Assessing Nutrient Limitation in Yeongsan River Estuary Using Bioassay Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Water Sampling
2.2. Experimental Method
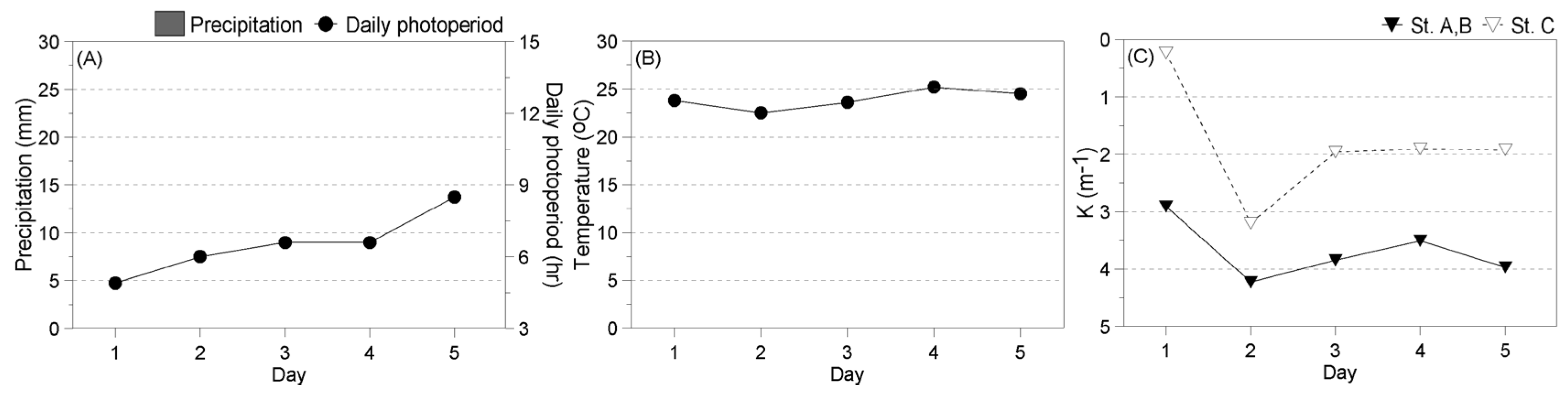
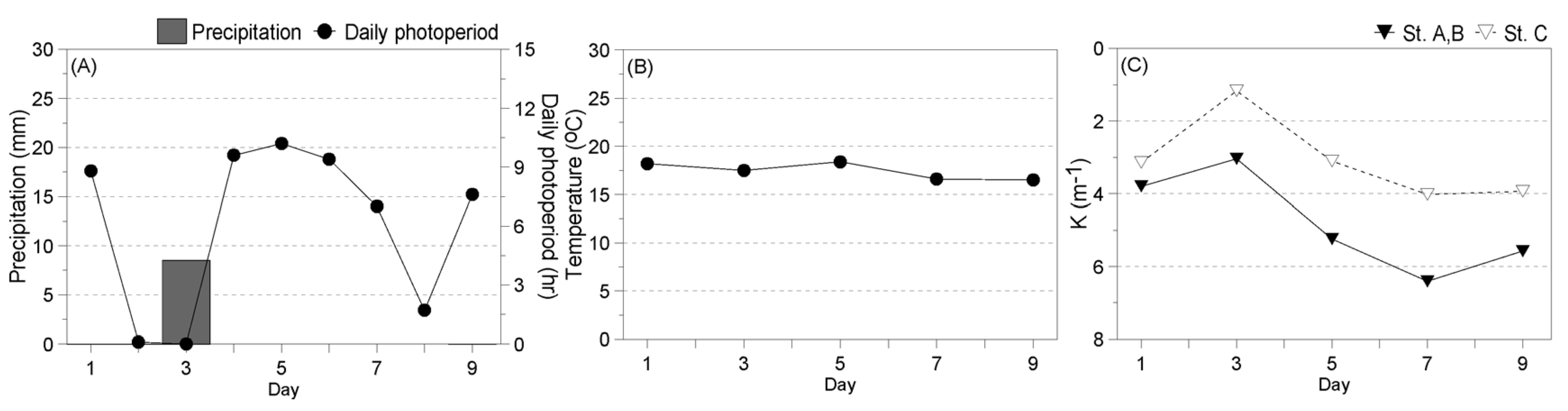
2.2.1. Environment during the Experiment
2.2.2. N:P Ratio Evaluation
2.2.3. Bioassay Experiment and Nutrient Addition
2.2.4. Phytoplankton Analysis
3. Results
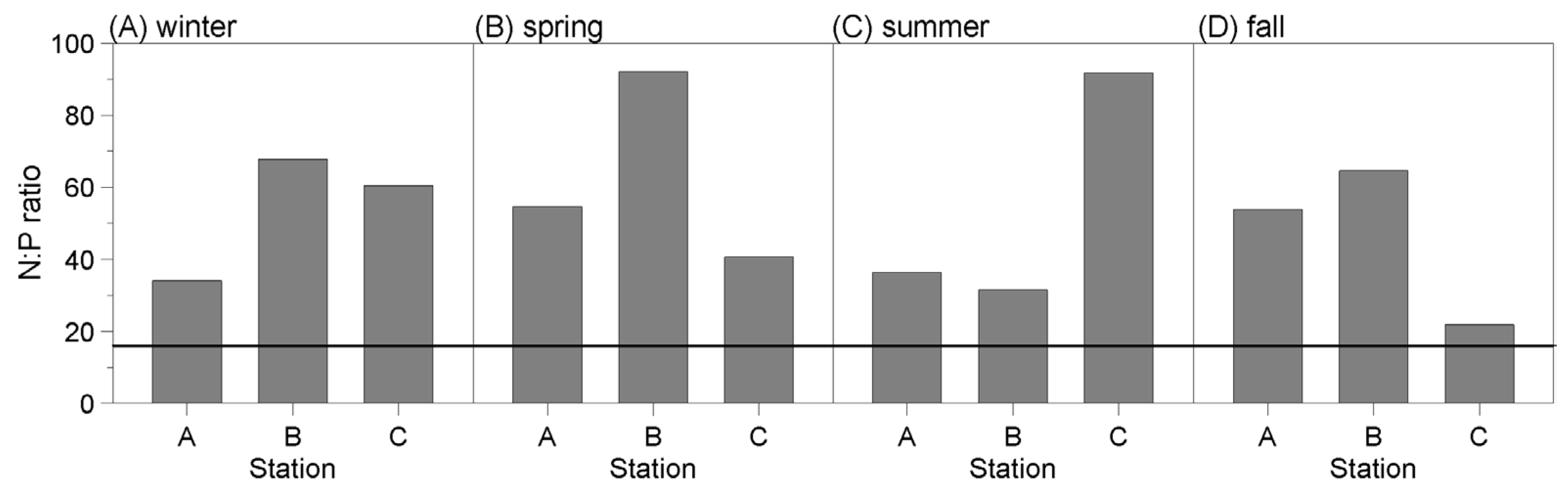
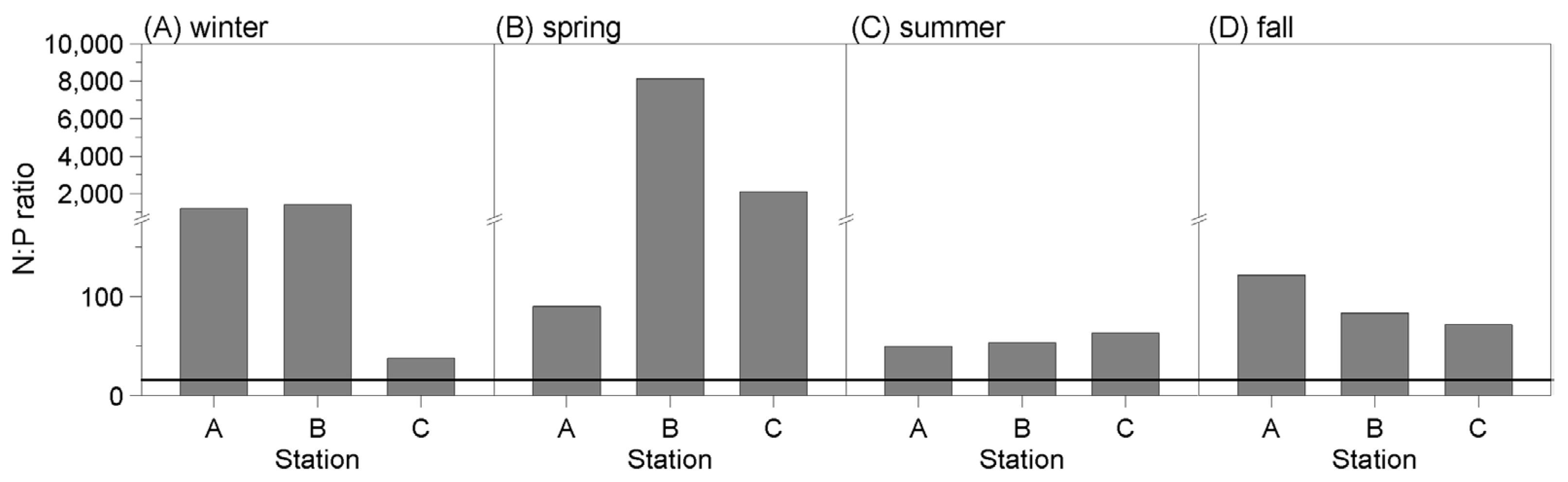
3.1. Assessment of N:P Ratio
3.1.1. N:P Ratio from 2004 to 2008
3.1.2. N:P Ratio during the Field Study
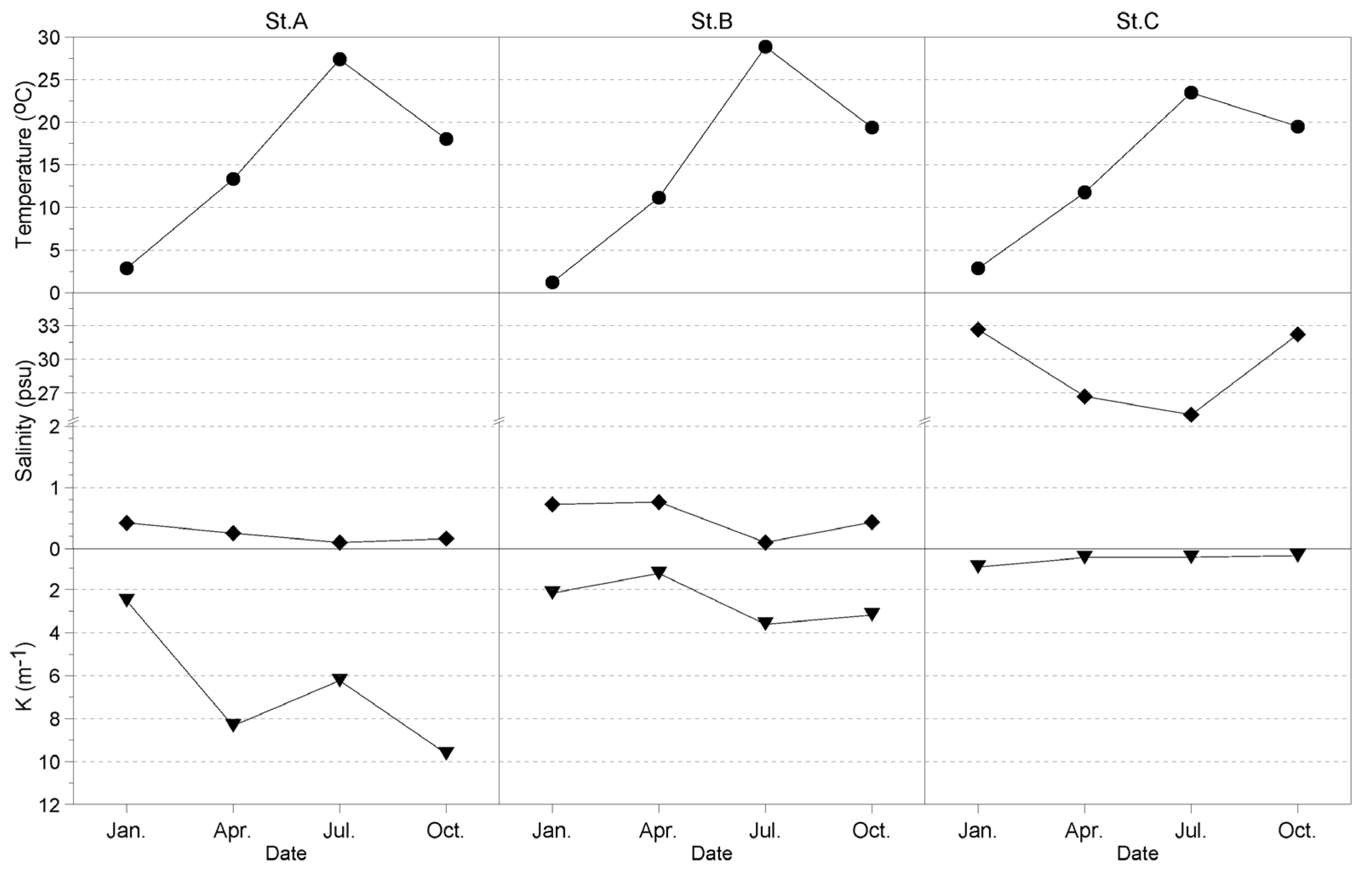
3.2. Environment during Field Survey
3.3. Bioassay Experiment
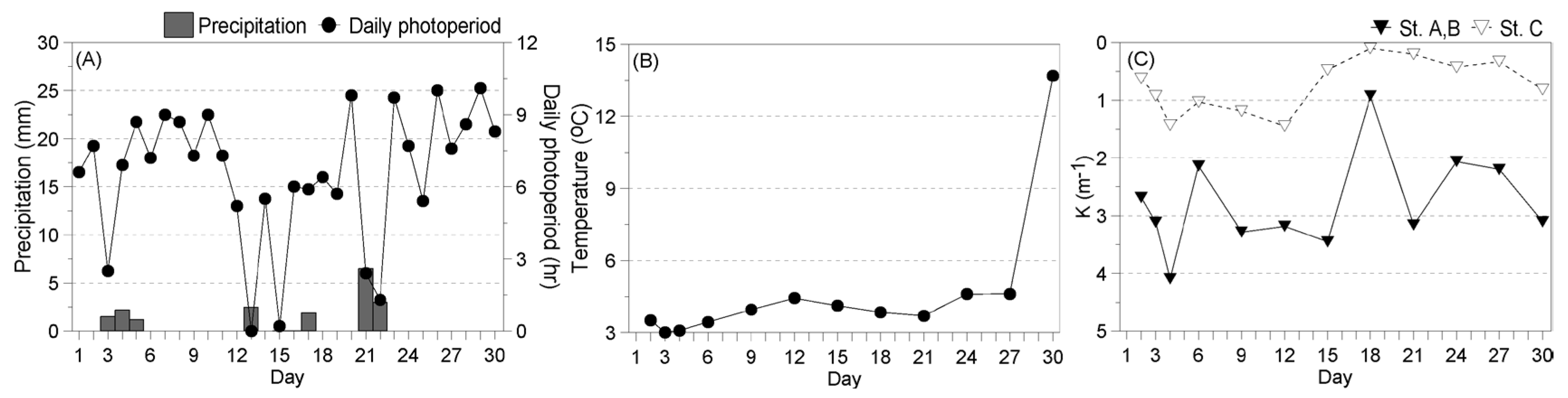
3.3.1. Environment during the Experiment
- Winter
- Spring
- Summer
- Fall
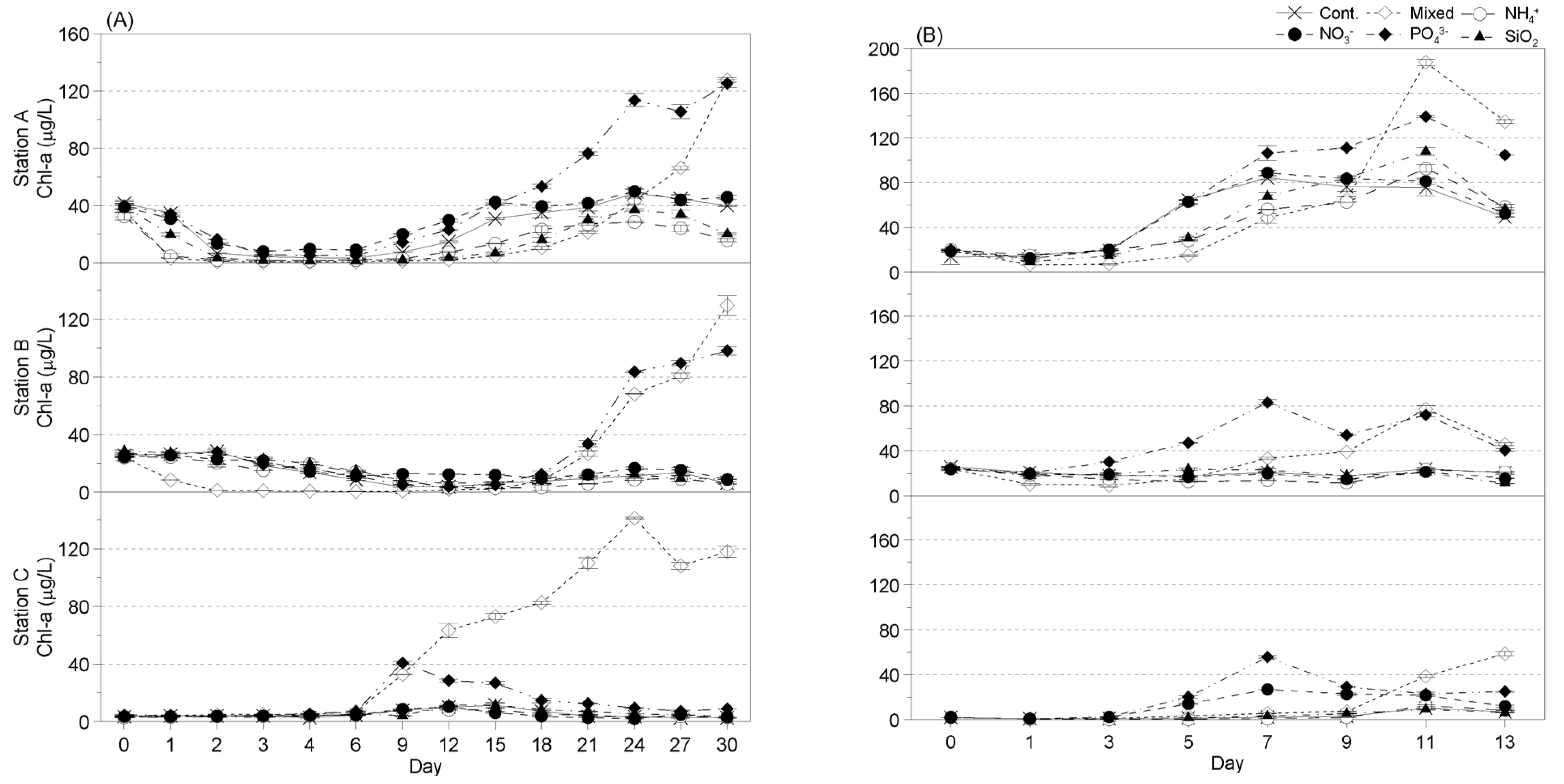
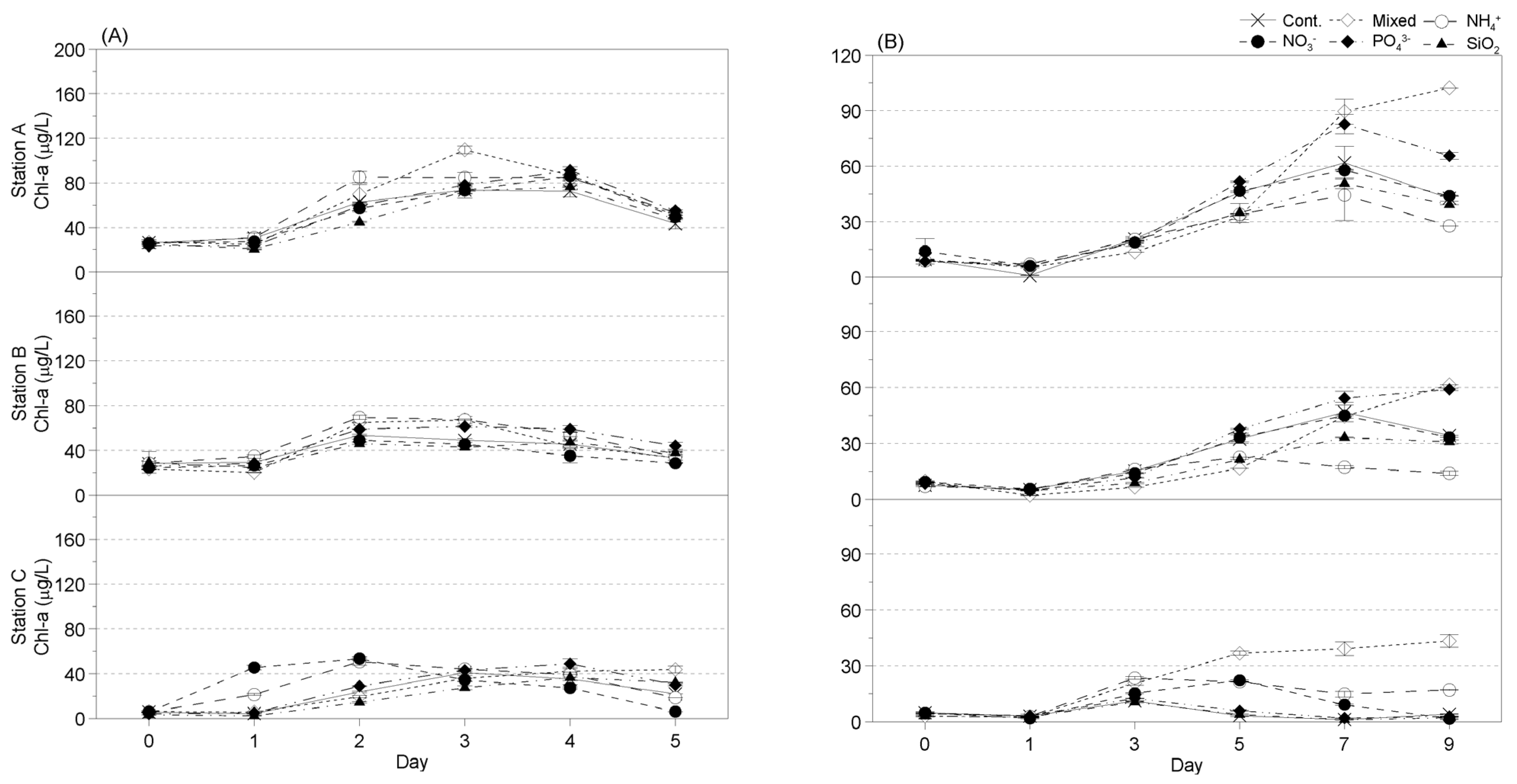
3.3.2. Seasonal Changes in Phytoplankton Chl-A Levels in Response to Nutrient Addition
- Winter
- Spring
- Summer
- Fall
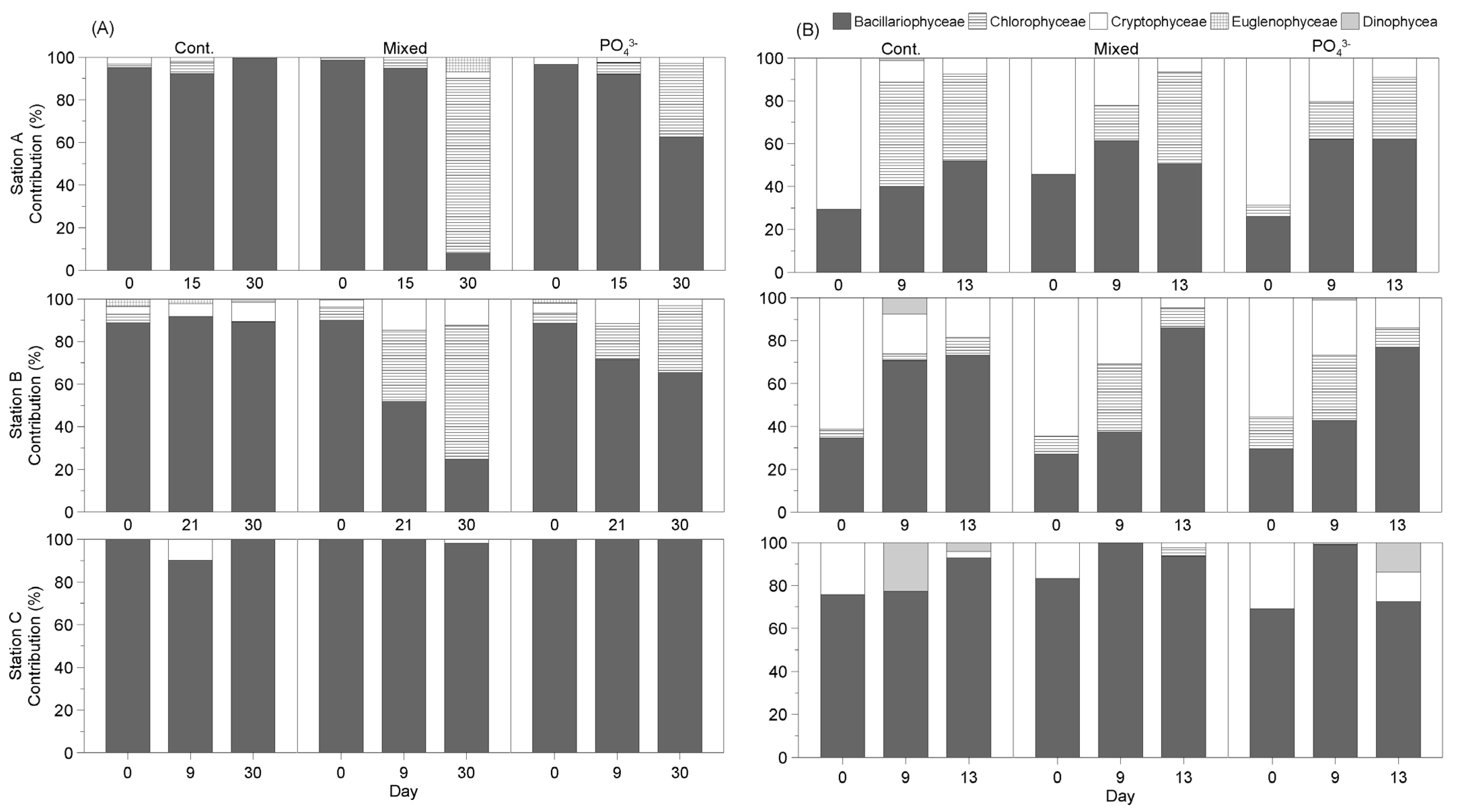
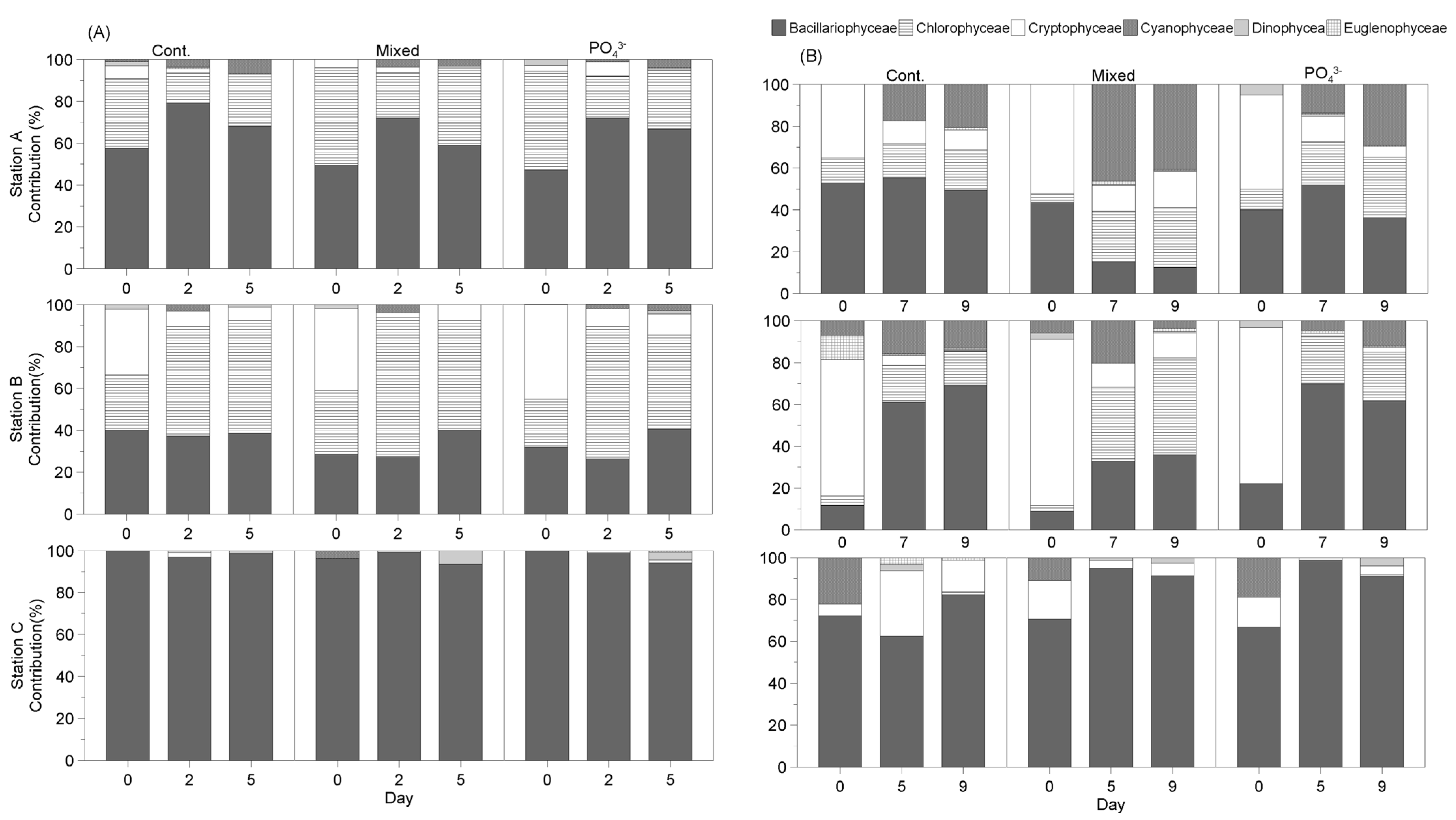
3.3.3. Seasonal Changes in Phytoplankton Community in Response to Nutrient Addition
- Winter
- Spring
- Summer
- Fall
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.S.; Kim, S.S. A study on sea water and ocean current in the Sea adjacent to Korea Peninsula. 1. Physical processes influencing the surface distributions of chlorophyll and nutrient in the southern sea of Korea in summer. Korean J. Fish Aquat. Sci. 1990, 23, 417–424. [Google Scholar]
- Kwon, K.Y.; Kim, C.H.; Kang, C.K.; Moon, C.H.; Park, M.O.; Yang, S.R. Limiting Nutrients for Phytoplankton Growth in the Seomjin River Estuary as Determined by Algal Bioassay experiment. Korean J. Fish Aquat. Sci. 2002, 35, 455–462. [Google Scholar]
- Song, E.; Shin, Y.S.; Jang, N.; Lee, J. Assessment of Nutrient and Light Limitation of Phytoplankton in the Youngsan Lake. Korean J. Limnol. 2010, 43, 35–43. [Google Scholar]
- Kagami, M.; Yoshida, T.; Gurung, T.B.; Urabe, J. Direct and indirect effects of zooplankton on algal composition in in situ grazing experiments. Oecologia 2002, 133, 356–363. [Google Scholar] [CrossRef]
- Keckeis, S.; Baranyi, C.; Hein, T.; Holarek, C.; Riedler, P.; Schiemer, F. The significance of zooplankton grazing in a floodplain system of the River Danube. J. Plankton Res. 2003, 25, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Uhm, S.H.; Hwang, S.J. Grazing Relationship between Phytoplankton and Zooplankton in Lake Paldang Ecosystem. Korean J. Limnol. 2006, 39, 390–401. [Google Scholar]
- Tilman, D. Resource competition between plankton algae: An experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Tilman, D. Ecological competition between algae: Experimental confirmation of resource-based competition. Theory Sci. 1978, 192, 463–465. [Google Scholar]
- Smith, V.H.; Willen, E.; Karlsson, B. Predicting the summer peak biomass of four species of blue-green algae (cyanphyta/cyanobacteria) in Swedish lakes. J. Am. Water Resour. Assoc. 1987, 23, 397–402. [Google Scholar] [CrossRef]
- Trimbee, A.M.; Prepas, E.E. Evaluation of total phosphorus as a predictor of the relative biomass of blue-green algae with emphasis on Alberta lakes. Can. J. Fish. Aquat. Sci. 1987, 44, 1337–1342. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; van Nes, E.H. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 1997, 78, 272–282. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P.; Li, S.; Tang, H.; Liu, H. The low TN:TP ratio, a cause or a result of Microcystis blooms? Water Res. 2003, 37, 2073–2080. [Google Scholar] [CrossRef]
- Choi, K.H.; Hwang, S.J.; Kim, H.S.; Han, M.S. Temporal changes of limiting nutrients and phytoplankton G-rowth rate in lake Paldang. Korean J. Limnol. 2003, 36, 139–149. [Google Scholar]
- Lee, S.H. Study on Nutrient Limitation and Trophic State in the Sumjin and Youngsan River System. Master’s Thesis, Mokpo National Maritime University, Mokpo, Republic of Korea, 2006. [Google Scholar]
- Kim, J.H.; Jeong, W.O.; Shin, Y.; Jeong, B. Evaluating Limiting nutrients through long-term data analyses and bioassay experiments in Cheonsu Bay and Taean Sea. J. Korean Soc. Mar. Environ. Saf. 2022, 28, 459–468. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–225. [Google Scholar]
- Kim, G.S. The Change of Pollution Loads flowing into Mokpo Harbour due to the Operation of Mokpo Municipal Sewage Treatment Plant. In Proceedings of the The Korean Society for Marine Environment and Energy, Ansan, Republic of Korea, 19–20 May 2000. [Google Scholar]
- Sin, Y.; Hyun, B.; Jeong, B.; Soh, H. Impacts of eutrophic freshwater inputs on water quality and phytoplankton size structure in a temperate estuary altered by a sea dike. Mar. Environ. Res. 2013, 85, 54–63. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yu, J.; Kwon, K.; Choi, Y.; Cho, E.S. Temporal and spatial variations of limiting nutrient on phytoplankton growth in the Gwangyang Bay, Korea. J. Korean Soc. Environ. Eng. 2004, 26, 890–895. [Google Scholar]
- Hyun, B.G.; Shin, K.; Kim, D.S.; Kim, Y.O.; Baek, S.H. Understanding of Phytoplankton Community Dynamic-s through Algae Bioassay Experiment during Winter Season of Jinhae bay, Korea. J. Korean Soc. Oceanogr. 2011, 16, 27–38. [Google Scholar]
- Son, M.; Baek, S.H. The distinct characteristics of phytoplankton growth response and their community struct-ure following seven different nutrients addition in spring season of Jinhae Bay. J. Korea Acad.-Ind. Coop. Soc. 2015, 16, 6567–6574. [Google Scholar]
- Yeongsan River Basin Environmental Office. Available online: http://www.me.go.kr/ysg (accessed on 20 April 2023).
- Ministry of Land. 2011 Long-Term Comprehensive Plan for Water Resources; Ministry of Land: Sejong, Republic of Korea, 2011; pp. 10–18.
- Kim, S.G.; Cha, G.S. Effect of water quality improvement with secure instream flow in Yeongsan river. J. Green Ind. Res. Honam Univ. 2011, 17, 45–51. [Google Scholar]
- Jung, J.W.; Lim, B.J.; Cho, S.H.; Choi, J.H.; Song, K.D.; Ha, D.W.; Kim, H.S.; Park, S.H.; Hwang, T.H.; Jung, S.J.; et al. The influence of land use on water quality in the tributary of the Yeongsan river basin. Korean J. Limnol. 2012, 45, 412–419. [Google Scholar]
- Song, J.J.; Kim, B.B.; Hong, S.G. Study on water quality change of Yeongsan river’s upstream. J. Korean Soc. Environ. Technol. 2015, 16, 154–159. [Google Scholar]
- Kim, J.S.; Kim, J.Y.; Seo, D.I. Effect of major pollution sources on algal blooms in the Seungchon weir and Juksan weir in the Yeongsan river Using EFDC. J. Korea Water Resour. Assoc. 2020, 53, 369–381. [Google Scholar]
- Kim, Y.W.; Lee, J.W.; Woo, S.Y.; Kim, S.J. Evaluation of stream flow and water quality changes of Yeongsan river basin by interbasin water transfer using SWAT. J. Korea Water Resour. Assoc. 2020, 53, 1081–1095. [Google Scholar]
- Korea Meteorological Administration. Available online: http://www.kma.go.kr (accessed on 25 April 2023).
- Marine Environmental Measurement Network. Available online: http://www.meis.go.kr (accessed on 21 March 2023).
- Water Environment Information System. Available online: http://water.nier.go.kr (accessed on 21 March 2023).
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical & Biological Methods for Seawater Analysis; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Jung, Y.H. Illustrated Encyclopedia of Fauna & Flora of Korea Vol. 9 Fresh Water Algae; The Ministry of Education: Sejong, Republic of Korea, 1968.
- Sim, J.H. Illustrated Encyclopedia of Fauna & Flora of Korea Vol. 34 Marine Phytoplankton; The Ministry of Education: Sejong, Republic of Korea, 1994.
- Schindler, D.W. Evolution of phosphate limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [Green Version]
- Fisher, T.R.; Peele, E.R.; Ammerman, J.W.; Harding, L. Nutrient limitation of phytoplankton in Chesapeake Bay. Mar. Ecol. Prog. Ser. 1992, 82, 51–63. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yamada, T.; Seiki, T.; Mukai, T.; Takimoto, K.K.; Okada, M. Effect of freshwater due to heavy rain on phytoplankton growth in Hiroshima Bay. Jap. Soc. Water Environ. (JSWE) 1996, 19, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of the sea water. Sea 1963, 2, 26–77. [Google Scholar]
- Jang, P.G.; Jang, M.C.; Lee, W.J.; Shin, K. Effects of nutrient property changes on summer phytoplankton community structure of Jangmok bay. Ocean Polar Res. 2010, 32, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.S. Variations in marine environmentes and phytoplankton community around Mokpo Harbour. J. Environ. Sci. Int. 2010, 19, 1323–1336. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.K. The Plankton Ecology of Korean Coastal Waters; Donghwa: Paju, Republic of Korea, 2011; pp. 30–104. [Google Scholar]
- Fisher, T.R.; Harding, L.W., Jr.; Stanley, D.W.; Ward, L.G. Phytoplankton, nutrients, and turbidity in the Chesapeake, Delaware, and Hudson estuaries. Estuar. Coast. Shelf Sci. 1988, 27, 61–93. [Google Scholar] [CrossRef]
- De Baar, H.J.W. von Liebig’s law of the minimum and plankton ecology (1899–1991). Prog. Oceanogr. 1994, 33, 347–386. [Google Scholar] [CrossRef] [Green Version]
- Begon, M.C.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; John Wiley & Sons: New York, NY, USA, 2012; pp. 574–583. [Google Scholar]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Stoecker, D.K. Mixotrophy among Dinoflagellates 1. J. Eukaryot. Microbiol. 1999, 46, 397–401. [Google Scholar] [CrossRef]
- Villiot, N.; Maas, A.E.; Poulton, A.J.; Blanco-Bercial, L. Organic and inorganic nutrients modulate taxonomic diversity and trophic strategies of small eukaryotes in oligotrophic oceans. FEMS Microbes 2023, 4, xtac029. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.S. The development of treatment system for removing the low concentrated nitrogen and phosphorus using phototrophic bacteria and media. Korean J. Microbiol. 2010, 46, 27–32. [Google Scholar]
- Baek, S.H.; You, K.; Han, M.S. Analysis of environmental factors related to seasonal variation of bacteria and heterotrophic nanoflagellate in Kyeonggi Bay, Korea. Korean J. Environ. Biol. 2017, 35, 198–206. [Google Scholar] [CrossRef]
- Kim, S.H.; Sin, Y.S. Bacterial distribution and relationship with phytoplankton in the Youngsan River estuary. J. Mar. Life Sci. 2019, 4, 53–62. [Google Scholar]



| NH4+ | NO3− | PO43− | SiO2 | Note | |
|---|---|---|---|---|---|
| Reagent added | NH4Cl | NaNO3 | K2HPO4 | Na2SiO3⋅9H2O | |
| Addition amount in freshwater zone (Station A and Station B) | 11 mg/L | 11 mg/L | 6 mg/L | 23 mg/L | Three times the maximum concentration in 2003–2010 |
| Addition amount in saltwater zone (Station C) | 4 mg/L | 5 mg/L | 2 mg/L | 18 mg/L | Twice the maximum concentration in 2003–2010 |
| Season | Station | Mixed | NH4+ | NO3− | PO43− | SiO2 |
|---|---|---|---|---|---|---|
| Winter | A | 0.016 | 0.002 | 0.126 | 0.103 | 0.012 |
| B | 0.098 | 0.552 | 0.082 | 0.026 | 0.231 | |
| C | 0.000 | 0.913 | 0.721 | 0.000 | 0.380 | |
| Spring | A | 0.474 | 0.706 | 0.637 | 0.152 | 0.895 |
| B | 0.327 | 0.065 | 0.093 | 0.000 | 0.806 | |
| C | 0.214 | 0.163 | 0.011 | 0.018 | 0.821 | |
| Summer | A | 0.564 | 0.248 | 0.931 | 1.000 | 0.686 |
| B | 0.954 | 0.184 | 0.133 | 0.386 | 0.525 | |
| C | 0.488 | 0.285 | 0.149 | 0.453 | 0.507 | |
| Fall | A | 0.817 | 0.419 | 0.840 | 0.564 | 0.525 |
| B | 0.908 | 0.272 | 0.954 | 0.488 | 0.326 | |
| C | 0.043 | 0.008 | 0.225 | 0.729 | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, B.B.; Jung, Y.-H.; Sin, Y. Assessing Nutrient Limitation in Yeongsan River Estuary Using Bioassay Experiments. J. Mar. Sci. Eng. 2023, 11, 1337. https://doi.org/10.3390/jmse11071337
Yoon BB, Jung Y-H, Sin Y. Assessing Nutrient Limitation in Yeongsan River Estuary Using Bioassay Experiments. Journal of Marine Science and Engineering. 2023; 11(7):1337. https://doi.org/10.3390/jmse11071337
Chicago/Turabian StyleYoon, Bo Bae, Yun-Hwan Jung, and Yongsik Sin. 2023. "Assessing Nutrient Limitation in Yeongsan River Estuary Using Bioassay Experiments" Journal of Marine Science and Engineering 11, no. 7: 1337. https://doi.org/10.3390/jmse11071337
APA StyleYoon, B. B., Jung, Y.-H., & Sin, Y. (2023). Assessing Nutrient Limitation in Yeongsan River Estuary Using Bioassay Experiments. Journal of Marine Science and Engineering, 11(7), 1337. https://doi.org/10.3390/jmse11071337








