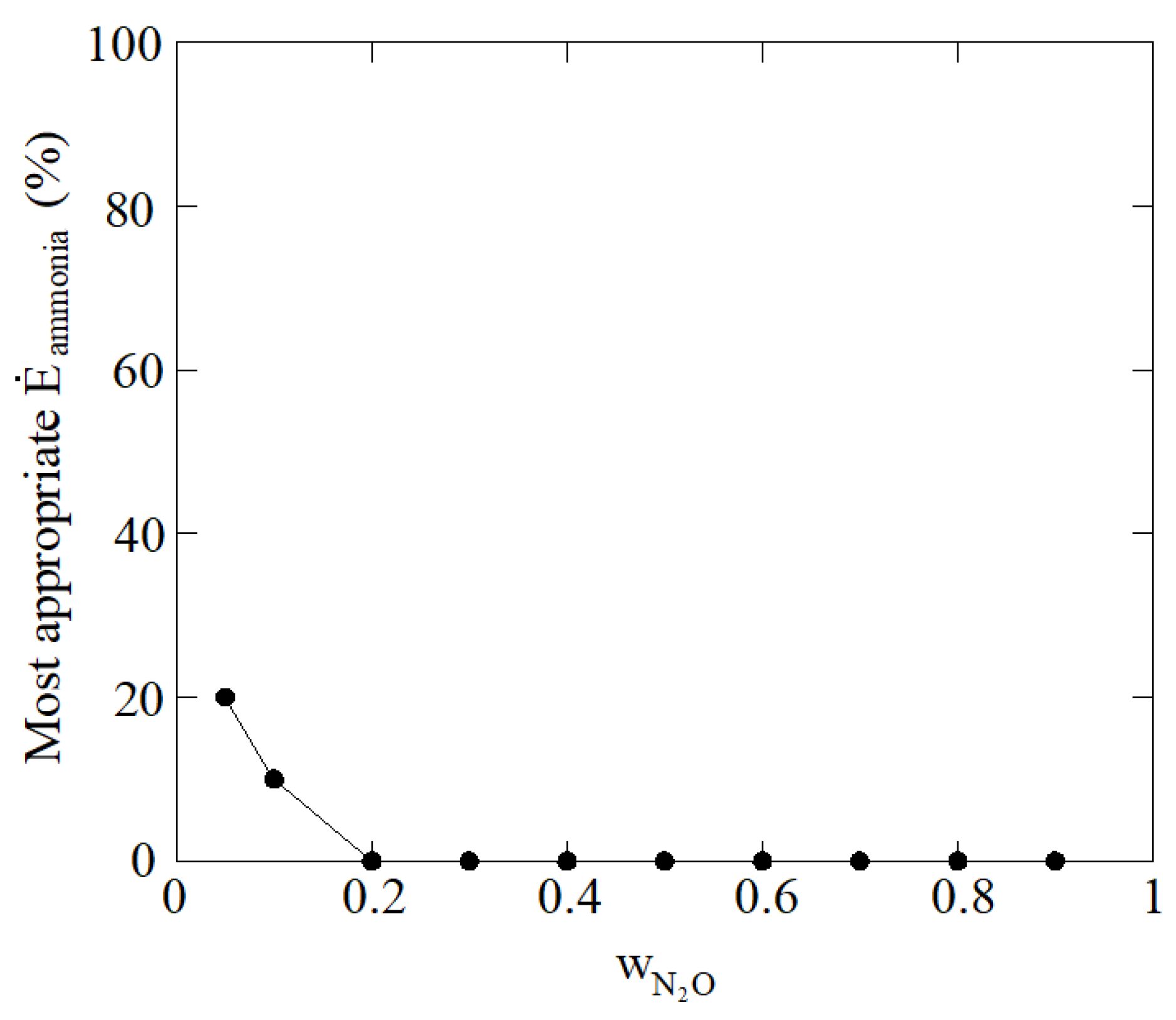
| 0.200 | 0.250 | 0.500 | 0.05 | 20 |
| 0.189 | 0.237 | 0.474 | 0.1 | 10 |
| 0.168 | 0.210 | 0.421 | 0.2 | 0 |
| 0.147 | 0.184 | 0.368 | 0.3 | 0 |
| 0.126 | 0.158 | 0.316 | 0.4 | 0 |
| 0.105 | 0.131 | 0.263 | 0.5 | 0 |
| 0.084 | 0.105 | 0.210 | 0.6 | 0 |
| 0.063 | 0.079 | 0.158 | 0.7 | 0 |
| 0.042 | 0.053 | 0.105 | 0.8 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.d.D.; Abbas, A. Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine. J. Mar. Sci. Eng. 2023, 11, 689. https://doi.org/10.3390/jmse11040689
Rodríguez CG, Lamas MI, Rodríguez JdD, Abbas A. Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine. Journal of Marine Science and Engineering. 2023; 11(4):689. https://doi.org/10.3390/jmse11040689
Chicago/Turabian StyleRodríguez, Carlos Gervasio, María Isabel Lamas, Juan de Dios Rodríguez, and Amr Abbas. 2023. "Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine" Journal of Marine Science and Engineering 11, no. 4: 689. https://doi.org/10.3390/jmse11040689
APA StyleRodríguez, C. G., Lamas, M. I., Rodríguez, J. d. D., & Abbas, A. (2023). Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine. Journal of Marine Science and Engineering, 11(4), 689. https://doi.org/10.3390/jmse11040689









