Artificial Reefs Reduce Morbidity and Mortality of Small Cultured Sea Cucumbers Apostichopus japonicus at High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
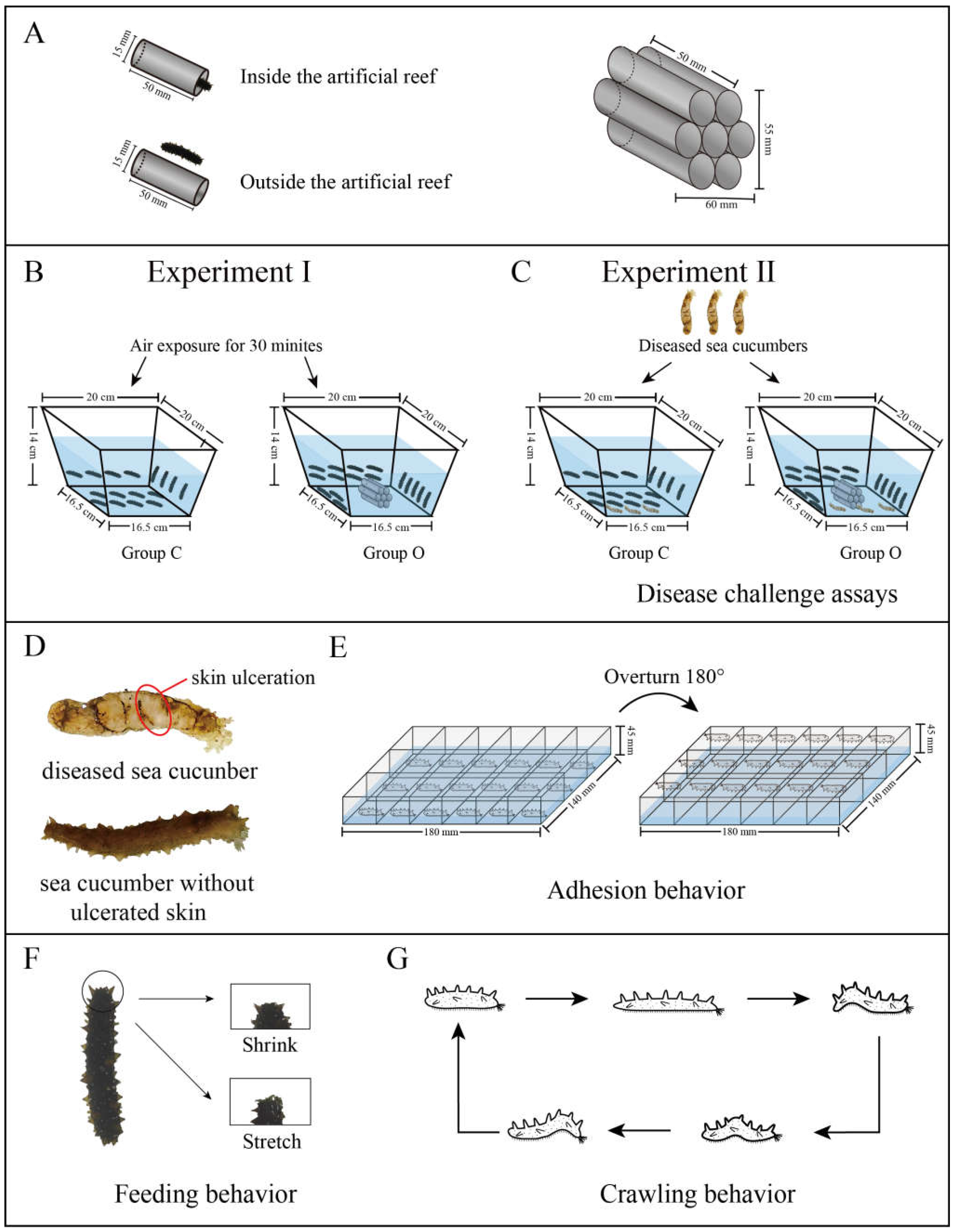
2.2. Experiment I
2.3. Experiment II
2.4. Mortality and Morbidity
2.5. Crawling Behavior
2.6. Feeding Behavior
2.7. Adhesion Behavior
2.8. Statistical Analysis
3. Results
3.1. Experiment I
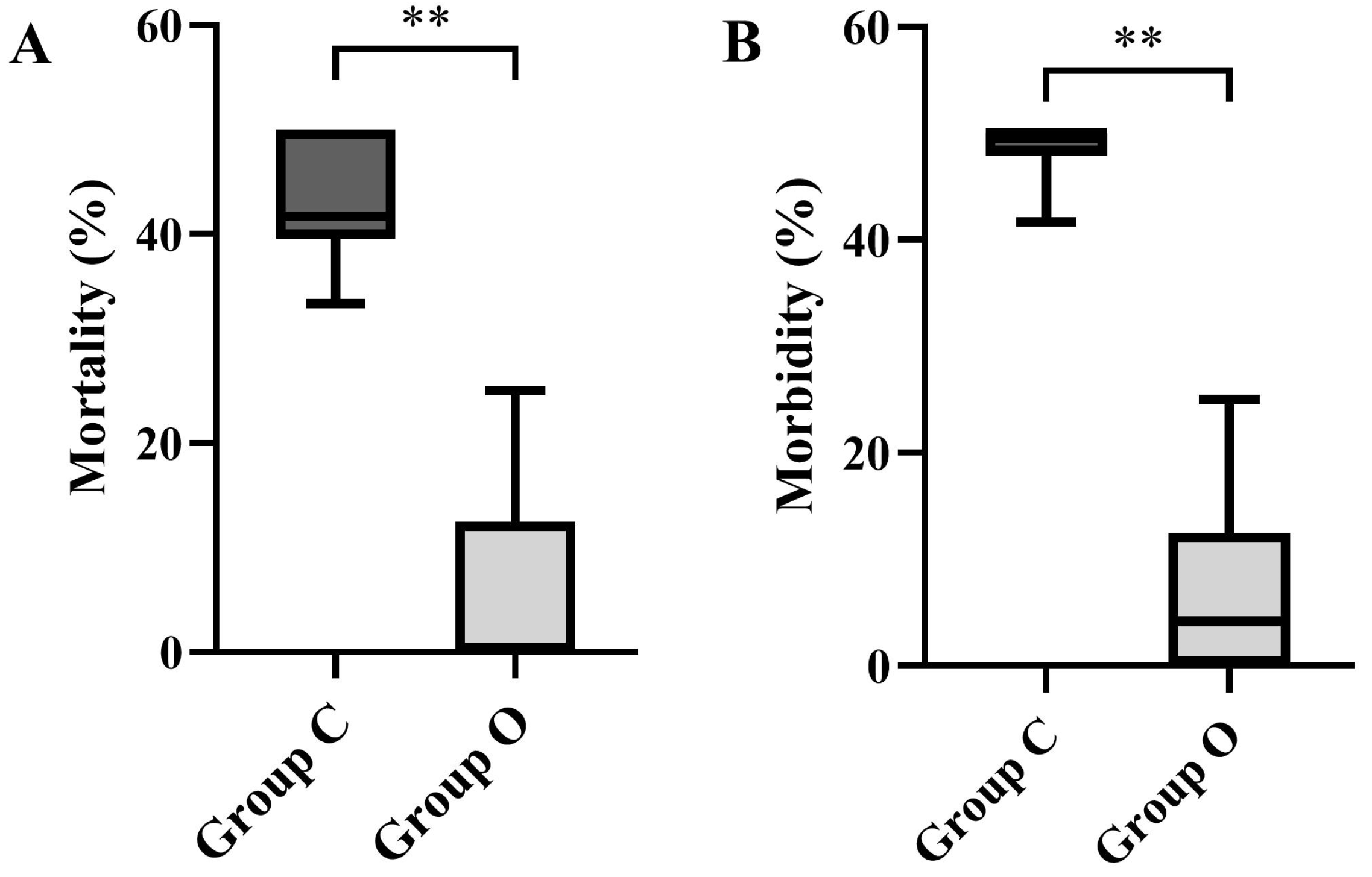
3.1.1. Mortality and Morbidity
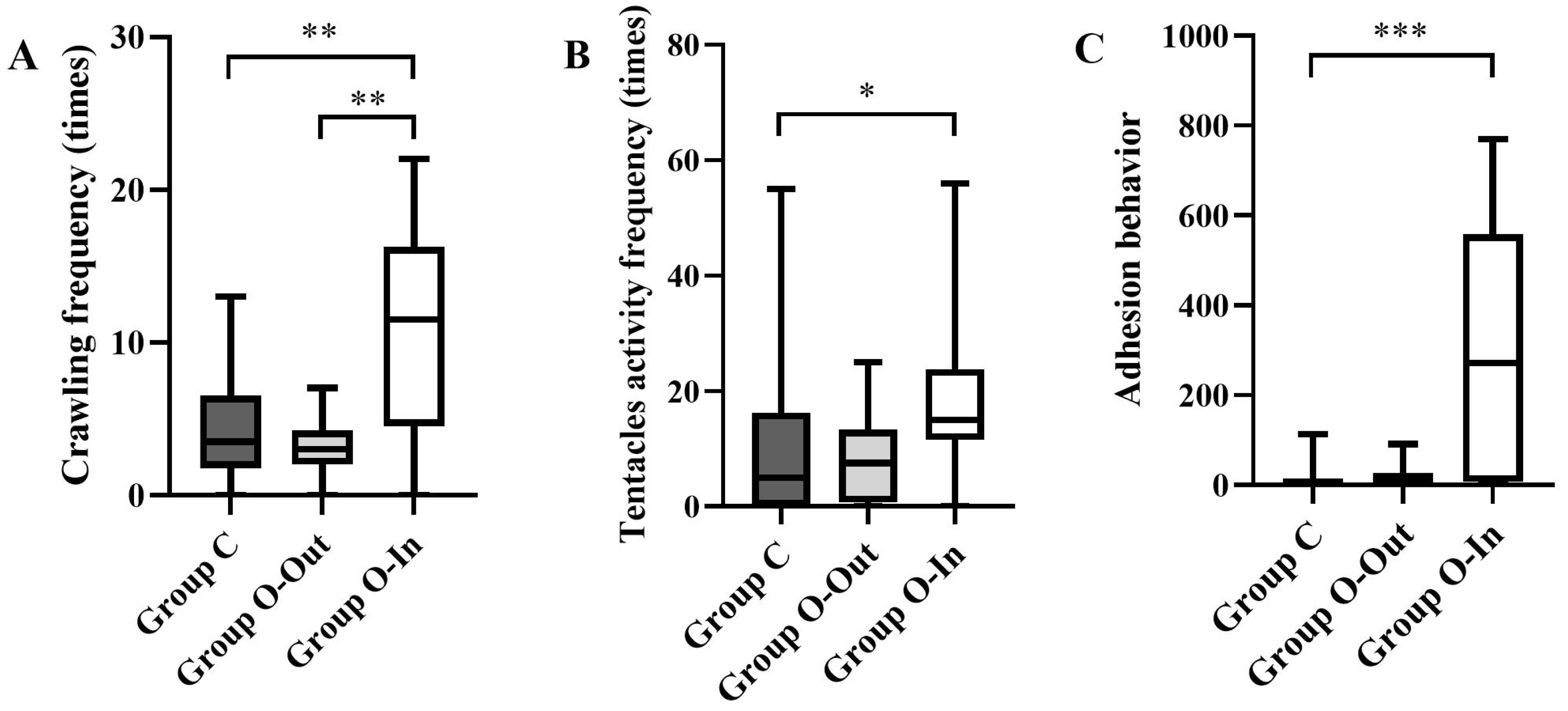
3.1.2. Crawling Frequency
3.1.3. Feeding Behavior
3.1.4. Adhesion Behavior
3.2. Experiment II
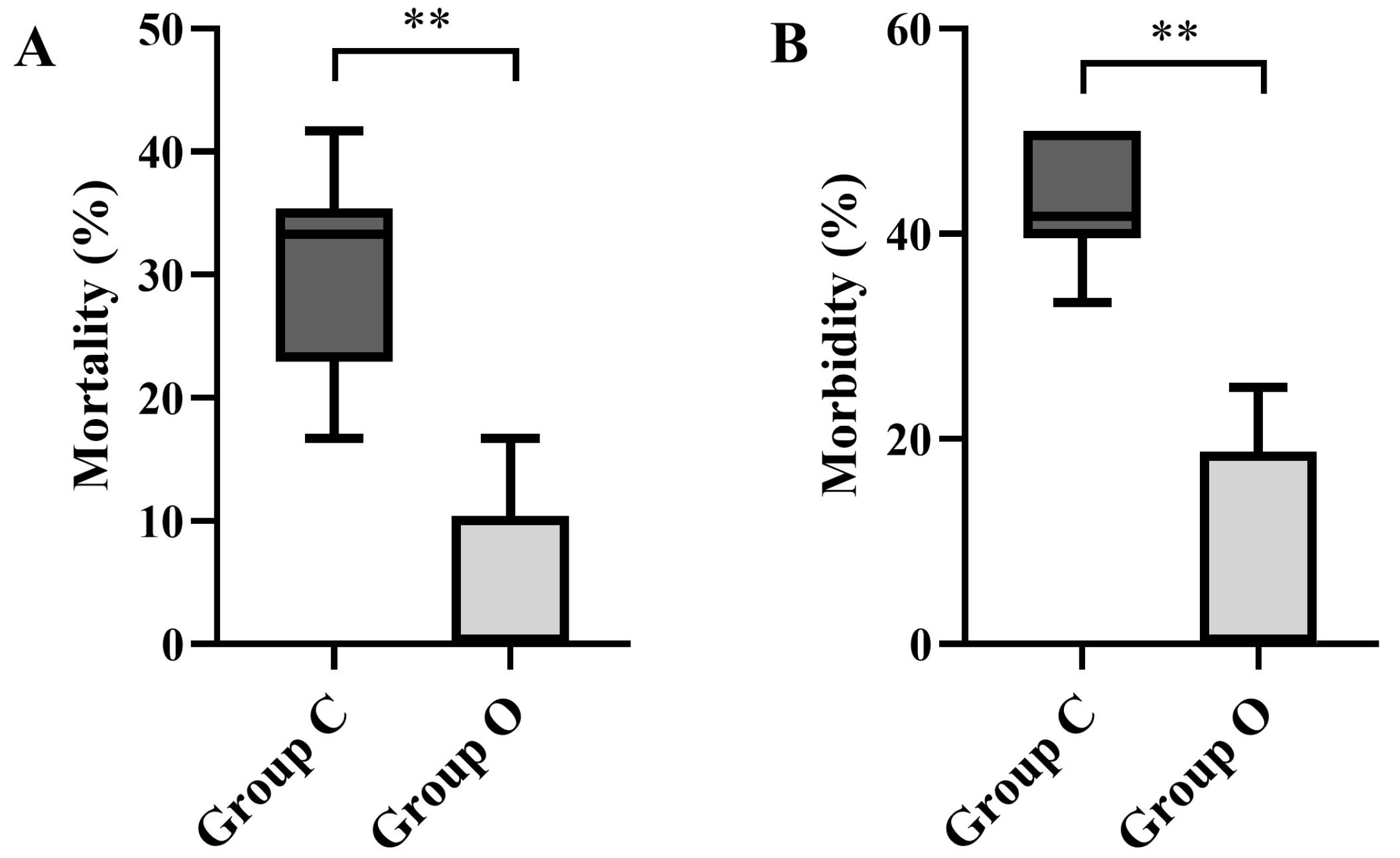
3.2.1. Mortality and Morbidity
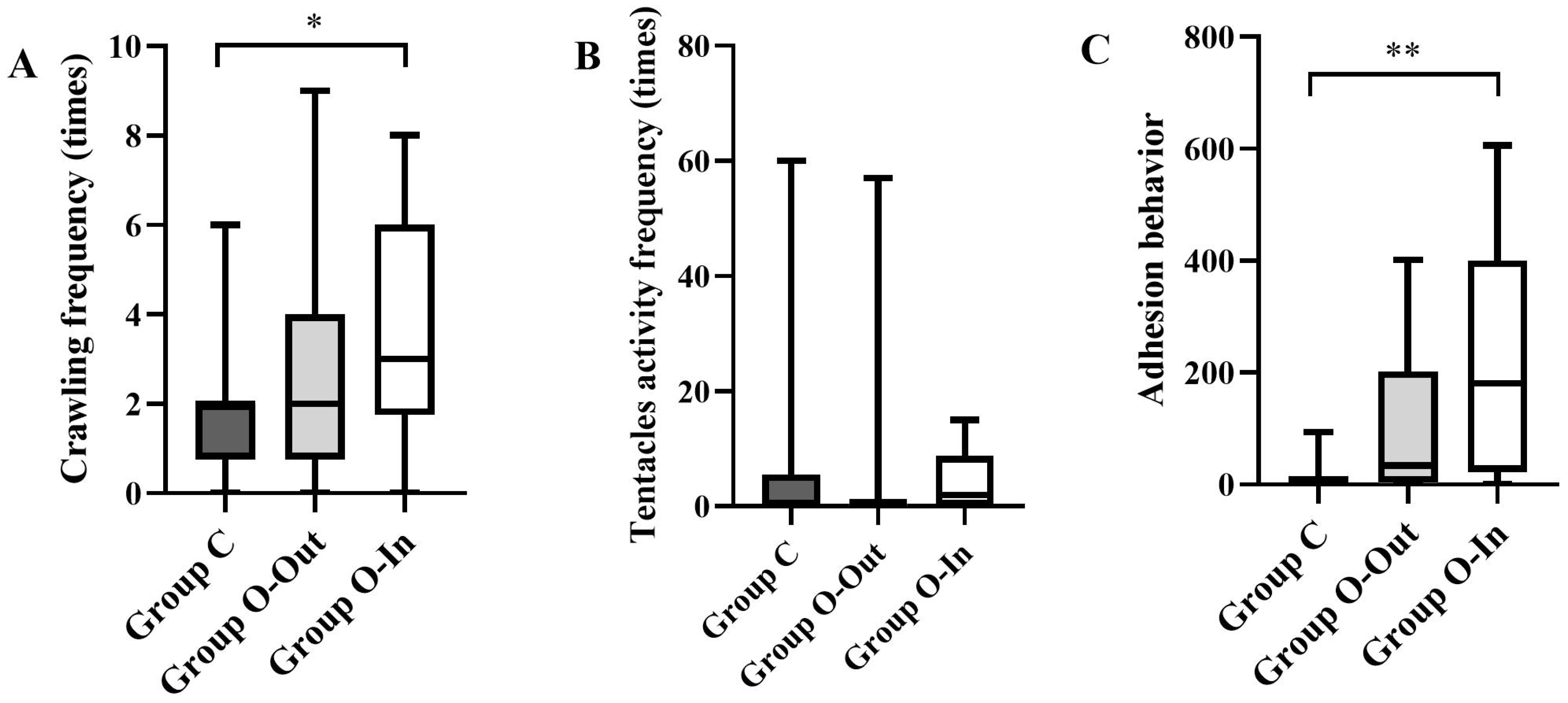
3.2.2. Crawling Frequency
3.2.3. Feeding Behavior
3.2.4. Adhesion Behavior
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.F. Study of Regional Differences in Nutrient Compositions and Bioactivities of Phospholipids in Sea Cucumber (Apostichopus japonicus). Ph.D. Thesis, Ocean University China, Qingdao, China, 2014. (In Chinese with an English Abstract). [Google Scholar]
- Yang, H.; Hamel, J.F.; Mercier, A. The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Jiang, S.H.; Ren, Y.C.; Tang, B.P.; Li, C.F.; Jiang, C.B. Development status and countermeasures of Apostichopus japonicus culture industry in China. J. Agric. Sci. Technol. 2017, 019, 15–23. [Google Scholar]
- Liu, X.Z. 2022 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2022; p. 23. (In Chinese) [Google Scholar]
- Chang, Y.; Yu, C.; Song, X. Pond Culture of Sea Cucumber, Apostichopus japonicus in Dalian. In Advances in Sea Cucumber Aquaculture and Management; Lovatelli, A., Conand, C., Purcell, S., Uthicke, S., Hamel, J.F., Mercier, A., Eds.; FAO: Rome, Italy, 2004; pp. 269–272. [Google Scholar]
- Li, C.; Feng, H.; Xu, D. Effect of seasonal high temperature on the immune response in Apostichopus japonicus by transcriptome analysis. Fish Shellfish Immunol. 2019, 92, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, S.; Li, D.; Liu, X.; Song, J. Research progress of effects and mechanism of environmental factors on physiology of sea cucumber Apostichopus japonicus. Hebei Fish. 2022, 11, 34–40, (In Chinese with an English Abstract). [Google Scholar]
- Hou, S.Y.; Jin, Z.W.; Jiang, W.W.; Chi, L.; Xia, B.; Chen, J.H. Physiological and immunological responses of sea cucumber Apostichopus japonicus during desiccation and subsequent resubmersion. PeerJ 2019, 7, e7427. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, Z.; Ren, Y.; Men, X.; Zheng, B.; Liu, P.; Xia, B. Effects of aerial exposure on oxidative stress, antioxidant and non-specific immune responses of juvenile sea cucumber Apostichopus japonicus under low temperature. Fish Shellfish Immunol. 2020, 101, 58–65. [Google Scholar] [CrossRef]
- Saeij, J.; Kemenade, V.V.; Muiswinkel, W.; Wiegertjes, G.F. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: In vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev. Comp. Immunol. 2003, 27, 233–245. [Google Scholar] [CrossRef]
- Tian, R.; Hu, F.; Wu, G.; Wang, H.; Ding, J.; Chang, Y.; Zhao, C. An effective approach to improving fitness-related behavior and digestive ability of small sea cucumbers Apostichopus japonicus at high temperature: New insights into seed production. Aquaculture 2023, 562, 738755. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Rheims, H.; Felske, A.; Pukall, R.; Tindall, B.J. Jannaschia helgolandensis, gen. nov., sp. nov., a novel abundant member of the marine Roseobacter clade from the North Sea. Int. J. Syst. Evol. Microbiol. 2003, 53, 731–738. [Google Scholar] [CrossRef]
- Wang, L.; Wei, C.; Chang, Y.; Ding, J. Response of bacterial community in sea cucumber Apostichopus japonicus intestine, surrounding water and sediment subjected to high-temperature stress. Aquaculture 2021, 535, 736353. [Google Scholar] [CrossRef]
- Eeckhaut, I.; Wayenberghe, K.V.; Nicolas, F.; Delroisse, J. Skin ulcerations in Holothuria scabra can be induced by various types of food. SPC Beche-De-Mer. Inf. Bull. 2019, 39, 31–35. [Google Scholar]
- Delroisse, J.; Wayneberghe, K.V.; Flammang, P.; Gillan, D.; Gerbaux, P.; Opina, N.; Todinanahary, G.G.B.; Eeck-haut, I. Epidemiology of a Skin Ulceration Disease (SKUD) in the Sea Cucumber Holothuria scabra with a Re-view on the SKUDs in Holothuroidea (Echinodermata). Sci. Rep. 2020, 10, 22150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Rong, X.J.; Zhang, C.Y.; Sun, S.F. Main diseases of cultured Apostichopus japonicus: Prevention and treatment. Mar. Sci. 2005, 9, 1–7, (In Chinese with an English Abstract). [Google Scholar]
- Deng, H.; He, C.; Zhou, Z.; Liu, C.; Tan, K.; Wang, N.; Jiang, B.; Gao, X.; Liu, W. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture 2009, 287, 18–27. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, C.; Ding, P.; Li, Y.; Tian, R.; Qiao, Y.; Chang, Y. An effective facility decreases disease transmission and promotes resistance ability of small sea urchins Strongylocentrotus intermedius: A potential application in the longline culture. Aquaculture 2021, 547, 737542. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, Z.; Tian, R.; Ding, P.; Yin, D.; Li, X.; Qiao, Y.; Chang, Y.; Zhao, C. A cost-effective approach to decreasing the disease transmission of the sea urchin Strongylocentrotus intermedius: New information for seed production and longline culture. Aquaculture 2021, 548, 737569. [Google Scholar] [CrossRef]
- Zhang, J.B. Experimental Studies on the Attractive Effects of Sea Cucumber Artificial Reefs on Apostichopus japonicus. Master’s Thesis, Ocean University China, Qingdao, China, 2011. (In Chinese with an English Abstract). [Google Scholar]
- Minami, K.; Masuda, R.; Takahashi, K.; Sawada, H.; Shirakawa, H.; Yamashita, Y. Seasonal and interannual variation in the density of visible Apostichopus japonicus (Japanese sea cucumber) in relation to sea water temperature. Estuar. Coast. Shelf Sci. 2019, 229, 106384. [Google Scholar] [CrossRef]
- Pei, S.; Dong, S.; Wang, F.; Tian, X.; Gao, Q. Effects of density on variation in individual growth and differentiation in endocrine response of Japanese sea cucumber (Apostichopus japonicus Selenka). Aquaculture 2012, 356–357, 398–403. [Google Scholar] [CrossRef]
- Xia, B.; Ren, Y.; Wang, Y.; Sun, Y.; Zhang, Z. Effects of feeding frequency and density on growth, energy budget and physiological performance of sea cucumber Apostichopus japonicus (Selenka). Aquaculture 2017, 466, 26–32. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, M.; Li, C.; Shao, Y.; Zhao, X.; Zhang, W. Divergent proteomics response of Apostichopus japonicus suffering from skin ulceration syndrome and pathogen infection. Comp. Biochem. 2019, 30, 196–205. [Google Scholar] [CrossRef]
- Lin, C.G. Effects of Four Physical Environment Factors on the Movement and Feeding Behavior of Sea Cucumber Apostichopus japonicus (Selenka). Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2014. (In Chinese with an English Abstract). [Google Scholar]
- Sun, J.M.; Zhang, L.B.; Pan, Y.; Lin, C.G.; Wang, F.; Yang, H.S. Feeding behavior and digestive physiology in sea cucumber Apostichopus japonicus. Physiol. Behav. 2015, 139, 336–343. [Google Scholar] [CrossRef]
- Yang, M.F.; Li, X.; Hu, F.Y.; Ning, Y.C.; Tian, R.H.; Ding, P.; Zhao, C. Effects of handling stresses on fitness related behaviors of small sea cucumbers Apostichopus japonicus: New insights into seed production. Aquaculture 2022, 546, 737321. [Google Scholar] [CrossRef]
- Chen, M.; Sun, S.; Xu, Q.; Gao, F.; Wang, H.; Wang, A. Influence of water temperature and flow velocity on locomotion behavior in tropical commercially important sea cucumber Stichopus monotuberculatus. Front. Mar. Sci. 2022, 9, 931430. [Google Scholar] [CrossRef]
- Clement, J.C.; Schagerström, E.; Dupont, S.; Jutfelt, F.; Ramesh, K. Roll, right, repeat: Short-term repeatability in the self-righting behaviour of a cold-water sea cucumber. J. Mar. Bio Assoc. 2020, 100, 115–120. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, X.; Zhou, Y.; Mao, Y.; Zhang, T.; Liu, Y. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (selenka) with special reference to aestivation. Aquac. Res. 2005, 36, 1085–1092. [Google Scholar] [CrossRef]
- Wang, F.Y.; Yang, H.S.; Gao, F.; Liu, G.B. Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 491–498. [Google Scholar] [CrossRef]
- Ross, E.; Behringer, D. Changes in temperature, pH, and salinity affect the sheltering responses of Caribbean spiny lobsters to chemosensory cues. Sci. Rep. 2019, 9, 4375. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Hu, F.; Qin, C.; Huang, X.; Sun, J.; Cui, Z.; Ding, J.; Yang, M.; Chang, Y.; Zhao, C. Conspecific alarm cues are a potential effective barrier to regulate foraging behavior of the sea urchin Mesocentrotus nudus. Mar. Environ. Res. 2021, 171, 105476. [Google Scholar] [CrossRef]
- Behringer, D.C.; Butler, M.J.; Shields, J.D. Avoidance of disease by social lobsters. Nature 2006, 441, 421. [Google Scholar] [CrossRef]
- Sun, J.; Yu, Y.; Zhao, Z.; Tian, R.; Li, X.; Chang, Y.; Zhao, C. Macroalgae and interspecific alarm cues regulate behavioral interactions between sea urchins and sea cucumbers. Sci. Rep. 2022, 12, 3971. [Google Scholar] [CrossRef] [PubMed]
- Vadas, R.L.; Elner, R.W. Responses to predation cues and food in two species of sympatric, tropical sea urchins. Mar. Ecol. 2003, 24, 101–121. [Google Scholar] [CrossRef]
- Cui, Y.; Guan, C.; Wang, R.; Tan, J.; Huang, B.; Li, J. The study of attractive effects of artificial reef models on Apostichopus japonicas. Prog. Mater. Sci. 2010, 31, 109–113, (In Chinese with an English Abstract). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, G.; Hu, F.; Tian, R.; Ding, J.; Chang, Y.; Su, Y.; Zhao, C. Artificial Reefs Reduce Morbidity and Mortality of Small Cultured Sea Cucumbers Apostichopus japonicus at High Temperature. J. Mar. Sci. Eng. 2023, 11, 948. https://doi.org/10.3390/jmse11050948
Wang H, Wu G, Hu F, Tian R, Ding J, Chang Y, Su Y, Zhao C. Artificial Reefs Reduce Morbidity and Mortality of Small Cultured Sea Cucumbers Apostichopus japonicus at High Temperature. Journal of Marine Science and Engineering. 2023; 11(5):948. https://doi.org/10.3390/jmse11050948
Chicago/Turabian StyleWang, Huiyan, Guo Wu, Fangyuan Hu, Ruihuan Tian, Jun Ding, Yaqing Chang, Yanming Su, and Chong Zhao. 2023. "Artificial Reefs Reduce Morbidity and Mortality of Small Cultured Sea Cucumbers Apostichopus japonicus at High Temperature" Journal of Marine Science and Engineering 11, no. 5: 948. https://doi.org/10.3390/jmse11050948
APA StyleWang, H., Wu, G., Hu, F., Tian, R., Ding, J., Chang, Y., Su, Y., & Zhao, C. (2023). Artificial Reefs Reduce Morbidity and Mortality of Small Cultured Sea Cucumbers Apostichopus japonicus at High Temperature. Journal of Marine Science and Engineering, 11(5), 948. https://doi.org/10.3390/jmse11050948






