Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets
Abstract
:1. Introduction
2. Materials and Method
2.1. Sampling
2.2. Rock Substrate Fragmentation and Sieving
2.3. Sand Sediment Lyophilization and Sieving
2.4. Extraction
2.5. Identification
3. Results and Discussion
3.1. Abundance, Shape and Size of Microplastics
3.2. Polymer Types of Microplastics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://plasticseurope.org/es/wpcontent/uploads/sites/4/2021/11/ES_Plastics_the_facts-WEB-2020_May21_final_updatedJuly2021.pdf (accessed on 21 May 2020).
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.forbes.com/sites/trevornace/2018/04/25/uk-to-ban-all-plastic-straws-q-tips-and-single-use-plastics/?sh=70d8d113831e (accessed on 25 April 2018).
- Macfadyen, G.; Huntington, T.; Cappell, R. Abandoned, Lost or Otherwise Discarded Fishing Gear (No. 523); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Thomas, S.N.; Sandhya, K.M. Netting Materials for Fishing Gear with Special Reference to Resource Conservation and Energy Saving; ICAR-Central Institute of Fisheries Technology: Kochi, India, 2019. [Google Scholar]
- Available online: https://www.redsinsa.com/en/fishing-nets-and-their-types.html (accessed on 13 July 2021).
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Gilman, E. Status of international monitoring and management of abandoned, lost and discarded fishing gear and ghost fishing. Mar. Policy 2015, 60, 225–239. [Google Scholar] [CrossRef]
- Beneli, T.M.; Pereira, P.H.C.; Nunes, J.A.C.C.; Barros, F. Ghost fishing impacts on hydrocorals and associated reef fish assemblages. Mar. Environ. Res. 2020, 161, 105129. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef] [PubMed]
- Gilman, E. Biodegradable fishing gear: Part of the solution to ghost fishing and marine pollution. Anim. Conserv. 2016, 19, 320–321. [Google Scholar] [CrossRef]
- Link, J.; Segal, B.; Casarini, L.M. Abandoned, lost or otherwise discarded fishing gear in Brazil: A review. Perspect. Ecol. Conserv. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Reineke, J.J.; Cho, D.Y.; Dingle, Y.-T.; Morello, A.P.; Jacob, J.; Thanos, C.G.; Mathiowitz, E. Unique insights into the intestinal absorption, transit, and subsequent biodistribution of polymer-derived microspheres. Proc. Natl. Acad. Sci. USA 2013, 110, 13803–13808. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://storage.googleapis.com/planet4-italy-stateless/2020/07/0e00e8d1-report-mayday-sos-plastic-cnr-ias.pdf (accessed on 17 July 2020).
- Available online: https://youtube.com/shorts/iSfm4BnyDdI (accessed on 29 December 2022).
- Houard, T.; Boudouresque, C.F.; Barcelo, A.; Cottalorda, J.M.; Formentin, J.Y.; Jullian, E.; Pironneau, E. Occurrence of a lost fishing net within the marine area of the Port-Cros national Park (Provence, northwestern Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park 2012, 26, 109–118. [Google Scholar]
- Perroca, J.F.; Giarrizzo, T.; Azzurro, E.; Rodrigues-Filho, J.L.; Silva, C.V.; Arcifa, M.S.; Azevedo-Santos, V.M. Negative effects of ghost nets on Mediterranean biodiversity. Aquat. Ecol. 2022. [Google Scholar] [CrossRef]
- Fan, J.; Zou, L.; Zhao, G. Microplastic abundance, distribution, and composition in the surface water and sediments of the Yangtze River along Chongqing City, China. J. Soils Sediments 2021, 21, 1840–1851. [Google Scholar] [CrossRef]
- Ranjbar Jafarabadi, A.; Mashjoor, S.; Riyahi Bakhtiari, A.; Cappello, T. Ecotoxico linking of phthalates and flame-retardant combustion byproducts with coral solar bleaching. Environ. Sci. Technol. 2021, 55, 5970–5983. [Google Scholar] [CrossRef]
- Sabdono, A.; Ayuningrum, D.; Sabdaningsih, A. First Evidence of Microplastics Presence in Corals of Jepara Coastal Waters, Java Sea: A Comparison Among Habitats Receiving Different Degrees of Sedimentations. Pol. J. Environ. Stud. 2022, 31, 825–832. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Šebek, O.; Nechutný, Z. Antimony availability in highly polluted soils and sediments–a comparison of single extractions. Chemosphere 2007, 68, 455–463. [Google Scholar] [CrossRef]
- Campo, P.; Holmes, A.; Coulon, F. A method for the characterisation of microplastics in sludge. MethodsX 2019, 6, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Microplastic sampling techniques in freshwaters and sediments: A review. Environ. Chem. Lett. 2021, 19, 4225–4252. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Tassin, B. Synthetic and nonsynthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci. Total Environ. 2018, 618, 157–164. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets 2017, 2, 395–409. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Liu, L.; Lu, H.; Wang, L.; Tang, J. Effects of microplastics on greenhouse gas emissions and microbial communities in sediment of freshwater systems. J. Hazard. Mater. 2022, 435, 129030. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Hu, H.; Ao, H.; Xiong, X.; Shi, H.; Wu, C. Transport, and fate of microplastics in constructed wetlands: A microcosm study. J. Hazard. Mater. 2021, 415, 125615. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, I.A.; Golsteijn, L.; Hendriks, A.J. Review of the partitioning of chemicals into different plastics: Consequences for the risk assessment of marine plastic debris. Mar. Pollut. Bull. 2016, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://encyclopedia.pub/entry/26640 (accessed on 13 September 2022).
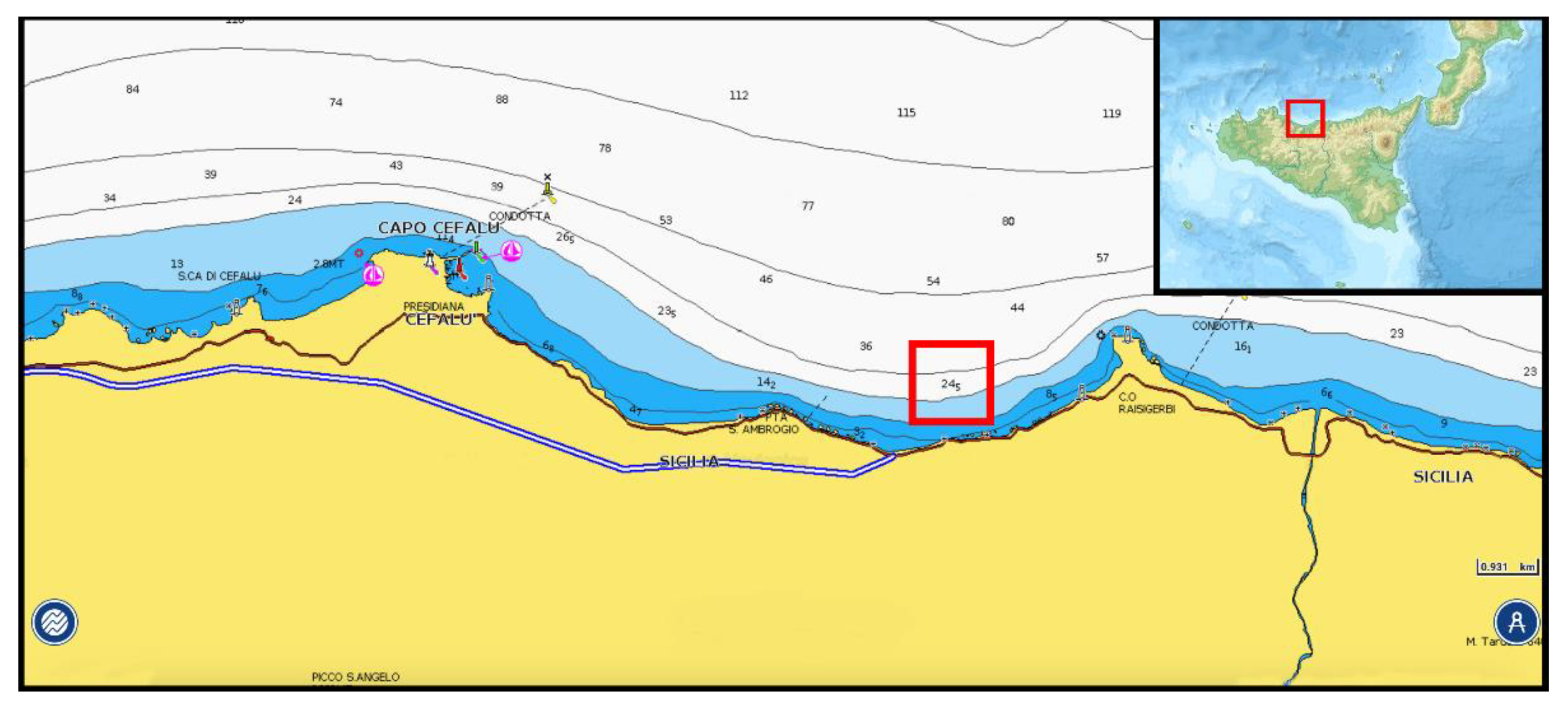
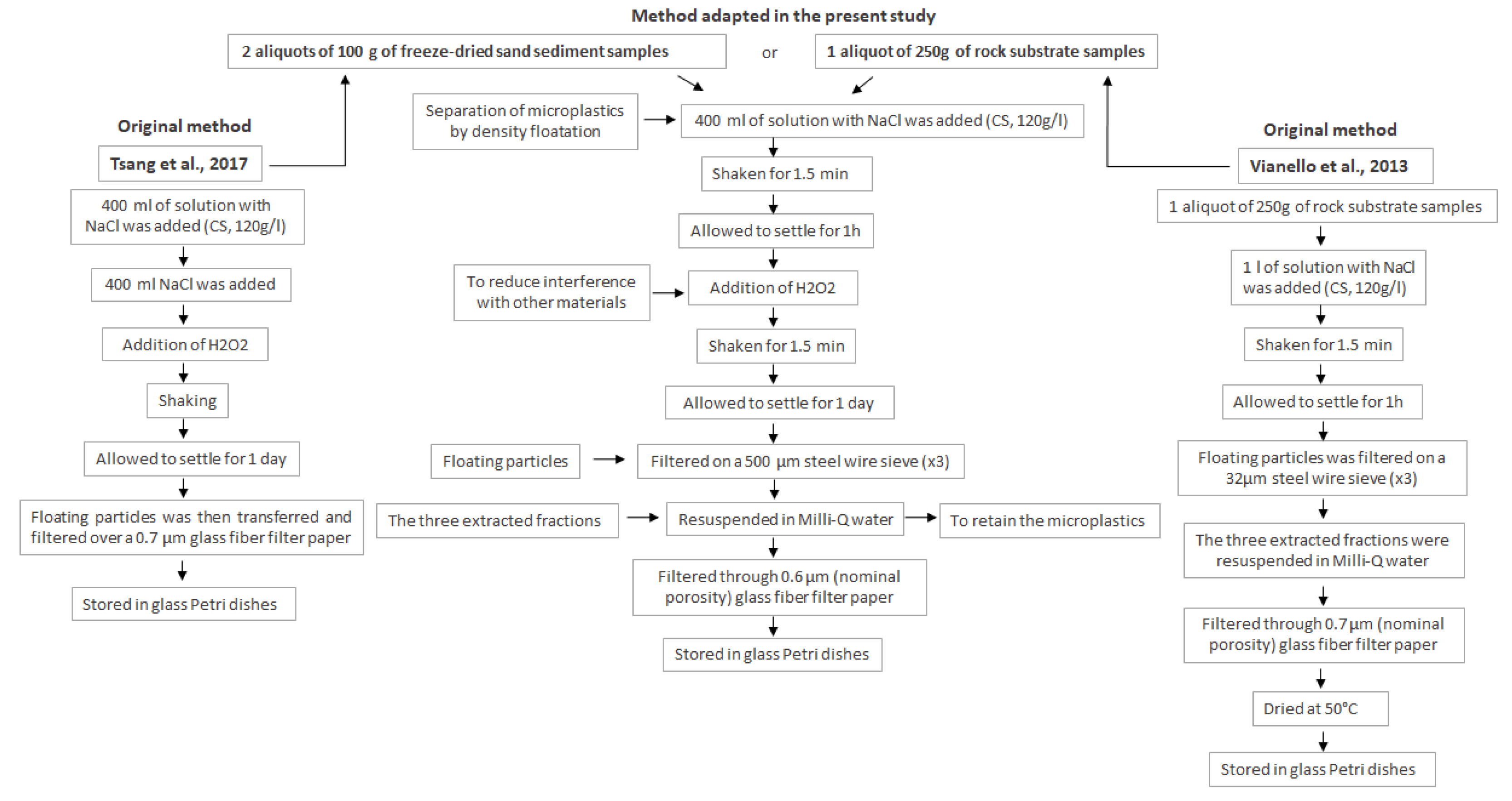


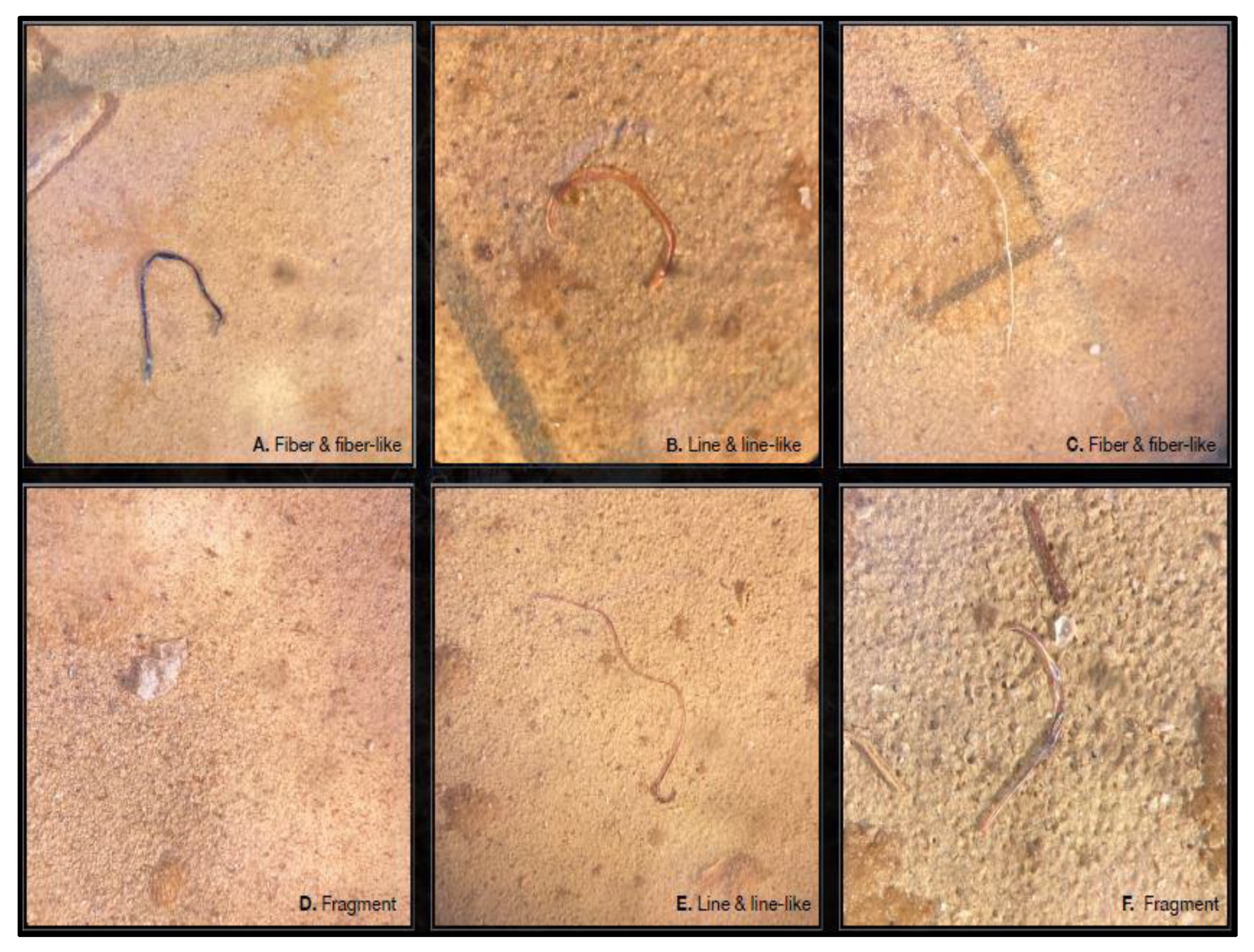
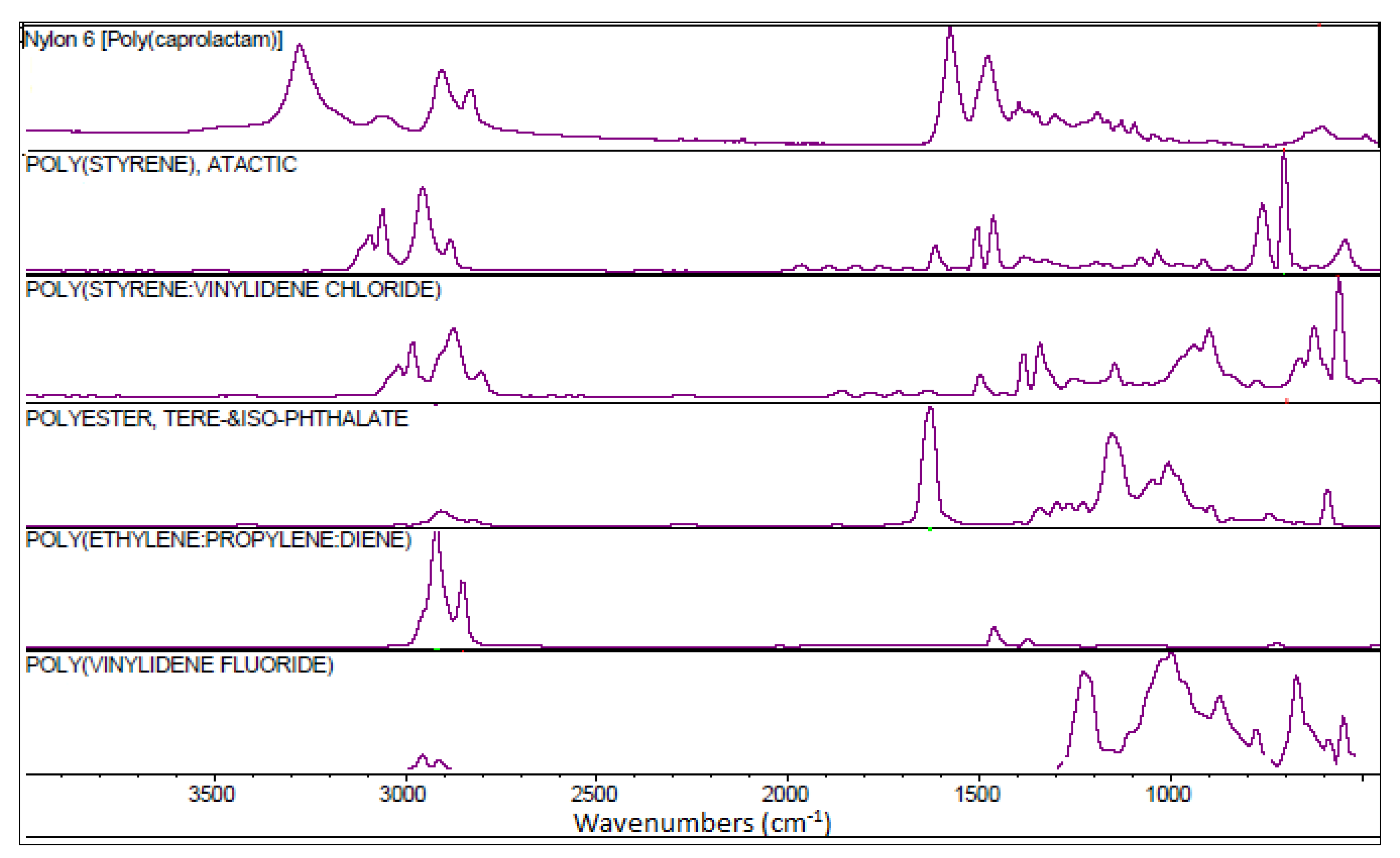
| Sampling Station (Rock Number) | Samples Type | Geographical Coordinates | Depth |
|---|---|---|---|
| 1 (control) | 2 Sediment/ 2 Rock substrate | 38.031065°N, 14.098783°E | 19 m |
| 2 | 4 Sediment/ 4 Rock substrate | 38.031065°N, 14.098783°E | 23 m |
| 3 | 4 Sediment/ 4 Rock substrate | 38.031065°N, 14.098783°E | 24 m |
| 4 | 4 Sediment/ 4 Rock substrate | 38.031065°N, 14.098783°E | 24 m |
| 5 (control) | 2 Sediment/ 2 Rock substrate | 38.031065°N, 14.098783°E | 26 m |
| 6 | 4 Sediment/ 4 Rock substrate | 38.031065°N, 14.098783°E | 28 m |
| 7 (control) | 2 Sediment/ 2 Rock substrate | 38.031065°N, 14.098783°E | 28 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, D.; Spinelli, A.; Picó, Y. Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets. J. Mar. Sci. Eng. 2023, 11, 750. https://doi.org/10.3390/jmse11040750
Vitale D, Spinelli A, Picó Y. Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets. Journal of Marine Science and Engineering. 2023; 11(4):750. https://doi.org/10.3390/jmse11040750
Chicago/Turabian StyleVitale, Dyana, Andrea Spinelli, and Yolanda Picó. 2023. "Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets" Journal of Marine Science and Engineering 11, no. 4: 750. https://doi.org/10.3390/jmse11040750
APA StyleVitale, D., Spinelli, A., & Picó, Y. (2023). Microplastics Detected in Sediments and Rocks Substrate of Marine Areas with Ghost Nets. Journal of Marine Science and Engineering, 11(4), 750. https://doi.org/10.3390/jmse11040750










