Anti-Inflammatory Activity of Mycobiont Extract of Parmotrema austrosinense (Zahlbr.) Hale in a Zebrafish Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Identification of P. austrosinense
2.3. Extraction of Bioactive Compound
2.4. Zebrafish Collection and Maintenance
2.5. Determination of LC50
2.6. Induction of Inflammation and Efficacy Analysis
2.7. Histopathology Study of Muscle Tissue
2.8. Genomic DNA Isolation
2.9. Quantification of Lipid Peroxidation
2.10. Amplification of Inflammatory Markers
2.11. Statistical Analysis
3. Results
3.1. Determination of LC50
3.2. Anti-Inflammatory Activity
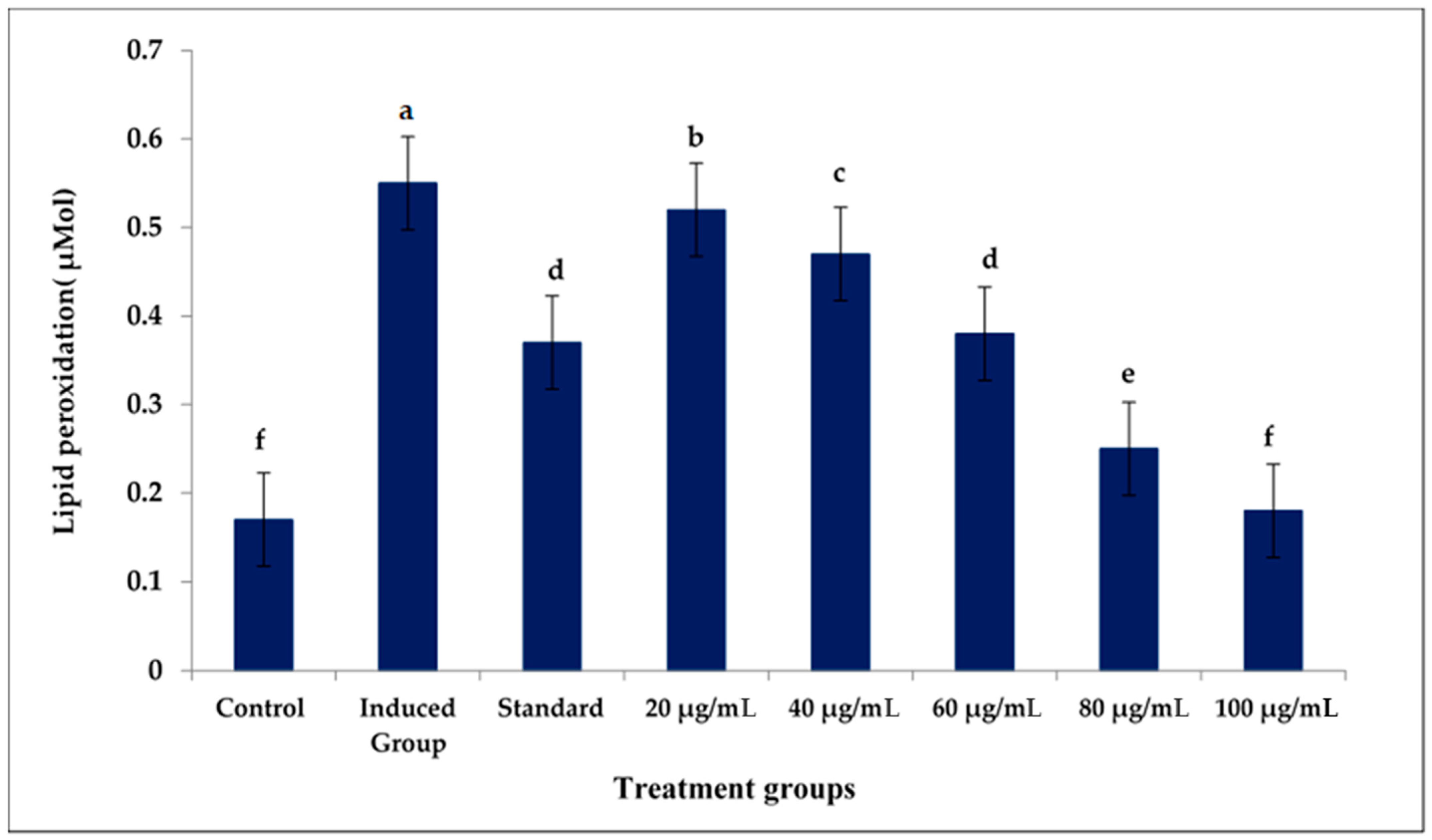
3.3. Lipid Peroxidation Assay
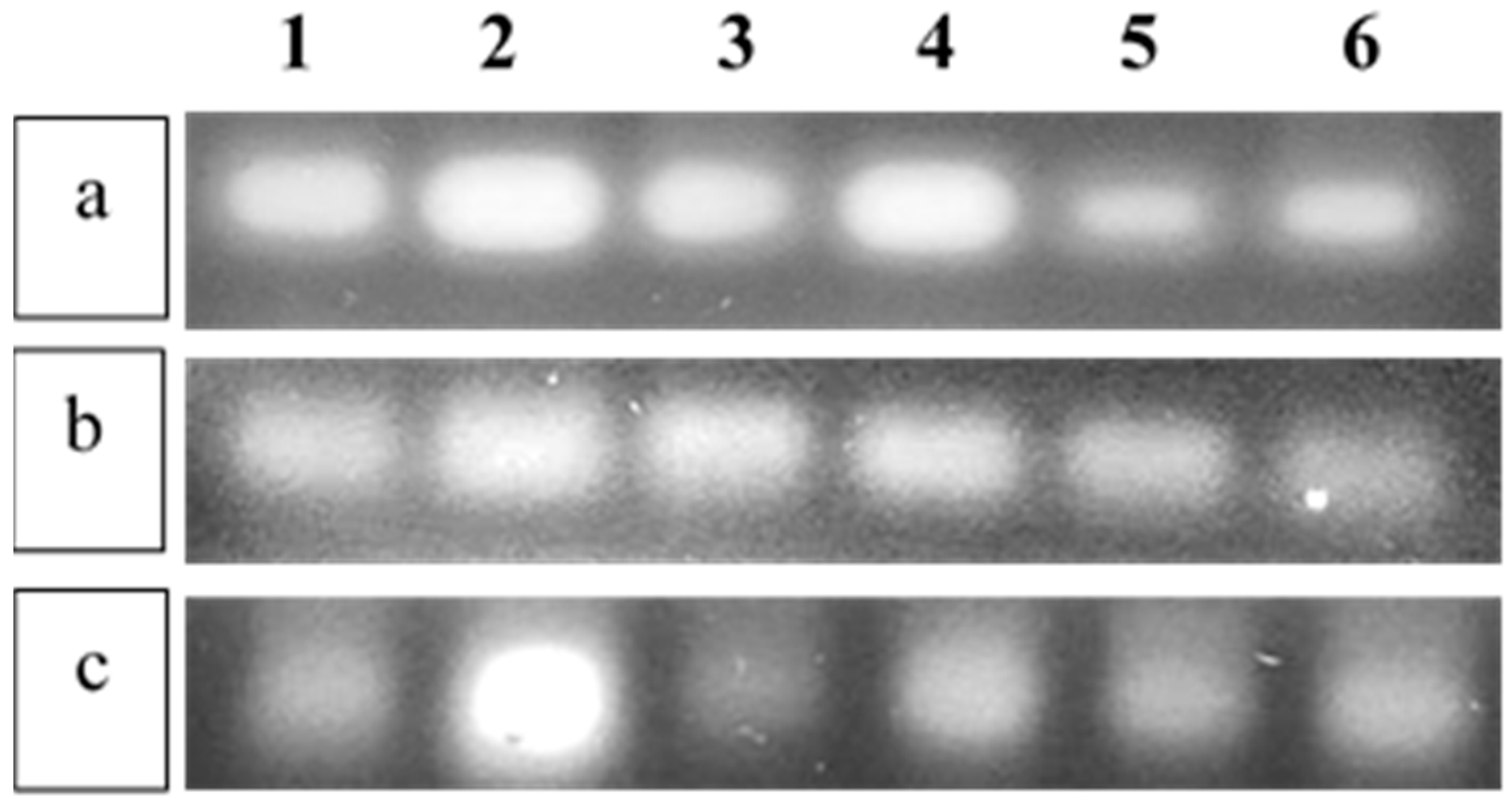
3.4. Amplification Studies of the Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phi, K.-H.; So, J.E.; Kim, J.H.; Koo, M.H.; Kim, J.-H.; Kim, D.; Lee, J.H.; Lee, S.; Youn, U.J. Chemical constituents from the Antarctic lichen Usnea aurantiaco-atra and their chemotaxonomic significance. Biochem. Syst. Ecol. 2023, 106, 104581. [Google Scholar] [CrossRef]
- Do, T.-H.; Duong, T.-H.; An, T.N.M.; Vo, T.-P.-G.; Do, V.-M.; Nguyen, N.-H.; Sichaem, J. Two new α-glucosidase inhibitory depsidones from the lichen Parmotrema cristiferum (Taylor) Hale. Chem. Biodivers. 2023, 20, e202201213. [Google Scholar] [CrossRef]
- Susithra, E.; Meena, S.; Chamundeeswari, D.; Chekkara, R.; Varalakshmi, E. Molecular docking studies of lichen metabolites as malarial protein inhibitors: Plasmepsin II protease and dihydrofolate reductase. J. Chem. Pharm. Res. 2014, 6, 501–512. [Google Scholar]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. J. Fungi 2023, 9, 116. [Google Scholar] [CrossRef]
- Bhat, N.B.; Das, S.; Sridevi, B.V.; Nayaka, S.; Birangal, S.R.; Shenoy, G.G.; Joseph, A. Molecular docking and dynamics supported investigation of antiviral activity of Lichen metabolites of Roccella montagnei: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2023, 1–14. [Google Scholar] [CrossRef]
- Chauhan, R.; Abraham, J. In vitro antimicrobial potential of the lichen Parmotrema sp. extracts against various pathogens. Iran. J. Basic Med. Sci. 2013, 16, 882. [Google Scholar]
- Yañez, O.; Osorio, M.I.; Osorio, E.; Tiznado, W.; Ruíz, L.; García, C.; Nagles, O.; Simirgiotis, M.J.; Castañeta, G.; Areche, C. Antioxidant activity and enzymatic of lichen substances: A study based on cyclic voltammetry and theoretical. Chem. Biol. Interact. 2023, 372, 110357. [Google Scholar] [CrossRef]
- Shukla, I.; Azmi, L.; Rao, C.V.; Jawaid, T.; Kamal, M.; Alkhamees, O.A.; Alaseem, A.M.; Alsanad, S.M. Inclusive roles of protocetraric acid, a secondary metabolite from the common green shield lichen Flavoparmelia caperata in alcohol-induced hepatic injury. Lat. Am. J. Pharm 2022, 41, 613–623. [Google Scholar]
- Morris Kupchan, S.; Kopperman, H.L. L-Usnic acid: Tumor inhibitor isolated from lichens. Experientia 1975, 31, 625. [Google Scholar] [CrossRef]
- Anand Mariadoss, A.V.; Krishnan Dhanabalan, A.; Munusamy, H.; Gunasekaran, K.; David, E. In Silico studies towards enhancing the anticancer activity of phytochemical phloretin against cancer drug targets. Curr. Drug Ther. 2018, 13, 174–188. [Google Scholar] [CrossRef]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of Novel Lichen Compounds as Inhibitors of SARS-CoV-2 Mpro: Ligand-Based Design, Molecular Dynamics, and ADMET Analyses. Appl. Biochem. Biotechnol. 2022, 194, 6386–6406. [Google Scholar] [CrossRef]
- Tripathi, A.H.; Negi, N.; Gahtori, R.; Kumari, A.; Joshi, P.; Tewari, L.M.; Joshi, Y.; Bajpai, R.; Upreti, D.K.; Upadhyay, S.K. A review of anti-cancer and related properties of lichen-extracts and metabolites. Anti-Cancer Agents Med. Chem. 2022, 22, 115–142. [Google Scholar]
- Awasthi, D.D. Key to the Microlichens of India, Nepal and Sri Lanka; J. Cramer: Stuttgart, Germany, 1991. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Armin, H.; Georg, B.; Elfie, S.-w. Investigations on secondary metabolites of lichens and liehen mycobionts in culture: Production and biological activity. Osterreichische 2008, 37, 323–324. [Google Scholar]
- Tanas, S.; Odabasoglu, F.; Halici, Z.; Cakir, A.; Aygun, H.; Aslan, A.; Suleyman, H. Evaluation of anti-inflammatory and antioxidant activities of Peltigera rufescens lichen species in acute and chronic inflammation models. J. Nat. Med. 2010, 64, 42–49. [Google Scholar] [CrossRef]
- Shrestha, G.; El-Naggar, A.M.; St. Clair, L.L.; O’Neill, K.L. Anticancer activities of selected species of North American lichen extracts. Phytother. Res. 2015, 29, 100–107. [Google Scholar] [CrossRef]
- Müller, K. Pharmaceutically relevant metabolites from lichens. Appl. Microbiol. Biotechnol. 2001, 56, 9–16. [Google Scholar] [CrossRef]
- Mitrović, T.; Stamenković, S.; Cvetković, V.; Nikolić, M.; Tošić, S.; Stojičić, D. Lichens as source of versatile bioactive compounds. Biol. Nyssana 2011, 2, 1–6. [Google Scholar]
- Studzińska-Sroka, E.; Dubino, A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018, 64, 56–64. [Google Scholar] [CrossRef]
- Studzińska, E.; Witkowska-Banaszczak, E.; Bylka, W. Związki biologicznie aktywne porostów. Herba Pol. 2008, 54, 80–88. [Google Scholar]
- Bugni, T.S.; Andjelic, C.D.; Pole, A.R.; Rai, P.; Ireland, C.M.; Barrows, L.R. Biologically active components of a Papua New Guinea analgesic and anti-inflammatory lichen preparation. Fitoterapia 2009, 80, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Shibamoto, N.; Naganawa, H.; Ayukawa, S.; Matsuzaki, M.; Takeuchi, T.; Kono, K.; Sakamoto, T. Isolation of lecanoric acid, an inhibitor of histidine decarboxylase from a fungus. J. Antibiot. 1974, 27, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Poornima, S.; Ponnusamy, P. Antimicrobial and Antiproliferative Activities of Depside Compound Isolated from the Mycobiont Culture of Parmotrema austrosinense (Zahlbr.) Hale. J. Pure Appl. Microbiol. 2020, 14, 2525–2542. [Google Scholar] [CrossRef]
- Kalidoss, R.; Mariraj, M.; Shenbagam, M.; Seles, J.M.; Prasath, K.A.; Rajaprabu, N.; Ponmurugan, P. Anti-microbial and Anti-oxidant Properties of Solvent Extract of Lichen Species Collected from Kodaikanal Hills, Western Ghats of Tamil Nadu. In Phytomedicine; CRC Press: Boca Raton, FL, USA, 2020; pp. 53–61. [Google Scholar]
- Wolseley, P. A Compendium of the Macrolichens from India, Nepal and Sri Lanka. Lichenologist 2008, 40, 267–268. [Google Scholar] [CrossRef]
- Cordeiro, L.M.; Iacomini, M.; Stocker-Wörgötter, E. Culture studies and secondary compounds of six Ramalina species. Mycol. Res. 2004, 108, 489–497. [Google Scholar] [CrossRef]
- Verma, N.; Behera, B.C. In Vitro culture of lichen partners: Need and Implications. In Recent Advances in Lichenology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 147–159. [Google Scholar]
- OECD Guideline for Testing Acute Toxicity in Fishes. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948241.pdf (accessed on 1 January 2023).
- Xie, Y.; Meijer, A.H.; Schaaf, M.J. Modeling inflammation in zebrafish for the development of anti-inflammatory drugs. Front. Cell Dev. Biol. 2021, 8, 620984. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.-A.; Bondesson, M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. JoVE (J. Vis. Exp.) 2010, 42, e2126. [Google Scholar]
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to histopathological evaluation. In Drug Safety Evaluation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 69–82. [Google Scholar]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. JoVE (J. Vis. Exp.) 2012, 62, e3923. [Google Scholar]
- Jain, A.; Bhandarkar, S.; Rai, G.; Yadav, A.; Lodhi, S. Evaluation of Parmotrema reticulatum taylor for antibacterial and antiinflammatory activities. Indian J. Pharm. Sci. 2016, 78, 94–102. [Google Scholar]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Für Nat. C 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen metabolites: An overview of some secondary metabolites and their biological potential. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 175–209. [Google Scholar]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A Comparative Study of Isolated Secondary Metabolites from Lichens and Their Antioxidative Properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Seçme, M.; Dodurga, Y. Usnic Acid Inhibits Cell Proliferation and Downregulates lncRNA UCA1 Expression in Ishikawa Endometrial Cancer Cells. Nat. Prod. Biotechnol. 2021, 1, 28–37. [Google Scholar]
- Ingelfinger, R.; Henke, M.; Roser, L.; Ulshöfer, T.; Calchera, A.; Singh, G.; Parnham, M.J.; Geisslinger, G.; Fürst, R.; Schmitt, I. Unraveling the pharmacological potential of lichen extracts in the context of cancer and inflammation with a broad screening approach. Front. Pharmacol. 2020, 11, 1322. [Google Scholar] [CrossRef]
- Shukla, V.; Joshi, G.P.; Rawat, M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Nguyen, H.-H.; Aree, T.; Nguyen, H.T.; Tran, T.-M.-D.; Nguyen, T.-P.; Vo, T.-P.G.; Nguyen, N.-H.; Duong, T.-H. Diorygmones AB, two new guaiane-sesquiterpenes from the cultured lichen mycobiont of Diorygma sp. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef]
- Patton, E.E.; Tobin, D.M. Spotlight on Zebrafish: The Next Wave of Translational Research; The Company of Biologists Ltd.: Cambridge, UK, 2019; Volume 12, p. dmm039370. [Google Scholar]
- Poornima, S.; Nagarjun, N.; Ponmurugan, P.; Gnanamangai, B.M.; Narasimman, S. Toxicity and anti-inflammatory study of Parmotrema austrosinense extract against oxozalone induced intestinal inflammation in zebrafish (Danio rerio) model. Biocatal. Agric. Biotechnol. 2019, 21, 101278. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Ketha, A.; Kukavica, B.; Tatipamula, V. Antiinflammatory potential of lichens and its substances. In Inflammatory Bowel Disease; MedDocs Publishers: Reno, NY, USA, 2021; pp. 1–9. [Google Scholar]
- Güvenç, A.; Akkol, E.K.; Süntar, İ.; Keleş, H.; Yıldız, S.; Çalış, İ. Biological activities of Pseudevernia furfuracea (L.) Zopf extracts and isolation of the active compounds. J. Ethnopharmacol. 2012, 144, 726–734. [Google Scholar] [CrossRef]
- Kosanić, M.M.; Ranković, B.R.; Stanojković, T.P. Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J. Sci. Food Agric. 2012, 92, 1909–1916. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z. Für Nat. C 2000, 55, 1018–1022. [Google Scholar] [CrossRef]
- Selvaraj, G.; Tinabaye, A.; Ananthi, R. In vitro antioxidant activities of Salazinic acid and its derivative hexaacetylsalazinic acid. Int. J. Res. Eng. Technol. 2015, 4, 345–355. [Google Scholar]
- Lee, A.S.; Jung, Y.J.; Kim, D.; Nguyen-Thanh, T.; Kang, K.P.; Lee, S.; Park, S.K.; Kim, W. SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochem. Biophys. Res. Commun. 2014, 450, 1363–1369. [Google Scholar] [CrossRef] [PubMed]


| Name of Primers | Sequences |
|---|---|
| iNOS | Forward—5′ACACTTCGAAAAGCAAGATGG 3′ |
| Reverse—5′ ACGGGCATCGAAAAGCTGTA 3′ | |
| TNF-α | Forward—5′ TCATTTTGGCTGTGGGCCTT 3′ |
| Reverse—5′ GGCGGTTCAAAATCTCACTCAC 3′ | |
| β-Actin | Forward—5′ CCATCGGCAATGAGCGTTTC3′ |
| Reverse—5′ CATCCTGAGTCAATGCGCCA 3′ |
| Dosage (µg/mL) | $ Weight before TNBS Injection (g) | Weight before Sample Treatment (g) | Weight after Sample Treatment (g) |
|---|---|---|---|
| Control | 0.62 ± 0.01 c | 0.64 ± 0.00 d | 0.64 ± 0.00 g |
| TNBS-induced group | 0.65 ± 0.01 b | 0.74 ± 0.01 bc | 0.77 ± 0.01 b |
| Standard | 0.61 ± 0.01 c | 0.72 ± 0.01 c | 0.67 ± 0.01 ef |
| 200 | 0.61 ± 0.00 c | 0.63 ± 0.01 d | 0.68 ± 0.00 e |
| 400 | 0.70 ± 0.01 a | 0.72 ± 0.01 c | 0.79 ± 0.01 a |
| 600 | 0.65 ± 0.01 b | 0.75 ± 0.01 b | 0.71 ± 0.00 d |
| 800 | 0.69 ± 0.01 a | 0.83 ± 0.01 a | 0.74 ± 0.01 c |
| 1000 | 0.62 ± 0.01 c | 0.72 ± 0.01 c | 0.66 ± 0.01 fg |
| F7,16 | 21.240 *** | 68.146 *** | 80.742 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, K.; Karuppiah, P.; Ponnusamy, P.; Shaik, M.R.; Khan, M.; Oh, T.H.; Shaik, B. Anti-Inflammatory Activity of Mycobiont Extract of Parmotrema austrosinense (Zahlbr.) Hale in a Zebrafish Model. J. Mar. Sci. Eng. 2023, 11, 1081. https://doi.org/10.3390/jmse11051081
Rajendran K, Karuppiah P, Ponnusamy P, Shaik MR, Khan M, Oh TH, Shaik B. Anti-Inflammatory Activity of Mycobiont Extract of Parmotrema austrosinense (Zahlbr.) Hale in a Zebrafish Model. Journal of Marine Science and Engineering. 2023; 11(5):1081. https://doi.org/10.3390/jmse11051081
Chicago/Turabian StyleRajendran, Kalidoss, Ponmurugan Karuppiah, Ponmurugan Ponnusamy, Mohammed Rafi Shaik, Mujeeb Khan, Tae Hwan Oh, and Baji Shaik. 2023. "Anti-Inflammatory Activity of Mycobiont Extract of Parmotrema austrosinense (Zahlbr.) Hale in a Zebrafish Model" Journal of Marine Science and Engineering 11, no. 5: 1081. https://doi.org/10.3390/jmse11051081
APA StyleRajendran, K., Karuppiah, P., Ponnusamy, P., Shaik, M. R., Khan, M., Oh, T. H., & Shaik, B. (2023). Anti-Inflammatory Activity of Mycobiont Extract of Parmotrema austrosinense (Zahlbr.) Hale in a Zebrafish Model. Journal of Marine Science and Engineering, 11(5), 1081. https://doi.org/10.3390/jmse11051081









