Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Growth Trial
2.3. Apparent Digestibility Measurements
2.4. Analytical Methods
2.5. Evaluation Criteria
2.6. Lysozyme
2.7. Fish and Fillet Quality Analysis
2.7.1. Body Indexes
2.7.2. Texture Profile Analysis (TPA)
- hardness (N), defined as the maximum force required to compress the sample;
- cohesiveness, defined as the area of work during the second compression divided by the area of work during the first compression (Area 2/Area 1);
- gumminess, calculated as hardness × cohesiveness;
- resilience (Nmm), calculated by dividing the upstroke energy of the first compression by the downstroke energy of the first compression (Area 4/Area 3);
- adhesiveness (Nmm), defined as the negative force area under the baseline between compression cycles.
2.7.3. Fillet Color
|
|
|
|
|
2.7.4. Sensory Analysis
2.8. Extrapolation of Trial Results Using a Nutrient-Based Model
2.8.1. Model Validation for Trial Conditions
2.8.2. Fish Performance under Commercial-like Farming Conditions: Extrapolation Scenarios
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Nutrient Retention
3.3. Apparent Digestibility Coefficients
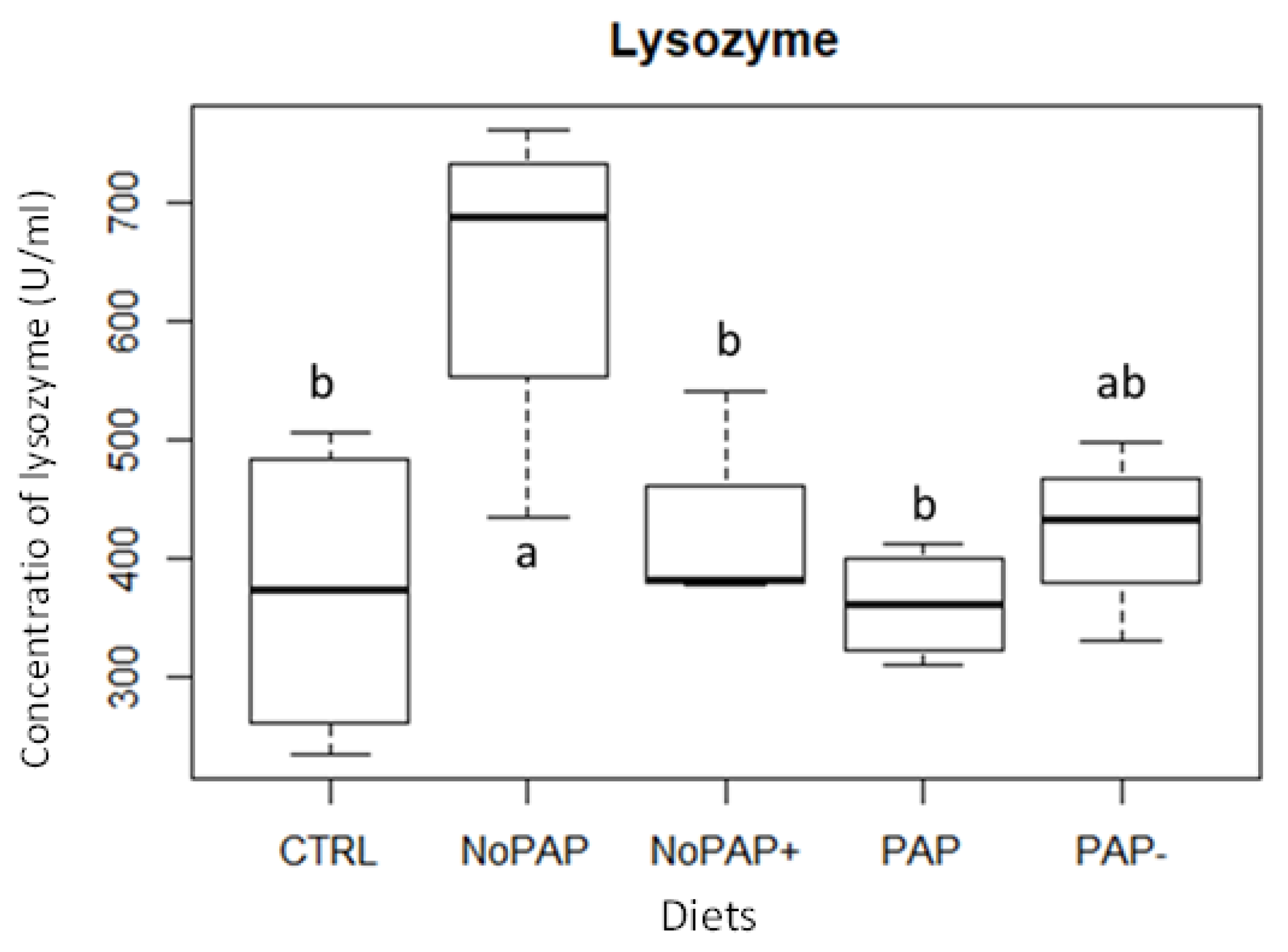
3.4. Lysozyme
3.5. Viscerosomatic Measurements
3.6. Fillet Quality
3.6.1. Fillet Texture
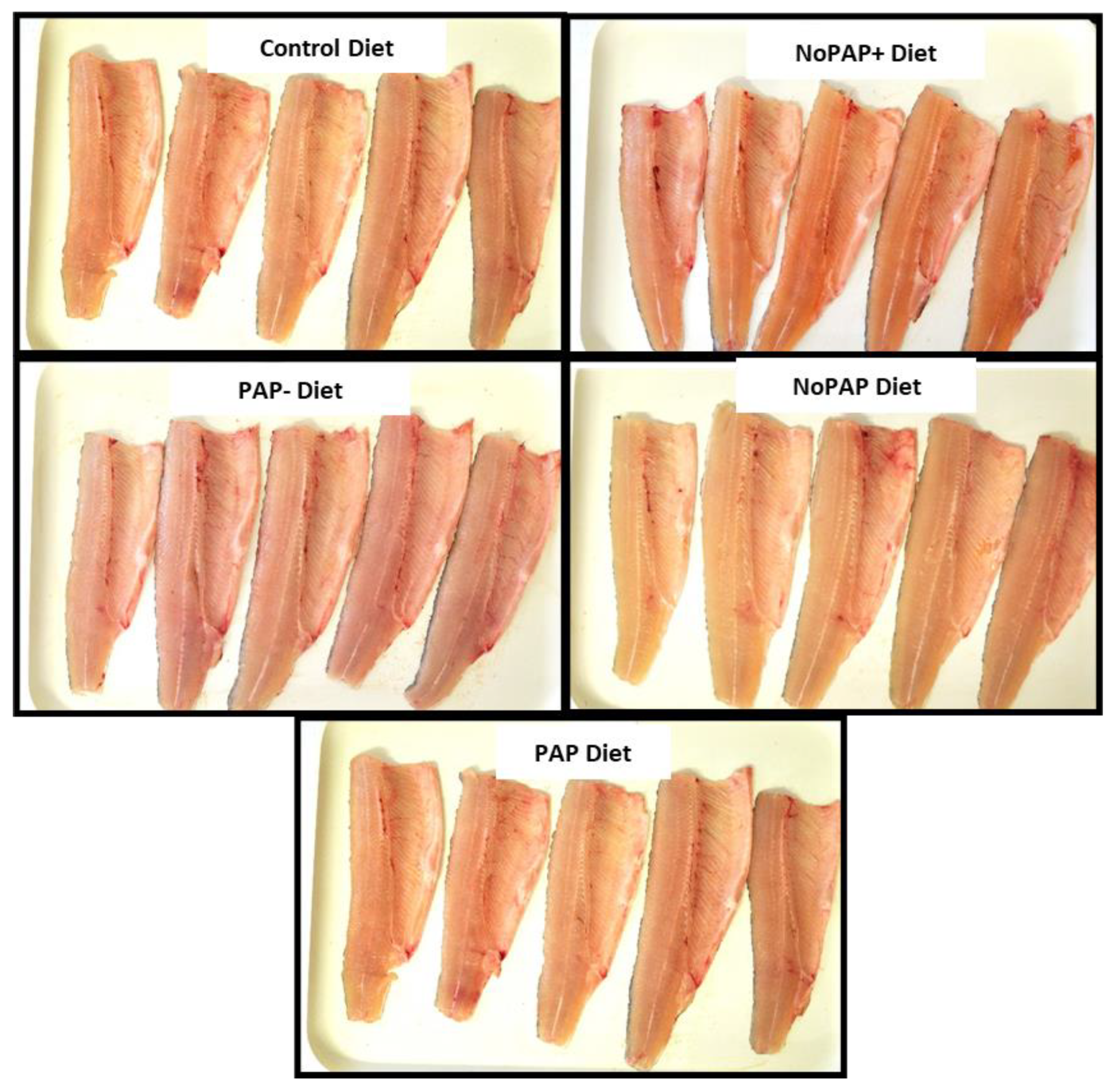
3.6.2. Fillet Color
3.7. Sensory Analysis
3.8. Extrapolation of Trial Results Using a Nutrient-Based Model
3.8.1. Model Validation for Trial Conditions
3.8.2. Fish Performance under Commercial-like Farming Conditions: Exploitation Scenarios
4. Discussion
4.1. Growth and Feed Performance
4.2. Welfare Indicators
4.3. Fillet Analysis
4.4. Sensory Analysis
4.5. Long-Term Prediction FEEDNETICSTM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients—A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Nichols, P.D. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Péron, G.; Mittaine, J.F.; Le Gallic, B. Where do fishmeal and fish oil products come from? An analysis of the conversion ratios in the global fishmeal industry. Mar. Policy 2010, 34, 815–820. [Google Scholar] [CrossRef]
- Bianchi, M.C.G.; Cjopin, F.; Farme, T.; Franz, N.; Fuentevilla, C.; Garibaldi, L.; Laurenti, A.L.G. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Hardy, R.W. Aquaculture feeds and ingredients: An overview. In New Technologies in Aquaculture; Burnell, G., Allan, G., Eds.; Woodhead Publishing Limited: New York, NY, USA, 2009; pp. 370–386. [Google Scholar]
- Lazzarotto, V.; Corraze, G.; Leprevost, A.; Quillet, E.; Dupont-Nivet, M.; Médale, F. Three-year breeding cycle of rainbow trout (Oncorhynchus mykiss) fed a plant-based diet, totally free of marine resources: Consequences for reproduction, fatty acid composition and progeny survival. PLoS ONE 2015, 10, e0117609. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef]
- Colombo, S.M. Physiological considerations in shifting carnivorous fishes to plant-based diets. In Fish Physiology; Benfey, T.J., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 53–82. [Google Scholar]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Di Marco, P.; Daniso, E.; Messina, M.; Donadelli, V.; Finoia, M.G.; Petochi, T.; Fava, F.; Faccenda, F.; Contò, M.; et al. Growth and Welfare of Rainbow Trout (Oncorhynchus mykiss) in Response to Graded Levels of Insect and Poultry By-Product Meals in Fishmeal-Free Diets. Animals 2022, 12, 1698. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Bandara, T. Alternative feed ingredients in aquaculture: Opportunities and challenges. J. Entomol. Zool. Stud. 2018, 6, 3087–3094. [Google Scholar]
- Siddik, M.A.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.; Cole, A.; Condon, K.; Jerry, D.; Mangott, A.; Praeger, C.; Vucko, M.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- NRC National Research Council. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011; p. 376.
- Regost, C.; Arzel, J.; Robin, J.; Rosenlund, G.; Kaushik, S.J. Total replacement of fish oil by soybean or linseed oil with a return to fish oil in turbot (Psetta maxima): 1. Growth performance, flesh fatty acid profile, and lipid metabolism. Aquaculture 2003, 217, 465–482. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; PerLues, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Davis, A., Ed.; Woodhead Publishing: Swaston, CA, USA, 2015; pp. 203–233. [Google Scholar]
- Ruiz-Lopez, N.; Usher, S.; Sayanova, O.V.; Napier, J.A.; Haslam, R.P. Modifying the lipid content and composition of plant seeds: Engineering the production of LC-PUFA. Appl. Microbiol. Biotechnol. 2015, 99, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.S.; Montero, D.; Robaina, L.; Caballero, M.J.; Rosenlund, G.; Ginés, R. Alterations in fillet fatty acid profile and flesh quality in gilthead seabream (Sparus aurata) fed vegetable oils for a long term period. Recovery of fatty acid profiles by fish oil feeding. Aquaculture 2005, 250, 431–444. [Google Scholar] [CrossRef]
- Carvalho, M.; Montero, D.; Rosenlund, G.; Fontanillas, R.; Ginés, R.; Izquierdo, M. Effective complete replacement of fish oil by combining poultry and microalgae oils in practical diets for gilthead sea bream (Sparus aurata) fingerlings. Aquaculture 2020, 529, 735696. [Google Scholar] [CrossRef]
- Santigosa, E.; Constant, D.; Prudence, D.; Wahli, T.; Verlhac-Trichet, V. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2020, 51, 649–665. [Google Scholar] [CrossRef]
- Bélanger, A.; Sarker, P.K.; Bureau, D.P.; Chouinard, Y.; Vandenberg, G.W. Apparent digestibility of macronutrients and fatty acids from microalgae (Schizochytrium sp.) fed to rainbow trout (Oncorhynchus mykiss): A potential candidate for fish oil substitution. Animals 2021, 11, 456. [Google Scholar] [CrossRef]
- Greene, D.H.; Selivonchick, D.P. Effects of dietary vegetable, animal and marine lipids on muscle lipid and hematology of rainbow trout (Oncorhynchus mykiss). Aquaculture 1990, 89, 165–182. [Google Scholar] [CrossRef]
- Richard, N.; Kaushik, S.; Larroquet, L.; Panserat, S.; Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2006, 96, 299–309. [Google Scholar] [CrossRef]
- Mancuso, T.; Baldi, L.; Gasco, L. An empirical study on consumer acceptance of farmed fish fed on insect meals: The Italian case. Aquac. Int. 2016, 24, 1489–1507. [Google Scholar] [CrossRef]
- Ferrer Llagostera, P.; Kallas, Z.; Reig, L.; Amores de Gea, D. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Hoerterer, C.; Petereit, J.; Krause, G. Informed choice: The role of knowledge in the willingness to consume aquaculture products of different groups in Germany. Aquaculture 2022, 556, 738319. [Google Scholar] [CrossRef]
- Petereit, J.; Hoerterer, C.; Krause, G. Country-specific food culture and scientific knowledge transfer events—Do they influence the purchasing behaviour of seafood products? Aquaculture 2022, 560, 738950. [Google Scholar] [CrossRef]
- Sanchez-Lozano, N.B.; Martinez-Llorens, S.; Tomas-Vidal, A.; Cerda, M.J. Effect of high-level fish meal replacement by pea and rice concentrate protein on growth, nutrient utilization and fillet quality in gilthead seabream (Sparus aurata L.). Aquaculture 2010, 298, 83–89. [Google Scholar] [CrossRef]
- Matos, E.; Gonçalves, A.; Bandarra, N.; Colen, R.; Nunes, M.L.; Valente, L.M.P.; Dinis, M.T.; Dias, J. Plant proteins and vegetable oil do not have detrimental effects on post-mortem muscle instrumental texture, sensory properties and nutritional value of gilthead seabream. Aquaculture 2012, 358, 205–212. [Google Scholar] [CrossRef]
- Calanche, J.B.; Beltran, J.A.; Hernandez, A.A.J. Aquaculture and sensometrics: The need to evaluate sensory attributes and the consumers’ preferences. Rev. Aquac. 2020, 12, 805–821. [Google Scholar] [CrossRef]
- De La Noüe, J.; Choubert, G. Digestibility in Rainbow Trout: Comparison of the Direct and Indirect Methods of Measurement, Prog. Fish Cult. 1986, 48, 190–195. [Google Scholar] [CrossRef]
- Austreng, E. Digestibility determination in fish using chromic oxide marking and analysis of contents from different segments of the gastrointestinal tract. Aquaculture 1978, 13, 265–272. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- ISO 27085:2009; Animal Feeding Stuffs. Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Iron, Zinc, copper, Manganese, Cobalt, Molybdenum, Arsenic, Lead and Cadmium by ICP-AES. International Organisation for Standardization: Geneva, Switzerland, 2009; p. 24.
- Reis, P.A.; Valente, L.M.P.; Almeida, C.M.R. A fast and simple methodology for determination of yttrium as an inert marker in digestibility studies. Food Chem. 2008, 108, 1094–1098. [Google Scholar] [CrossRef]
- Milla, S.; Mathieu, C.; Wang, N.; Lambert, S.; Nadzialek, S.; Massart, S.; Henrotte, E.; Douxfils, J.; Mélard, C.; Mandiki, S.N.; et al. Spleen immune status is affected after acute handling stress but not regulated by cortisol in Eurasian perch, Perca fluviatilis. Fish Shellfish. Immunol. 2010, 28, 931–941. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- C.I.E. Compagnie Internationale de l’Eclairage. International. In Commission on Illumination, Recommendations on Uniform Colorspaces; Supplement No. 2 to CIE Publication No. 15.2; Bureaucentral: Paris, France, 1978. [Google Scholar]
- Mokrzycki, W.; Tatol, M. Color difference ΔE: A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Soares, F.M.R.C.; Nobre, A.M.D.; Raposo, A.I.G.; Mendes, R.C.P.; Engrola, S.A.D.; Rema, P.J.A.P.; Conceição, L.E.C.; Silva, T.S. Development and Application of a Mechanistic Nutrient-Based Model for Precision Fish Farming. J. Mar. Sci. Eng. 2023, 11, 472. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Naya-Català, F.; Pereira, G.V.; Estensoro, I.; Del Pozo, R.; Calduch-Giner, J.A.; Nuez-Ortín, W.G.; Palenzuela, O.; Sitjà-Bobadilla, A.; Dias, J.; et al. A novel fish meal-free diet formulation supports proper growth and does not impair intestinal parasite susceptibility in gilthead sea bream (Sparus aurata) with a reshape of gut microbiota and tissue-specific gene expression patterns. Aquaculture 2022, 558, 738362. [Google Scholar] [CrossRef]
- Hoerterer, C.; Petereit, J.; Lannig, G.; Johansen, J.; Pereira, G.V.; Conceição, L.E.C.; Pastres, R.; Buck, B.H. Sustainable fish feeds: Potential of emerging protein sources in diets for juvenile turbot (Scophthalmus maximus) in RAS. Aquac. Int. 2022, 30, 1481–1504. [Google Scholar] [CrossRef]
- Petereit, J.; Hoerterer, C.; Bischoff-Lang, A.A.; Conceição, L.E.C.; Pereira, G.; Johansen, J.; Pastres, R.; Buck, B.H. Adult European Seabass (Dicentrarchus labrax) Perform Well on Alternative Circular-Economy-Driven Feed Formulations. Sustainability 2022, 14, 7279. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Cardinaletti, G.; Belloni, A.; Giorgini, E.; Faccenda, F.; Cerri, R.; Tibaldi, E.; Olivotto, I. Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture 2021, 538, 736550. [Google Scholar] [CrossRef]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Simon, C.J.; Salini, M.J.; Irvin, S.; Blyth, D.; Bourne, N.; Smullen, R. The effect of poultry protein concentrate and phosphorus supplementation on growth, digestibility and nutrient retention efficiency in barramundi Lates calcarifer. Aquaculture 2019, 498, 305–314. [Google Scholar] [CrossRef]
- Øverland, M.; Sørensen, M.; Storebakken, T.; Penn, M.; Krogdahl, Å.; Skrede, A. Pea protein concentrate substituting fish meal or soybean meal in diets for Atlantic salmon (Salmo salar)—Effect on growth performance, nutrient digestibility, carcass composition, gut health, and physical feed quality. Aquaculture 2009, 288, 305–311. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Shearer, K.D.; Øverland, M.; Penn, M.H.; Krogdahl, Å.; Mydland, L.T.; Storebakken, T. Replacement of LT fish meal with a mixture of partially deshelled krill meal and pea protein concentrates in diets for Atlantic salmon (Salmo salar). Aquaculture 2011, 315, 275–282. [Google Scholar] [CrossRef]
- Thiessen, D.L.; Campbell, G.L.; Addizi, P.D. Digestibility and growth performance of juvenile rainbow trout (Oncohychus mykiss) fed with pea and canola products. Aqua. Nutr. 2003, 9, 67–75. [Google Scholar] [CrossRef]
- Sallam, A.E.; El-feky, M.M.; Ahmed, M.S.; Mansour, A.T. Potential use of whey protein as a partial substitute of fishmeal on growth performance, non-specific immunity and gut histological status of juvenile European seabass, Dicentrarchus labrax. Aquac. Res. 2021, 53, 1527–1541. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Velázquez-Fernández, J.; Díaz-Resendiz, K.; Diaz-Salas, F.; Canto-Montero, C.; Medina-Diaz, I.; Zaitseva, G. Immunologic parameters evaluations in Nile tilapia (Oreochromis niloticus) exposed to sublethal concentrations of diazinon. Fish Shellfish Immunol. 2009, 27, 383–385. [Google Scholar] [CrossRef]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Heidary, A.A.; Ghanei-Motlagh, R.; Hosseini, S.S. Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol. 2019, 86, 269–279. [Google Scholar] [CrossRef]
- Ramos, M.A.; Gonçalves, J.F.M.; Batista, S.; Costas, B.; Pires, M.A.; Rema, P.; Ozório, R.O.A. Growth, immune responses and intestinal morphology of rainbow trout (Oncorhynchus mykiss) supplemented with commercial probiotics. Fish Shellfish Immunol. 2015, 45, 19–26. [Google Scholar] [CrossRef]
- Davies, S.J.; El-Haroun, E.R.; Hassaan, M.S.; Bowyer, P.H. A Solid-State Fermentation (SSF) supplement improved performance, digestive function and gut ultrastructure of rainbow trout (Oncorhynchus mykiss) fed plant protein diets containing yellow lupin meal. Aquaculture 2021, 545, 737177. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Morais, R.; Choubert, G. Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 176, 331–341. [Google Scholar] [CrossRef]
- Skrede, G.; Storebakken, T. Instrumental colour analysis of farmed and wild Atlantic salmon when raw, baked and smoked. Aquaculture 1986, 53, 279–286. [Google Scholar] [CrossRef]
- Choubert, G.; Blanc, J.-M.; Courvalin, C. Muscle carotenoid content and colour of farmed rainbow trout fed astaxanthin or canthaxanthin as affected by cooking and smoke-curing procedures. Int. J. Food Sci. 1992, 27, 277–284. [Google Scholar] [CrossRef]
- Christiansen, R.; Struksnæs, G.; Estermann, R.; Torrissen, O.J. Assessment of flesh colour in Atlantic salmon, Salmo salar L. Aquac. Res. 1995, 26, 311–321. [Google Scholar] [CrossRef]
- Wathne, E.; Bjerkeng, B.; Storebakken, T.; Vassvik, V.; Odland, A.B. Pigmentation of Atlantic salmon (Salmo salar) fed astaxanthin in all meals or in alternating meals. Aquaculture 1998, 159, 217–231. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Texture Profile Analysis Texture Technologies Corp. and Stable Micro Systems, Ltd. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 15 May 2023).
- Mørkøre, T.; Hansen, A.Å.; Unander, E.; Einen, O. Composition, liquid leakage, and mechanical properties of farmed rainbow trout: Variation between fillet sections and the impact of ice and frozen storage. J. Food Sci. 2006, 67, 1933–1938. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Kenney, P.B.; Weber, G.M.; Yao, J.; Slider, S.D.; Manor, M.L.; Salem, M. Effect of sexual maturation on growth, fillet composition, and texture of female rainbow trout (Oncorhynchus mykiss) on a high nutritional plane. Aquaculture 2010, 317, 79–88. [Google Scholar] [CrossRef]
- Ginés, R.; Valdimarsdottir, T.; Sveinsdottir, K.; Thorarensen, H. Effects of rearing temperature and strain on sensory characteristics, texture, colour and fat of Arctic charr (Salvelinus alpinus). Food Qual. Prefer. 2004, 15, 177–185. [Google Scholar] [CrossRef]
- Martelli, R.; Franci, O.; Lupi, P.; Faccenda, F.; Parisi, G. Physico-Chemical Traits of Raw and Cooked Fillets of Rainbow Trout (Oncorhynchus mykiss) from Different Strains and Farms. Ital. J. Anim. Sci. 2014, 13, 3417. [Google Scholar] [CrossRef]
- Husein, Y.; Secci, G.; Dinnella, C.; Parisi, G.; Fusi, R.; Monteleone, E.; Zanoni, B. Enhanced utilisation of nonmarketable fish: Physical, nutritional and sensory properties of ‘clean label’ fish burgers. Int. J. Food Sci. Technol. 2019, 54, 593–601. [Google Scholar] [CrossRef]
- Bruni, L.; Secci, G.; Husein, Y.; Faccenda, F.; Medeiros, A.C.L.; Parisi, G. Is it possible to cut down fishmeal and soybean meal use in aquafeed limiting the negative effects on rainbow trout (Oncorhynchus mykiss) fillet quality and consumer acceptance? Aquaculture 2021, 543, 736996. [Google Scholar] [CrossRef]
- Morales, J.B.C.; Tomás-Vidal, A.; Cusiyunca, P.E.R.; Martínez-Llorens, S.; Marquina, P.L.; Jover-Cerdá, M.; Beltrán, J.A. An Approach to the Spanish Consumer’s Perception of the Sensory Quality of Environmentally Friendly Seabass. Foods 2021, 10, 2694. [Google Scholar] [CrossRef] [PubMed]
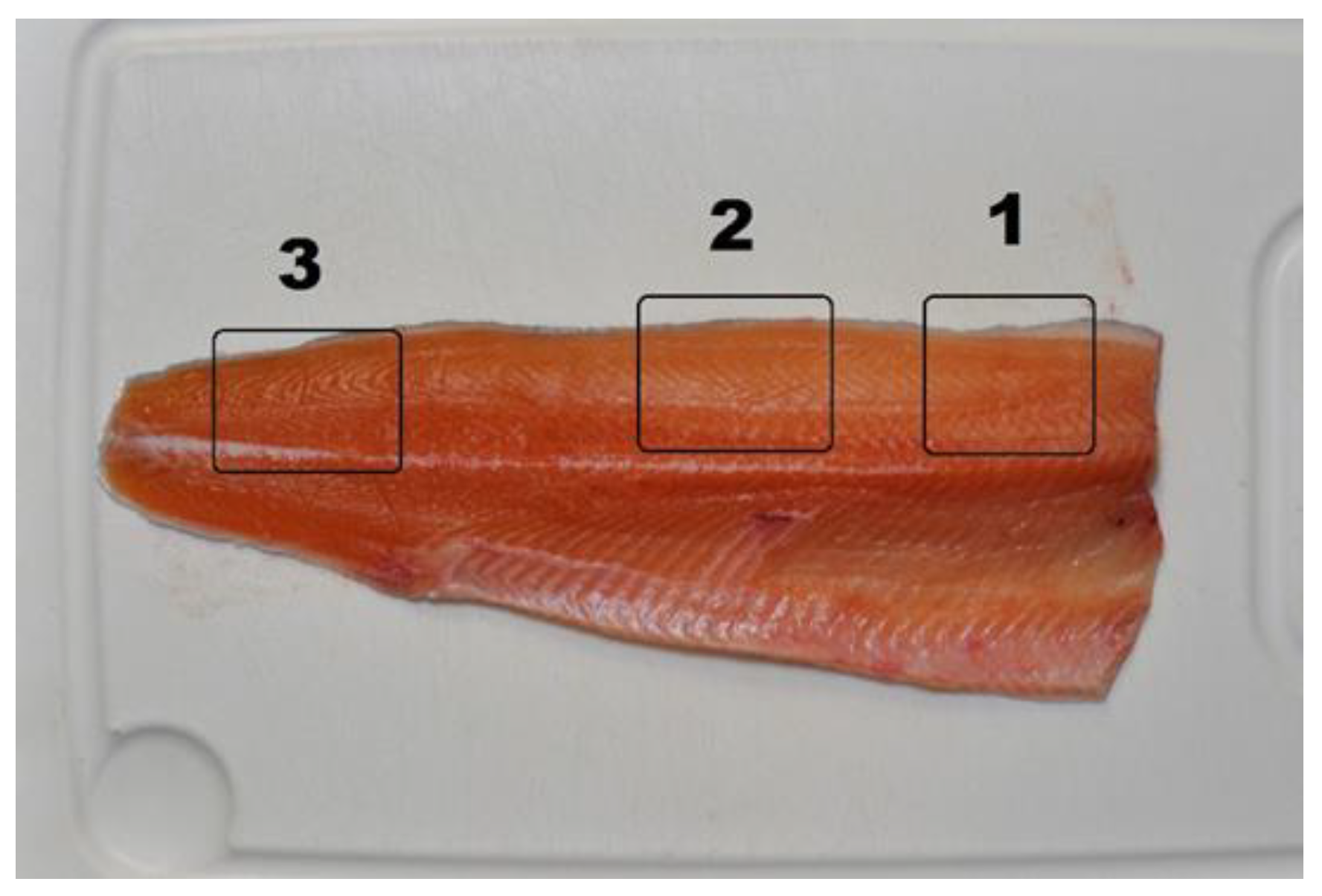
| Ingredients (%) | CTRL | NoPAP | NoPAP+ | PAP | PAP− |
|---|---|---|---|---|---|
| Fish meal Super Prime 1 | 15.00 | 12.50 | |||
| Krill meal 2 | 5.00 | ||||
| Fish protein hydrolysate 3 | 2.00 | ||||
| FPH-TURBOT-HEAD 4 | 0.250 | 0.250 | 0.250 | 0.250 | |
| FPH-TURBOT-TF 5 | 0.250 | 0.250 | 0.250 | 0.250 | |
| FPH-SALMON-HEAD 6 | 0.250 | 0.250 | 0.250 | 0.250 | |
| FPH-SALMON-TF 7 | 0.250 | 0.250 | 0.250 | 0.250 | |
| FPH-BREAM/BASS 8 | 1.00 | 1.00 | 1.00 | 1.000 | |
| Feathermeal hydrolysate 9 | 7.50 | 15.00 | |||
| Porcine blood meal 10 | 2.00 | 4.00 | |||
| Poultry meal 11 | 5.500 | 11.00 | |||
| Insect meal (Black soldier fly) 12 | 16.00 | 10.00 | 16.00 | 5.00 | |
| Fermentation biomass (M. capsulatus) 13 | 16.00 | 10.00 | 16.00 | 5.00 | |
| Soy protein concentrate 14 | 20.00 | 10.00 | 10.00 | ||
| Pea protein concentrate 15 | 2.55 | 10.00 | |||
| Wheat gluten 16 | 6.00 | 3.00 | 3.00 | ||
| Corn gluten meal 17 | 7.00 | 3.50 | |||
| Soybean meal 48 18 | 10.00 | ||||
| Wheat meal 19 | 11.20 | ||||
| Whole peas 20 | 12.03 | 20.84 | 31.77 | ||
| Pea starch (raw) 21 | 4.00 | 7.00 | 13.35 | 4.00 | |
| Vit and Min Premix-WITH I and Se 22 | 1.00 | ||||
| Vit and Min Premix-NO I and Se 23 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Macroalgae SHP 24 | 2.00 | 2.00 | 2.00 | 2.00 | |
| Macroalgae SHP + Se 25 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Microalgae Se-rich 26 | 0.20 | 0.20 | 0.30 | 0.30 | |
| Vitamin E50 27 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Betaine HCl 28 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Antioxidant 29 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium propionate 30 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Monocalcium phosphate 31 | 1.04 | 2.26 | 0.70 | 1.80 | 1.70 |
| L-Lysine HCl 99% 32 | 0.50 | 0.60 | 0.45 | 0.65 | |
| L-Tryptophan 33 | 0.04 | 0.01 | 0.04 | 0.17 | |
| DL-Methionine 34 | 0.12 | ||||
| L-Taurine 35 | 0.17 | 0.20 | 0.10 | 0.12 | 0.04 |
| Yttrium oxide 36 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Fish oil 37 | 5.30 | 2.65 | 2.65 | 2.65 | 2.65 |
| Salmon oil (by-products) 38 | 10.00 | 10.00 | 10.00 | 10.00 | |
| Algae oil (Veramaris) 39 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Rapeseed oil 40 | 16.30 | 7.50 | 6.00 | 6.30 | 6.10 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Composition (feed basis) | |||||
| Crude protein, % feed | 39.6 | 39.3 | 43.1 | 41.5 | 38.4 |
| Crude fat, % feed | 21.5 | 22.1 | 22.2 | 23.6 | 22.1 |
| Fiber, % feed | 1.6 | 1.6 | 1.3 | 1.4 | 2.5 |
| Ash, % feed | 6.3 | 6.2 | 5.9 | 6.0 | 5.8 |
| Gross Energy, MJ/kg feed | 23.4 | 22.8 | 23.3 | 22.8 | 23.5 |
| Category | Inputs | |||
|---|---|---|---|---|
| Farming conditions | Initial body weight: 50 g Initial number of fish: 20,000 Mortality rate: 1%/month | |||
| Diet properties | Price * (% of change in relation to CTRL) | Proximate composition: analyzed values Digestibility: measured values Amino acid profile: estimated values Fatty acid profile: default values | ||
| CTRL | 0.0% | |||
| NoPAP | +22.92% | |||
| NoPAP+ | +48.53% | |||
| PAP | −11.78% | |||
| PAP− | −6.70% | |||
| Feeding regime | Number of meals: 2 meals/day Feeding type: feeding table Feed waste: 0% | |||
| Temperature | Daily water temperature: constant profiles of 12 °C and 16 °C | |||
| Growth Parameters | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| IBW (g) | 58.2 ± 1.81 | 58.1 ± 1.43 | 58.6 ± 1.38 | 59.4 ± 1.40 | 59.9 ± 0.69 | 0.342 |
| FBW (g) | 292 ± 7.25 c | 297 ± 11.3 bc | 336 ± 6.17 a | 309 ± 5.30 b | 297 ± 4.45 bc | <0.001 |
| Weight gain (g) | 234 ± 5.97 c | 239 ± 10.0 bc | 277 ± 4.79 a | 250 ± 4.70 b | 237 ± 3.95 bc | <0.001 |
| RGR (%BW/day) | 1.79 ± 0.02 c | 1.8 ± 0.02 bc | 1.94 ± 0.01 a | 1.83 ± 0.02 b | 1.78 ± 0.01 c | <0.001 |
| FCR | 0.82 ± 0.01 b | 0.84 ± 0.02 b | 0.76 ± 0.01 c | 0.83 ± 0.01 b | 0.87 ± 0.01 a | <0.001 |
| VFI (%/day) | 1.46 ± 0.03 c | 1.51 ± 0.02 ab | 1.48 ± 0.01 bc | 1.52 ± 0.01 ab | 1.55 ± 0.01 a | <0.001 |
| PER | 3.09 ± 0.04 a | 3.04 ± 0.06 ab | 3.04 ± 0.03 ab | 2.90 ± 0.05 bc | 2.99 ± 0.01 bc | <0.001 |
| Survival (%) | 100 ± 0.00 | 98.5 ± 1.91 | 98.0 ± 1.63 | 99.5 ± 1.00 | 99.0 ± 2.00 | 0.306 |
| Retention (% of Intake) | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| Crude Protein | 48.1 ± 2.6 a | 43.7 ± 0.7 ab | 46.5 ± 1.3 ab | 42.8 ± 1.2 b | 43.1 ± 3.1 b | 0.020 |
| Crude Fat | 75.6 ± 6.7 | 65.6 ± 6.2 | 71.1 ± 5.8 | 69.8 ± 1.4 | 68.6 ± 3.1 | 0.271 |
| Energy | 47.2 ± 2.7 | 44.1 ± 2.3 | 47.8 ± 2.2 | 47.5 ± 1.0 | 42.9 ± 1.2 | 0.065 |
| Digestibility (%) | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| Crude protein | 89.2 ± 0.1 a | 88.9 ± 0.5 a | 89.8 ± 0.3 a | 90.3 ± 0.7 a | 86.2 ± 1.3 b | <0.001 |
| Crude fat | 97.8 ± 0.5 b | 98.8 ± 0.1 a | 98.2 ± 0.3 ab | 98.7 ± 0.2 a | 98.2 ± 0.5 ab | 0.01 |
| Energy | 82.3 ± 1.6 b | 84.4 ± 1.1 ab | 83.3 ± 1.2 ab | 86.1 ± 1.2 a | 81.4 ± 1.6 b | 0.002 |
| Total P | 42.1 ± 4.7 a | 48.1 ± 2.8 a | 51.5 ± 2.6 a | 63.7 ± 3.5 a | 47.1 ± 4.5 b | <0.001 |
| Indexes | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| CY (%) | 90.5 ± 1.3 a | 90.3 ± 1.2 ab | 90.5 ± 1.1 a | 89.5 ± 0.1 b | 89.7 ± 1.1 b | 0.0209 |
| HSI (%) | 0.91 ± 0.1 b | 0.99 ± 0.1 ab | 0.94 ± 0.09 b | 1.04 ± 0.10 a | 1.04 ± 0.12 a | <0.001 |
| MFI (%) | 1.5 ± 0.5 | 1.5 ± 0.4 | 1.7 ± 0.66 | 1.6 ± 0.56 | 1.7 ± 0.47 | 0.72 |
| VSI (%) | 9.5 ± 1.3 b | 9.7 ± 1.2 ab | 9.53 ± 1.1 b | 10.4 ± 0.9 a | 10.32 ± 1.1 a | 0.0209 |
| Texture Parameters | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| Hardness (N) | 6.46 ± 1.79 | 6.93 ± 2.13 | 6.32 ± 1.47 | 6.56 ± 2.05 | 5.89 ± 1.88 | 0.604 |
| Cohesiveness | 0.22 ± 0.04 | 0.24 ± 0.04 | 0.23 ± 0.03 | 0.22 ± 0.04 | 0.21 ± 0.03 | 0.345 |
| Resilience | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.493 |
| Adhesiveness (Nmm) | 0.44 ± 0.10 a | 0.39 ± 0.08 abc | 0.44 ± 0.13 ab | 0.36 ± 0.09 bc | 0.33 ± 0.06 c | <0.001 |
| Gumminess (N) | 1.45 ± 0.51 | 1.66 ± 0.53 | 1.43 ± 0.41 | 1.50 ± 0.60 | 1.26 ± 0.46 | 0.233 |
| Colour Parameters | CTRL | NoPAP | NoPAP+ | PAP | PAP− | p |
|---|---|---|---|---|---|---|
| L* | 43.19 ± 1.93 c | 43.90 ± 2.19 b | 42.65 ± 1.49 c | 44.12 ± 1.73 ab | 44.55 ± 1.49 a | p < 0.001 |
| a* | 2.18 ± 1.19 d | 2.48 ± 1.22 cd | 3.87 ± 1.97 a | 2.68 ± 1.36 bc | 3.02 ± 1.43 b | p < 0.001 |
| b* | −0.19 ± 1.70 bc | −0.01 ± 1.87 b | 0.92 ± 2.38 a | −0.27 ± 1.49 bc | −0.68 ± 1.41 c | p < 0.001 |
| Chroma | 2.79 ± 1.14 b | 3.04 ± 1.35 b | 4.41 ± 2.44 a | 3.09 ± 1.33 b | 3.48 ± 1.23 a | p < 0.001 |
| Hue (rad) | −0.26 ± 0.67 b | −0.21 ± 0.61 b | 0.01 ± 0.48 a | −0.22 ± 0.52 b | −0.35 ± 0.47 b | p < 0.001 |
| ∆E Lab | CTRL | NoPAP+ | NoPAP | PAP | PAP− |
|---|---|---|---|---|---|
| CTRL | |||||
| NoPAP+ | 2.09 | ||||
| NoPAP | 0.79 | 2.09 | |||
| PAP | 1.06 | 2.23 | 0.39 | ||
| PAP− | 1.68 | 2.63 | 1.08 | 0.69 |
| Sensory Analysis | CTRL | PAP | NoPAP | p |
|---|---|---|---|---|
| Appearance | 7.8 ± 1.3 | 8.0 ± 1.0 | 8.0 ± 1.0 | 0.106 |
| Odor | 7.9 ± 1.1 | 7.9 ± 1.0 | 8.0 ± 0.9 | 0.932 |
| Texture | 7.9 ± 1.2 ab | 7.9 ± 1.1 b | 8.1 ± 0.9 a | 0.009 |
| Taste | 7.0 ± 1.2 | 7.9 ± 1.1 | 8.1 ± 0.9 | 0.898 |
| Global acceptance | 7.9 ± 1.0 ab | 7.9 ± 0.9 b | 8.1 ± 0.7 a | 0.043 |
| 12 °C | 16 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | Unit | CTRL | NoPAP | NoPAP+ | PAP | PAP− | CTRL | NoPAP | NoPAP+ | PAP | PAP− |
| Time to reach 400 g | days | 176 (0.0%) | 179 (+1.7%) | 160 (−9.1%) | 158 (−10.2%) | 166 (−5.7%) | 139 (0.0%) | 140 (+0.7%) | 127 (−8.6%) | 129 (−7.2%) | 134 (−3.6%) |
| Growth rate | %/day | 1.19 (0.0%) | 1.17 (−1.6%) | 1.31 (+9.9%) | 1.33 (+11.7%) | 1.27 (+6.3%) | 1.52 (0.0%) | 1.50 (−1.0%) | 1.66 (+9.6%) | 1.63 (+7.5%) | 1.57 (+3.5%) |
| Feed conversion ratio (FCR) | g feed/g weight gain | 1.19 (0.0%) | 1.21 (+1.4%) | 1.08 (−9.3%) | 1.04 (−12.2%) | 1.10 (−7.3%) | 1.00 (0.0%) | 1.00 (+0.5%) | 0.90 (−9.4%) | 0.90 (−9.4%) | 0.94 (−5.3%) |
| Economic conversion ratio (ECR) | - | - (0.0%) | - (+25.0%) | - (+34.8%) | - (−2.3%) | - (−13.8%) | - (0.0%) | - (+22.9%) | - (+33.7%) | - (+0.6%) | - (−12.3%) |
| Total N waste | kg N/ton biomass gain | 52.14 (0.0%) | 52.60 (+0.9%) | 51.14 (−1.9%) | 46.09 (−11.6%) | 44.51 (−4.6%) | 39.82 (0.0%) | 39.68 (−0.3%) | 38.95 (−2.2%) | 36.67 (−7.9%) | 34.67 (−12.9%) |
| Total P waste | kg P/ton biomass gain | 8.96 (0.0%) | 8.25 (−7.9%) | 6.53 (−27.1%) | 7.28 (−18.8%) | 7.75 (−3.4%) | 7.63 (0.0%) | 6.58 (−13.8%) | 4.99 (−34.6%) | 6.41 (−6.0%) | 6.75 (−11.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale Pereira, G.d.; Conceição, L.E.C.; Soares, F.; Petereit, J.; Buck, B.H.; Johansen, J.; Dias, J.; Faccenda, F. Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus mykiss). J. Mar. Sci. Eng. 2023, 11, 1135. https://doi.org/10.3390/jmse11061135
Vale Pereira Gd, Conceição LEC, Soares F, Petereit J, Buck BH, Johansen J, Dias J, Faccenda F. Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus mykiss). Journal of Marine Science and Engineering. 2023; 11(6):1135. https://doi.org/10.3390/jmse11061135
Chicago/Turabian StyleVale Pereira, Gabriella do, Luis E. C. Conceição, Filipe Soares, Jessica Petereit, Bela H. Buck, Johan Johansen, Jorge Dias, and Filippo Faccenda. 2023. "Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus mykiss)" Journal of Marine Science and Engineering 11, no. 6: 1135. https://doi.org/10.3390/jmse11061135
APA StyleVale Pereira, G. d., Conceição, L. E. C., Soares, F., Petereit, J., Buck, B. H., Johansen, J., Dias, J., & Faccenda, F. (2023). Alternative Feed Formulations Impact Growth Performance, Flesh Quality and Consumer Acceptance of Rainbow Trout (Oncorhynchus mykiss). Journal of Marine Science and Engineering, 11(6), 1135. https://doi.org/10.3390/jmse11061135







