Experimental Study on CH4 Hydrate Dissociation by the Injection of Hot Water, Brine, and Ionic Liquids
Abstract
:1. Introduction
2. Experimental Section
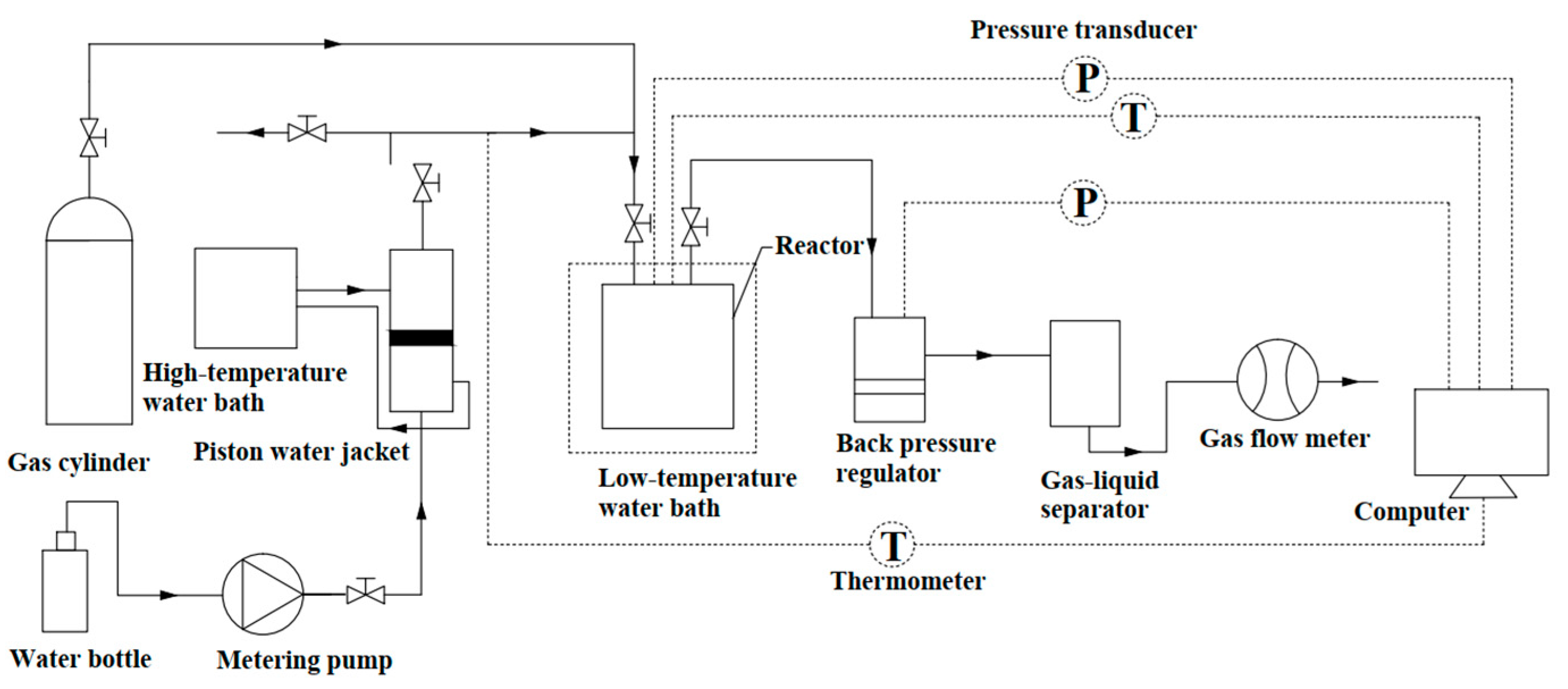
2.1. Apparatus and Materials
2.2. Procedure
2.3. Calculation of Hydrate Saturation and Thermal Efficiency
2.3.1. Calculation of Hydrate Saturation
2.3.2. Calculation of Thermal Efficiency
3. Results and Discussion
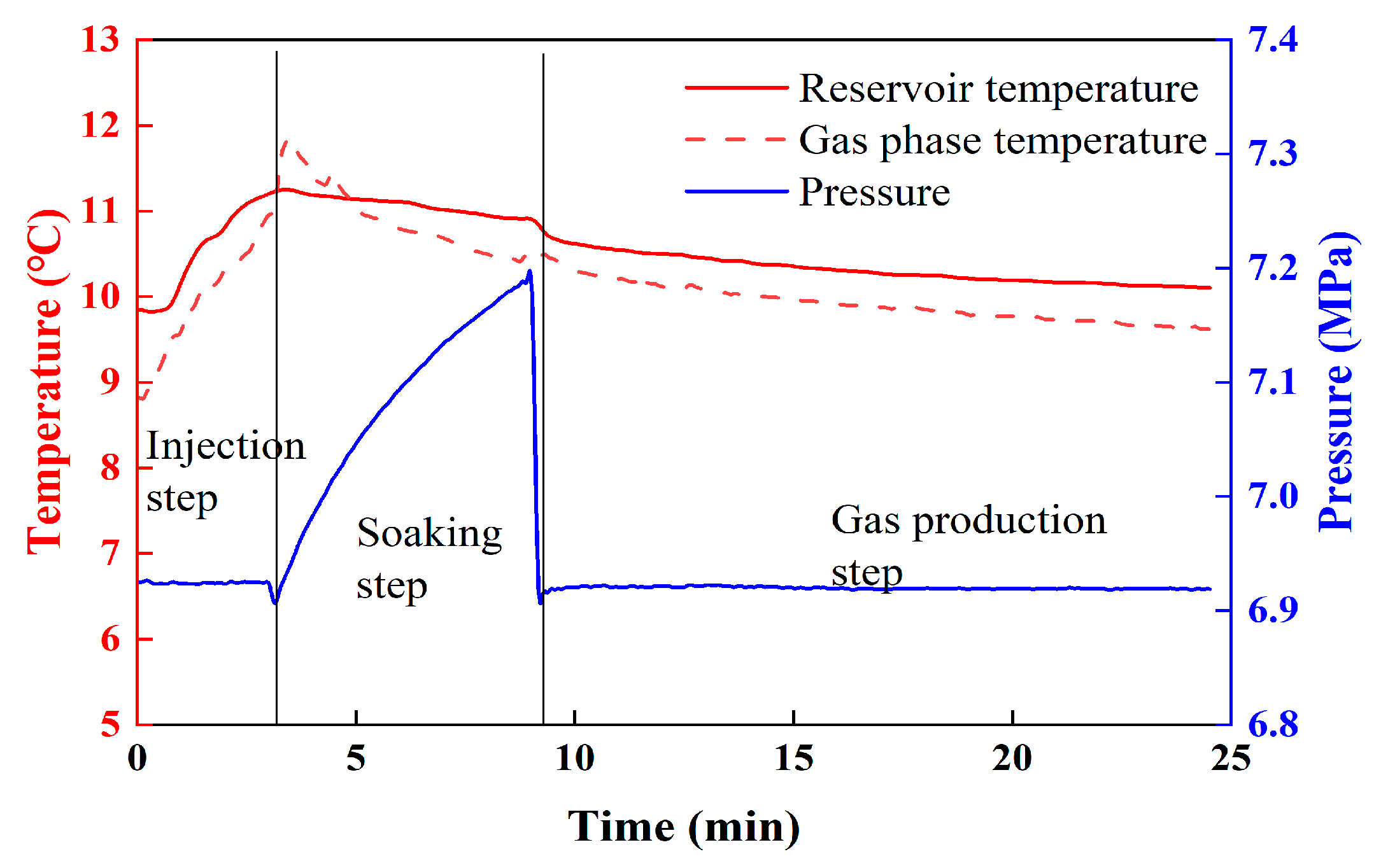
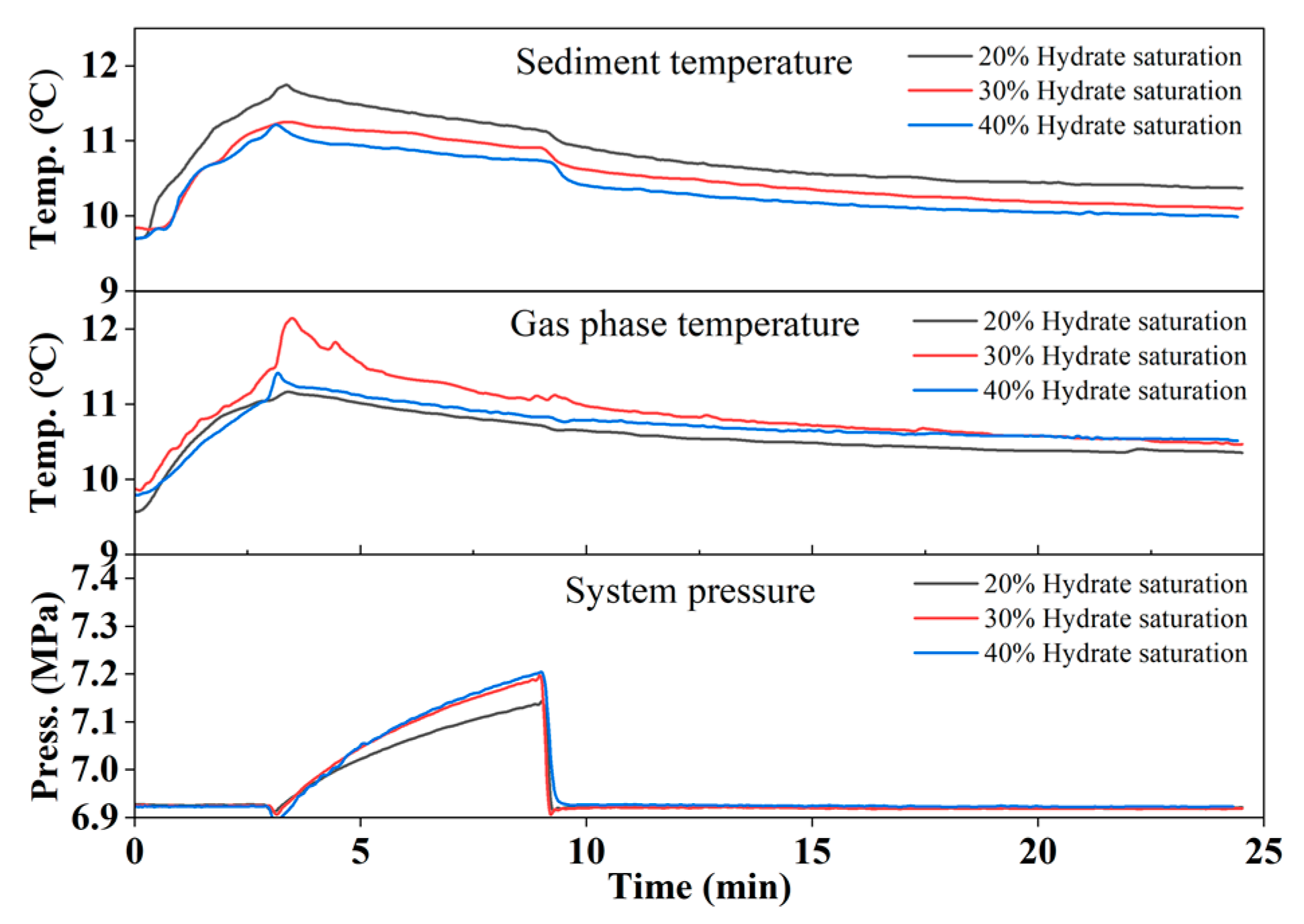
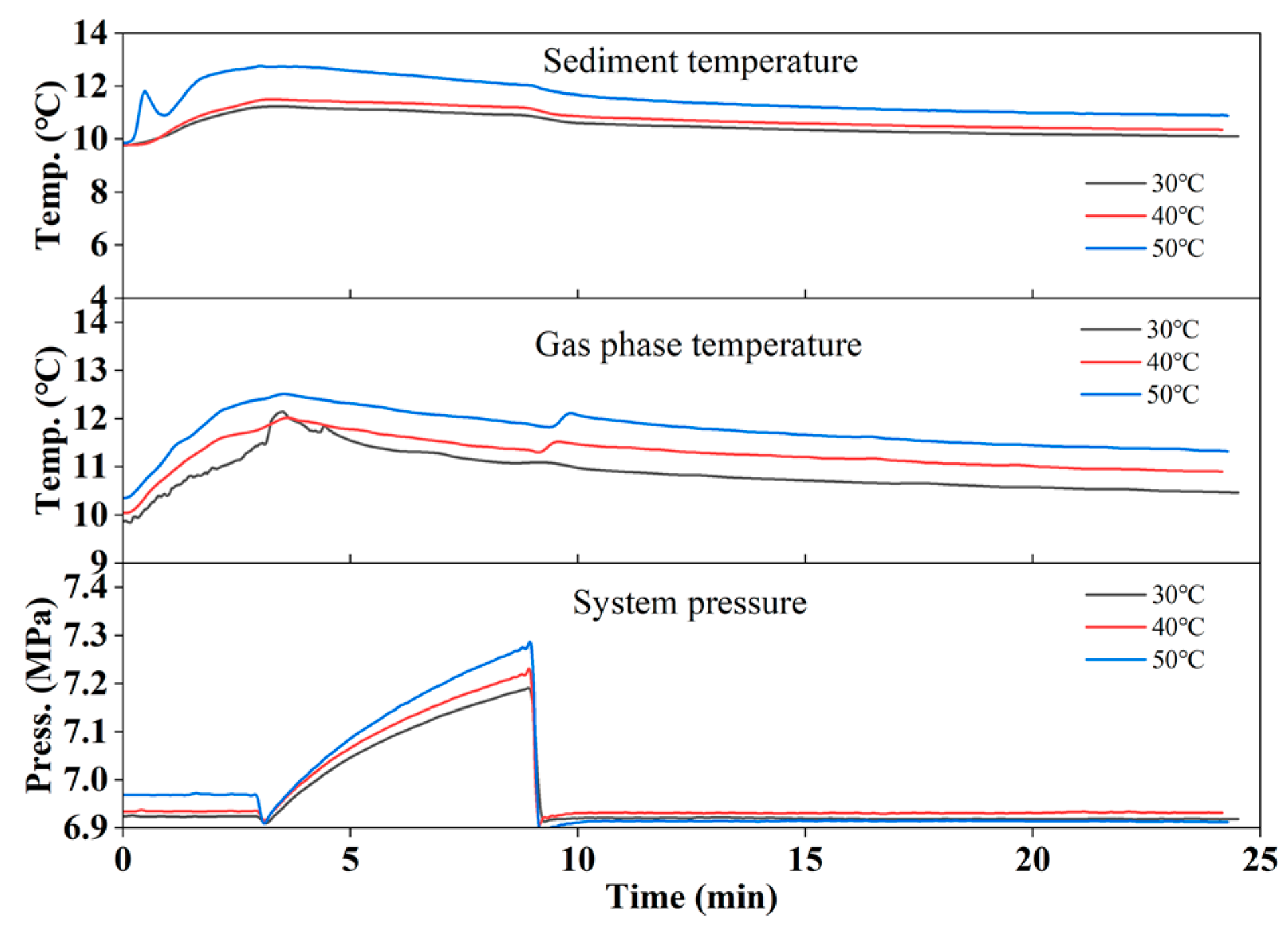
3.1. Gas Production by Hot Water Injection
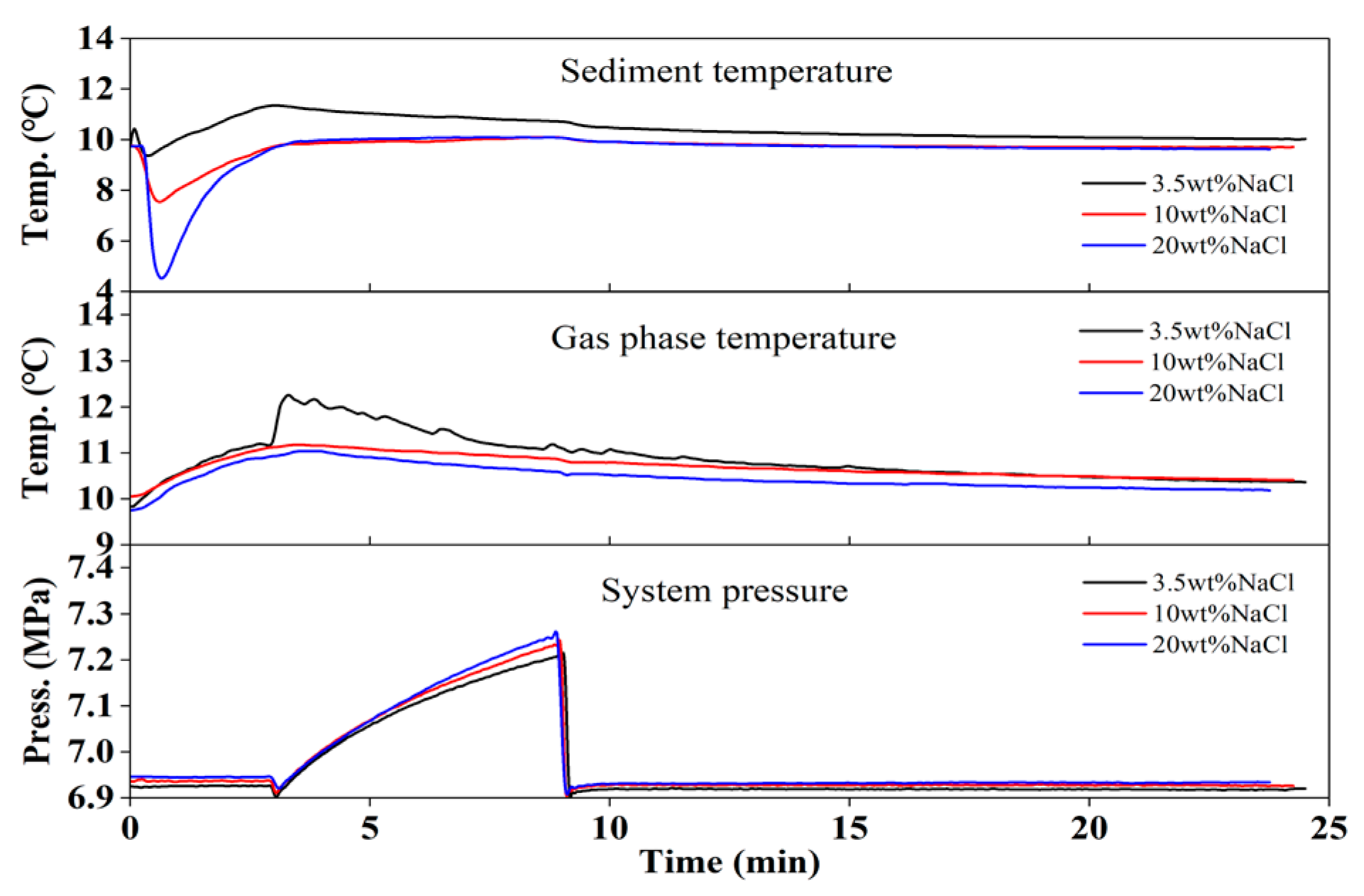
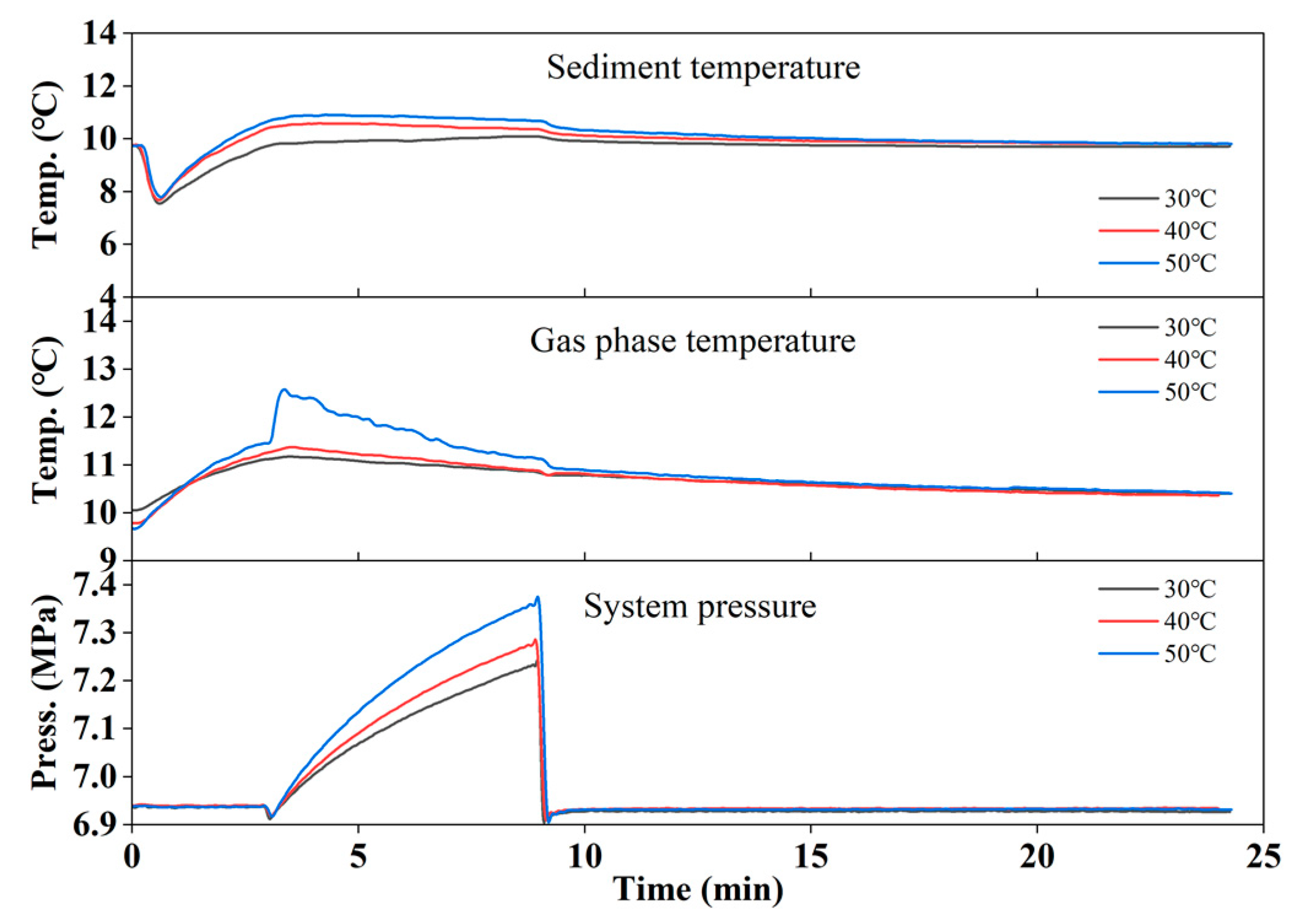
3.2. Gas Production by Hot Brine Injection
3.3. Gas Production and the Injection of Ionic Liquids
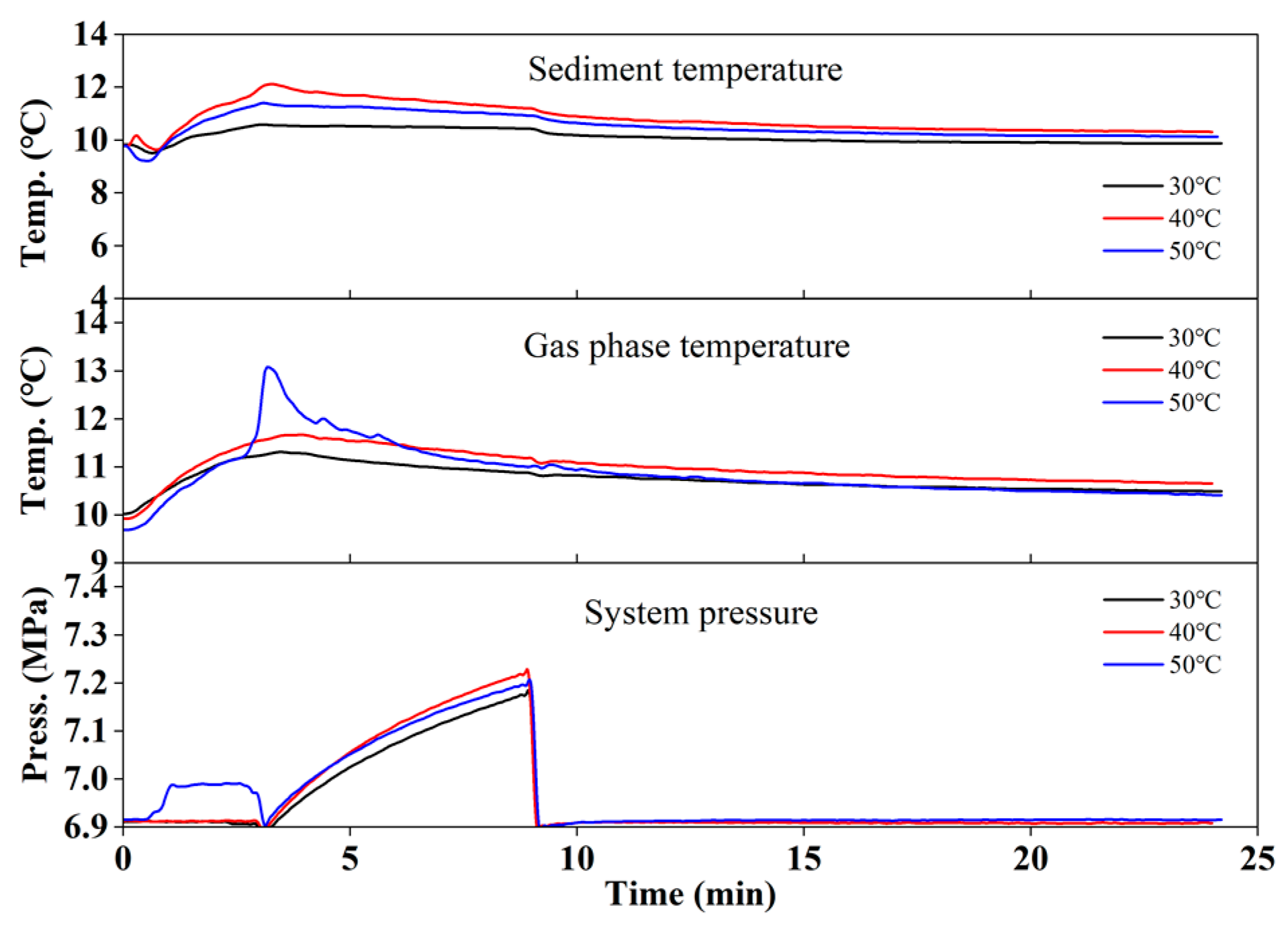
3.3.1. BMIM-Cl Injection
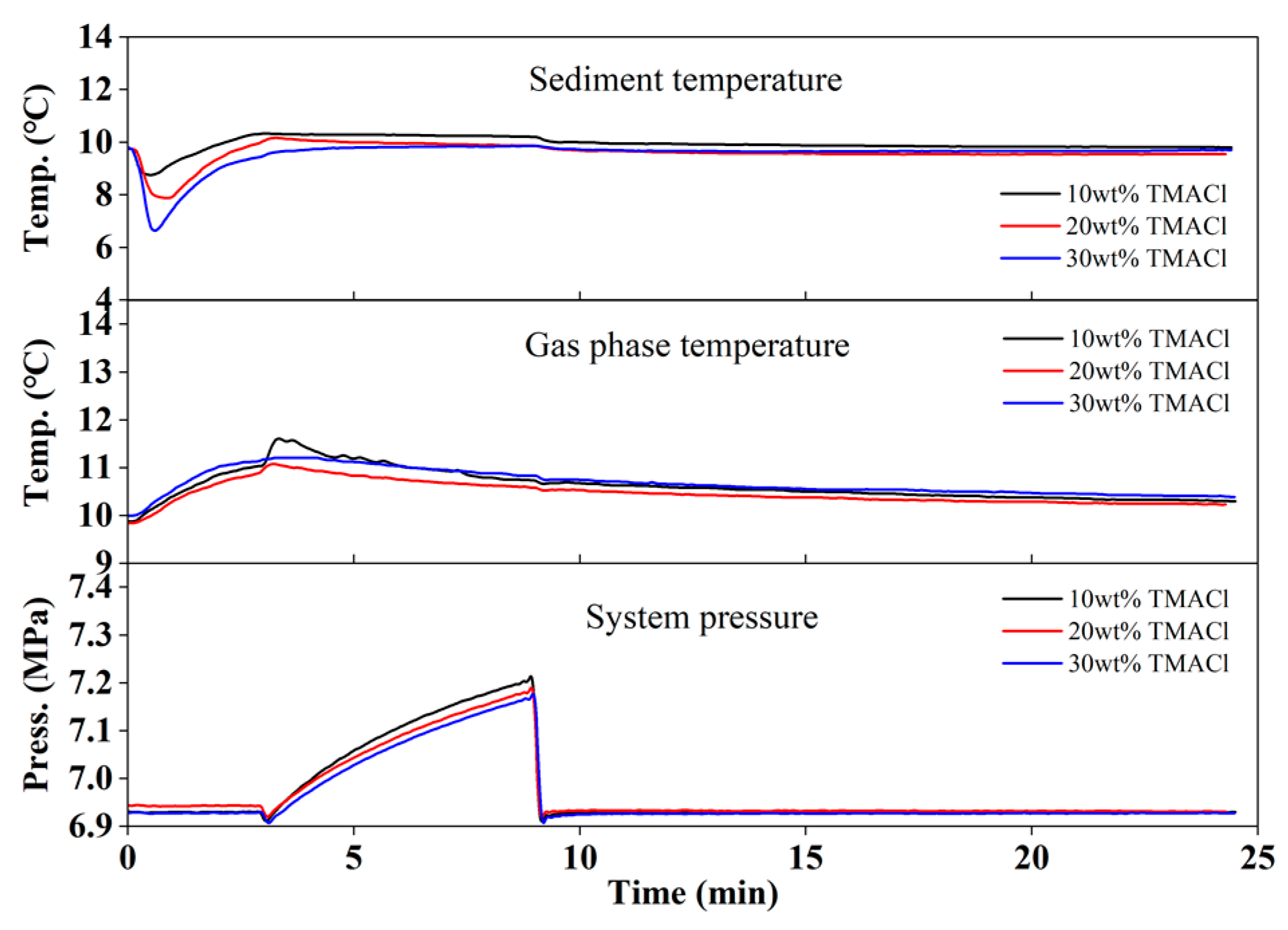
3.3.2. TMACl Injection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.H.; Lang, X.M.; Fan, S.S.; Wang, S.L.; Yu, C.; Li, G. Review on Enhanced Technology of Natural Gas Hydrate Recovery by Carbon Dioxide Replacement. Energy Fuels 2021, 35, 3659–3674. [Google Scholar] [CrossRef]
- Yu, Y.S.; Zhang, X.W.; Liu, J.W.; Lee, Y.H.; Li, X.S. Natural gas hydrate resources and hydrate technologies: A review and analysis of the associated energy and global warming challenges. Energy Environ. Sci. 2021, 14, 5611–5668. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth-Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Zhao, J.F.; Song, Y.C.; Lim, X.L.; Lam, W.H. Opportunities and challenges of gas hydrate policies with consideration of environmental impacts. Renew. Sustain. Energy Rev. 2017, 70, 875–885. [Google Scholar] [CrossRef]
- Ahlbrandt, T.S.; Charpentier, R.R.; Klett, T.R.; Schmoker, J.W.; Schenk, C.J.; Ulmishek, G.F. Future oil and gas resources of the world. Geotimes 2000, 45, 24–25. [Google Scholar]
- Liu, Y.S.; Zhu, Y.H.; Wu, B.H. Natural gas hydrate—New energy in the 21st century. Depos. Qual. 2012, 31, 401–405. [Google Scholar]
- Farahani, M.V.; Hassanpouryouzband, A.; Yang, J.H.; Tohidi, B. Insights into the climate-driven evolution of gas hydrate-bearing permafrost sediments: Implications for prediction of environmental impacts and security of energy in cold regions. RSC Adv. 2021, 11, 14334–14356. [Google Scholar] [CrossRef]
- Farahani, M.V.; Guo, X.W.; Zhang, L.X.; Yang, M.Z.; Hassanpouryouzband, A.; Zhao, J.F.; Yang, J.H.; Song, Y.C.; Tohidi, B. Effect of thermal formation/dissociation cycles on the kinetics of formation and pore-scale distribution of methane hydrates in porous media: A magnetic resonance imaging study. Sustain. Energy Fuels 2021, 5, 1567–1583. [Google Scholar] [CrossRef]
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Li, Z.F.; Han, J. Environmental safety and low velocity of the development of submarine natural gas hydrate with examples of test production in South China Sea. Environ. Sci. Pollut. Res. 2021, 28, 6259–6265. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S. Pilot-scale experimental test on gas production from methane hydrate decomposition using depressurization assisted with heat stimulation below quadruple point. Int. J. Heat Mass Transf. 2019, 131, 965–972. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, L.; Feng, J.C.; Li, X.S. Influence of the Particle Size of Sandy Sediments on Heat and Mass Transfer Characteristics during Methane Hydrate Dissociation by Thermal Stimulation. Energies 2019, 12, 4227. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.C.; Cheng, C.X.; Zhao, J.F.; Zhu, Z.H.; Liu, W.G.; Yang, M.J.; Xue, K.H. Evaluation of gas production from methane hydrates using depressurization, thermal stimulation and combined methods. Appl. Energy 2015, 145, 265–277. [Google Scholar] [CrossRef]
- Huang, L.; Yin, Z.Y.; Linga, P.; Veluswamy, H.P.; Liu, C.L.; Chen, Q.; Hu, G.W.; Sun, J.Y.; Wu, N.Y. Experimental investigation on the production performance from oceanic hydrate reservoirs with different buried depths. Energy 2022, 242, 122542. [Google Scholar] [CrossRef]
- Huang, L.; Su, Z.; Wu, N.Y.; Cheng, J.W. Analysis on geologic conditions affecting the performance of gas production from hydrate deposits. Mar. Petrol. Geol. 2016, 77, 19–29. [Google Scholar] [CrossRef]
- Wang, D.D.; Ning, F.L.; Lu, J.G.; Lu, H.F.; Kang, D.J.; Xie, Y.F.; Li, J.; Sun, J.X.; Ou, W.J.; Liu, Z.C.; et al. Reservoir characteristics and critical influencing factors on gas hydrate accumulations in the Shenhu area, South China Sea. Mar. Petrol. Geol. 2021, 133, 105238. [Google Scholar] [CrossRef]
- Song, Y.C.; Yang, L.; Zhao, J.F.; Liu, W.G.; Yang, M.J.; Li, Y.H.; Liu, Y.; Li, Q.P. The status of natural gas hydrate research in China: A review. Renew. Sustain. Energy Rev. 2014, 31, 778–791. [Google Scholar] [CrossRef]
- Fang, B.; Lu, T.; Cheng, L.W.; Wang, D.D.; Ni, Y.; Fan, B.W.; Meng, J.L.; Vlugt, T.J.H.; Ning, F.L. Negative Effects of Inorganic Salt Invasion on the Dissociation Kinetics of Silica-Confined Gas Hydrate via Thermal Stimulation. Energy Fuels 2022, 36, 6216–6228. [Google Scholar] [CrossRef]
- Li, G.; Li, X.S.; Wang, Y.; Zhang, Y. Production behavior of methane hydrate in porous media using huff and puff method in a novel three-dimensional simulator. Energy 2011, 36, 3170–3178. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S.; Chen, Z.Y.; Li, G.; Zhang, Y. Investigation into optimization condition of thermal stimulation for hydrate dissociation in the sandy reservoir. Appl. Energy 2015, 154, 995–1003. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, B.; Lv, Q.N. Preparation of Warm Brine in Situ Seafloor Based on the Hydrate Process for Marine Gas Hydrate Thermal Stimulation. Ind. Eng. Chem. Res. 2014, 53, 14142–14157. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.Q.; Xu, X.H.; Zheng, R.Y.; Hou, J. Experimental study on dissociation of hydrate reservoirs with different saturations by hot brine injection. J. Nat. Gas Sci. Eng. 2017, 46, 555–562. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.B.; Shi, L.L.; Long, Z.; Lu, J.S.; Liang, D.Q. Study on the hydrate inhibition effect of nano-silica in drilling fluids. J. Nat. Gas Sci. Eng. 2022, 105, 104688. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Q.; Long, Z.; Liang, D.Q. In situ PXRD analysis on the kinetic effect of PVP-K90 and PVCap on methane hydrate dissociation below ice point. Fuel 2021, 286, 119491. [Google Scholar] [CrossRef]
- Paz, P.; Netto, T.A. On the Rheological Properties of Thermodynamic Hydrate Inhibitors Used in Offshore Oil and Gas Production. J. Mar. Sci. Eng. 2020, 8, 878. [Google Scholar] [CrossRef]
- Li, G.; Wu, D.M.; Li, X.S.; Zhang, Y.; Lv, Q.N.; Wang, Y. Experimental Investigation into the Production Behavior of Methane Hydrate under Methanol Injection in Quartz Sand. Energy Fuels 2017, 31, 5411–5418. [Google Scholar] [CrossRef]
- Dong, F.H.; Zang, X.Y.; Li, D.L.; Fan, S.A.S.; Liang, D.Q. Experimental Investigation on Propane Hydrate Dissociation by High Concentration Methanol and Ethylene Glycol Solution Injection. Energy Fuels 2009, 23, 1563–1567. [Google Scholar] [CrossRef]
- Chen, X.J.; Lu, H.L.; Gu, L.J.; Shang, S.L.; Zhang, Y.; Huang, X.; Zhang, L. Preliminary evaluation of the economic potential of the technologies for gas hydrate exploitation. Energy 2022, 243, 123007. [Google Scholar] [CrossRef]
- Xiao, C.W.; Wibisono, N.; Adidharma, H. Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2010, 65, 3080–3087. [Google Scholar] [CrossRef]
- Sulaimon, A.A.; Tajuddin, M.Z.M. Application of COSMO-RS for pre-screening ionic liquids as thermodynamic gas hydrate inhibitor. Fluid Phase Equilibria 2017, 450, 194–199. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies 2022, 15, 8551. [Google Scholar] [CrossRef]
- Long, Z.; He, Y.; Zhou, X.B.; Li, D.L.; Liang, D.Q. Phase behavior of methane hydrate in the presence of imidazolium ionic liquids and their mixtures. Fluid Phase Equilibria 2017, 439, 1–8. [Google Scholar] [CrossRef]
- Yu, D.M.; Wang, M.; Wang, S.L.; Zhao, S.H.; Lv, X.F.; Chen, S. Experimental study on the dissociation of hydrate by using ionic liquid as plugging removal agent in pipelines. Ind. Saf. Environ. Prot. 2018, 44, 27–30. [Google Scholar]
- Zhou, X.B.; Kang, Z.X.; Lu, J.S.; Fan, J.T.; Zang, X.Y.; Liang, D.Q. Recyclable and efficient hydrate-based CH4 storage strengthened by fabrics. Appl. Energy 2023, 336, 120820. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Zhang, H.Q.; Xiao, B.; Guo, X.W.; Wei, R.P.; Xu, L.; Sun, L.J.; Yu, B.; Leng, S.D.; et al. The status of exploitation techniques of natural gas hydrate. Chin. J. Chem. Eng. 2019, 27, 2133–2147. [Google Scholar] [CrossRef]
- Yang, M.J.; Zhao, J.; Zheng, J.N.; Song, Y.C. Hydrate reformation characteristics in natural gas hydrate dissociation process: A review. Appl. Energy 2019, 256, 113878. [Google Scholar] [CrossRef]
- Kondori, J.; Zendehboudi, S.; Hossain, M.E. A review on simulation of methane production from gas hydrate reservoirs: Molecular dynamics prospective. J. Petrol. Sci. Eng. 2017, 159, 754–772. [Google Scholar] [CrossRef]
- Su, Z.; Huang, L.; Wu, N.Y.; Yang, S.X. Effect of thermal stimulation on gas production from hydrate deposits in Shenhu area of the South China Sea. Sci. China Earth Sci. 2013, 56, 601–610. [Google Scholar] [CrossRef]
- Wu, T.W.; Wan, K.; Li, X.S.; Wang, Y.; Chen, Z.Y. Heat utilization efficiency analysis of gas production from hydrate reservoir by depressurization in conjunction with heat stimulation. Energy 2023, 263, 125625. [Google Scholar] [CrossRef]
- Yang, X.; Sun, C.Y.; Yuan, Q.; Ma, P.C.; Chen, G.J. Experimental Study on Gas Production from Methane Hydrate-Bearing Sand by Hot-Water Cyclic Injection. Energy Fuels 2010, 24, 5912–5920. [Google Scholar] [CrossRef]
- Ma, X.L.; Sun, Y.H.; Liu, B.C.; Guo, W.; Jia, R.; Li, B.; Li, S.L. Numerical study of depressurization and hot water injection for gas hydrate production in China’s first offshore test site. J. Nat. Gas Sci. Eng. 2020, 83, 103530. [Google Scholar] [CrossRef]
- Tang, L.G.; Xiao, R.; Li, G.; Feng, Z.P.; Li, X.S.; Fan, S.S. Experimental Investigation of Production Behavior of Gas Hydrate under Thermal Stimulation. Chin. J. Process Eng. 2006, 4, 548–553. [Google Scholar]
- Feng, J.C.; Wang, Y.; Li, X.S.; Li, G.; Chen, Z.Y. Production behaviors and heat transfer characteristics of methane hydrate dissociation by depressurization in conjunction with warm water stimulation with dual horizontal wells. Energy 2015, 79, 315–324. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S.; Zhang, Y. Influence of Hydrate Saturation on Methane Hydrate Dissociation by Depressurization in Conjunction with Warm Water Stimulation in the Silica Sand Reservoir. Energy Fuels 2015, 29, 7875–7884. [Google Scholar] [CrossRef]
- Zeng, H.P.; Zhang, Y.; Li, X.S.; Chen, C.; Zhang, L.; Chen, Z.Y. Experimental study on the influence of brine concentration on the dissociation characteristics of methane hydrate. J. Nat. Gas Sci Eng 2022, 100, 104492. [Google Scholar] [CrossRef]
- Shao, Q. On the influence of hydrated imidazolium-based ionic liquid on protein structure stability: A molecular dynamics simulation study. J. Chem. Phys. 2013, 139, 115102. [Google Scholar] [CrossRef]



| Chemical | Formula | Supplier | Purity |
|---|---|---|---|
| CH4 | CH4 | Guangzhou Yuejia | 99.5 mol% |
| H2O | H2O | Laboratory made | 18 MΩ/cm |
| NaCl | NaCl | TCI | 99.5 wt% |
| BMIM-Cl | C8H15ClN2 | TCI | >98 wt% |
| TMACl | C4H12ClN | TCI | >98 wt% |
| Exp. No. | Hydrate Saturation (%) | Hot Water Temperature (°C) | Volume of Injection (mL) | Gas Production (L) | Thermal Efficiency (%) |
|---|---|---|---|---|---|
| 1 | 30 | 30 | 30 | 0.392 | 37.3 |
| 2 | 30 | 30 | 35 | 0.540 | 44.1 |
| 3 | 30 | 30 | 40 | 0.512 | 36.8 |
| 4 | 20 | 30 | 30 | 0.352 | 33.1 |
| 5 | 40 | 30 | 30 | 0.404 | 38.4 |
| 6 | 30 | 40 | 30 | 0.444 | 28.5 |
| 7 | 30 | 50 | 30 | 0.488 | 23.6 |
| Exp. No. | NaCl Concentration (wt%) | Brine Temperature (°C) | Gas Production (L) | Thermal Efficiency (%) |
|---|---|---|---|---|
| 8 | 10 | 30 | 0.444 | 42.7 |
| 9 | 3.5 | 30 | 0.396 | 37.6 |
| 10 | 20 | 30 | 0.464 | 44.0 |
| 11 | 10 | 40 | 0.452 | 28.7 |
| 12 | 10 | 50 | 0.530 | 25.2 |
| Exp. No. | BMIM-Cl Concentration (wt%) | Injection Temperature (°C) | Gas Production (L) | Thermal Efficiency (%) |
|---|---|---|---|---|
| 13 | 10 | 30 | 0.456 | 43.8 |
| 14 | 20 | 30 | 0.388 | 36.8 |
| 15 | 30 | 30 | 0.500 | 48.9 |
| 16 | 10 | 40 | 0.440 | 28.1 |
| 17 | 10 | 50 | 0.352 | 16.8 |
| Exp. No. | TMACl Concentration (wt%) | Injection Temperature (°C) | Gas Production (L) | Thermal Efficiency (%) |
|---|---|---|---|---|
| 18 | 10 | 30 | 0.500 | 47.7 |
| 19 | 20 | 30 | 0.276 | 36.3 |
| 20 | 30 | 30 | 0.500 | 45.7 |
| 21 | 10 | 40 | 0.428 | 27.2 |
| 22 | 10 | 50 | 0.268 | 12.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhou, X.; Lu, J.; Liang, D.; Li, D. Experimental Study on CH4 Hydrate Dissociation by the Injection of Hot Water, Brine, and Ionic Liquids. J. Mar. Sci. Eng. 2023, 11, 713. https://doi.org/10.3390/jmse11040713
Wu S, Zhou X, Lu J, Liang D, Li D. Experimental Study on CH4 Hydrate Dissociation by the Injection of Hot Water, Brine, and Ionic Liquids. Journal of Marine Science and Engineering. 2023; 11(4):713. https://doi.org/10.3390/jmse11040713
Chicago/Turabian StyleWu, Siting, Xuebing Zhou, Jingsheng Lu, Deqing Liang, and Dongliang Li. 2023. "Experimental Study on CH4 Hydrate Dissociation by the Injection of Hot Water, Brine, and Ionic Liquids" Journal of Marine Science and Engineering 11, no. 4: 713. https://doi.org/10.3390/jmse11040713






