Taxonomic and Morpho-Functional Photosynthetic Patterns of 18 Intertidal Macroalgal Species in the Guangdong–Hong Kong–Macao Greater Bay Area, China
Abstract
:1. Introduction
2. Materials and Methods
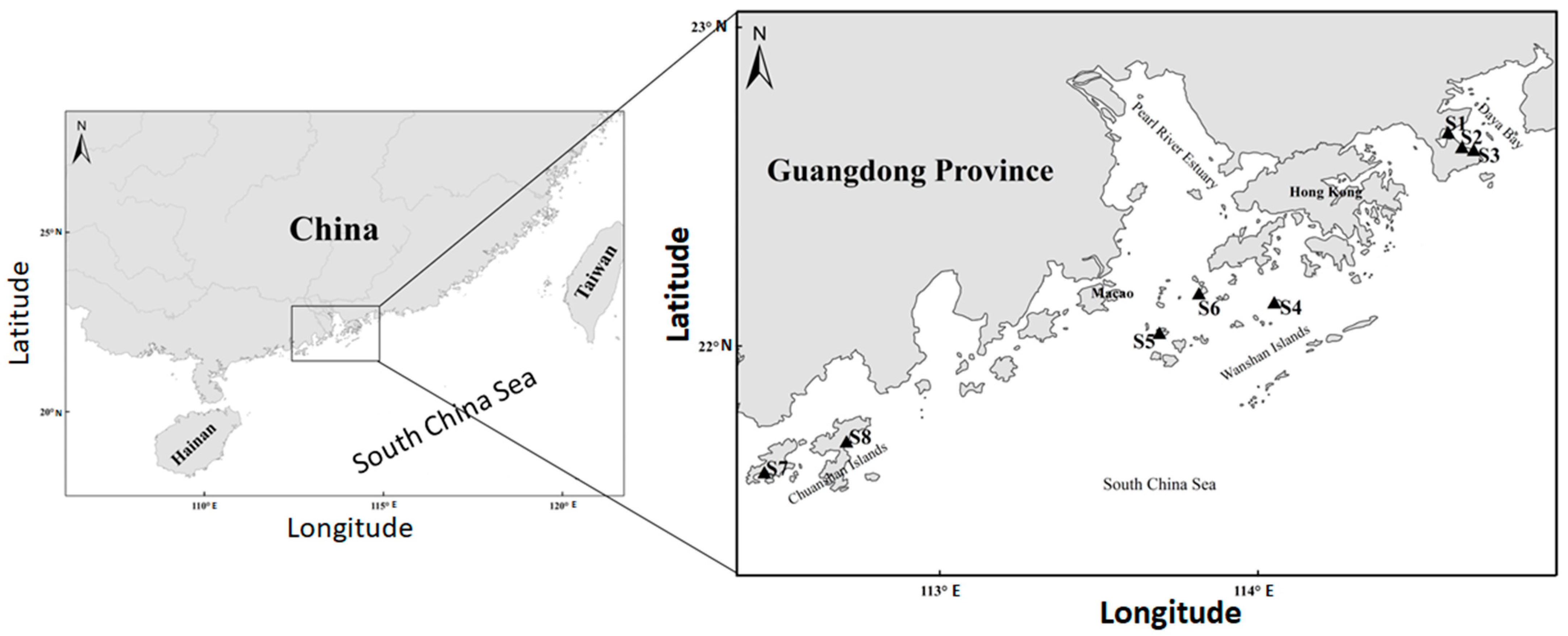
2.1. Sample Collection and Pre-Culture Protocol
2.2. Pigment Content Measurements
2.3. P vs. E Curve and Dark Respiration Measurements
2.4. Statistical Analysis
3. Results
3.1. Macroalgal Species and Biomass
3.2. Patterns of Pigments and Photosynthesis
3.3. Multivariate Analysis of Photosynthetic Patterns
3.4. Relationship of Algal Biomass and Photosynthetic Pattern
4. Discussion
4.1. Photosynthetic Patterns of Macroalgae in the Greater Bay Area
4.2. Predictability of Macroalgal Biomass through Their Photosynthetic Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, Y.; Gao, K. Effects of climate change factors on marine macroalgae: A review. Adv. Mar. Biol. 2020, 88, 91–136. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Ware, C.; Dijkstra, J.A.; Mello, K.; Stevens, A.; O’Brien, B.; Ikedo, W. A novel three-dimensional analysis of functional architecture that describes the properties of macroalgae as a refuge. Mar. Ecol. Prog. Ser. 2019, 608, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef]
- Nejrup, L.B.; Staehr, P.A.; Thomsen, M.S. Temperature- and light-dependent growth and metabolism of the invasive red algae Gracilaria vermiculophylla—A comparison with two native macroalgae. Eur. J. Phycol. 2013, 48, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Iñiguez, C.; Galmés, J.; Gordillo, F.J.L. Rubisco carboxylation kinetics and inorganic carbon utilization in polar versus cold-temperate seaweeds. J. Exp. Bot. 2019, 70, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Qin, Z.; Zhang, J.; Lin, Q.; Ni, G.; Tan, Y.; Zou, D. Algal density mediates the photosynthetic responses of a marine macroalga Ulva conglobata (Chlorophyta) to temperature and pH changes. Algal Res. 2020, 46, 101797. [Google Scholar] [CrossRef]
- Varela, D.A.; Santelices, B.; Correa, J.A.; Arroyo, M.K. Spatial and temporal variation of photosynthesis in intertidal Mazzaella laminarioides (Bory) Fredericq (Rhodophyta, Gigartinales). J. Appl. Phycol. 2006, 18, 827–838. [Google Scholar] [CrossRef]
- Gómez, I.; Navarro, N.P.; Huovinen, P. Bio-optical and physiological patterns in Antarctic seaweeds: A functional trait based approach to characterize vertical zonation. Prog. Oceanogr. 2019, 174, 17–27. [Google Scholar] [CrossRef]
- Borum, J.; Pedersen, M.; Krause-Jensen, D.; Christensen, P.; Nielsen, K. Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord, NE Greenland. Mar. Biol. 2002, 141, 11–19. [Google Scholar] [CrossRef]
- Pärnoja, M.; Kotta, J.; Orav-Kotta, H.; Paalme, T. Comparisons of individual and community photosynthetic production indicate light limitation in the shallow water macroalgal communities of the Northern Baltic Sea. Mar. Ecol. Evol. Persp. 2013, 35, 19–27. [Google Scholar] [CrossRef]
- Kaewsrikhaw, R.; Ritchie, R.J.; Prathep, A. Variations of tidal exposures and seasons on growth, morphology, anatomy and physiology of the seagrass Halophila ovalis (R.Br.) Hook. f. in a seagrass bed in Trang Province, Southern Thailand. Aquat. Bot. 2016, 130, 11–20. [Google Scholar] [CrossRef]
- Sant, N.; Ballesteros, E. Depth distribution of canopy-forming algae of the order Fucales is related to their photosynthetic features. Mar. Ecol. 2021, 42, e12651. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.W.; Kim, Y.K.; Park, S.R.; Lee, K.-S. Factors controlling the vertical zonation of the intertidal seagrass, Zostera japonica in its native range in the northwestern Pacific. Mar. Environ. Res. 2020, 157, 104959. [Google Scholar] [CrossRef]
- Bruno, J.F.; Lee, S.C.; Kertesz, J.S.; Carpenter, R.C.; Long, Z.T.; Duffy, J.E. Partitioning the effects of algal species identity and richness on benthic marine primary production. Oikos 2006, 115, 170–178. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014; p. 562. [Google Scholar]
- Gómez, I.; Huovinen, P. Morpho-functional patterns and zonation of South Chilean seaweeds: The importance of photo-synthetic and bio-optical traits. Mar. Ecol. Prog. Ser. 2011, 422, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Bruno, J.F.; Boyer, K.E.; Duffy, J.E.; Lee, S.C.; Kertesz, J.S. Effects of macroalgal species identity and richness on primary production in benthic marine communities. Ecol. Lett. 2005, 8, 1165–1174. [Google Scholar] [CrossRef]
- Migné, A.; Delebecq, G.; Davoult, D.; Spilmont, N.; Menu, D.; Gévaert, F. Photosynthetic activity and productivity of inter-tidal macroalgae: In situ measurements, from thallus to community scale. Aquat. Bot. 2015, 123, 6–12. [Google Scholar] [CrossRef]
- Rodgers, K.L.; Shears, N.T. Modelling kelp forest primary production using in situ photosynthesis, biomass and light measurements. Mar. Ecol. Prog. Ser. 2016, 553, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Ning, J.; Bao, W.; Yan, A.; Yin, Q. A study on the marine ecological security assessment of Guangdong-Hong Kong-Macao Great Bay Area. Mar. Pollut. Bull. 2022, 176, 113416. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, B.; Zheng, H.; Ouyang, Z. Evolution and simulation of ecosystem patterns in Guangdong-Hong Kong-Macau Bay Area. Acta Ecol. Sin. 2020, 40, 3364–3374. [Google Scholar]
- Wang, Y.; Lou, Z.; Sun, C.; Sun, S. Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar. Pollut. Bull. 2008, 56, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Huang, L.; Zhang, J.; Huang, X.; Zhang, J.; Yin, J.; Tan, Y.; Liu, S. Variation of phytoplankton biomass and primary production in Daya Bay during spring and summer. Mar. Pollut. Bull. 2004, 49, 1036–1044. [Google Scholar] [CrossRef]
- Liu, H.; Huang, L.; Song, X.; Zhong, Y. Using primary productivity as an index of coastal eutrophication: A case study in Daya Bay. Water Environ. J. 2012, 26, 235–240. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, A.; Qin, C.; Guo, Y.; Pan, W.; Chen, J.; Yu, G.; Li, C. Seasonal and spatial variation of protist communities from reef water and open ocean water in patchy coral reef areas of a semi-enclosed bay. Mar. Environ. Res. 2021, 169, 105407. [Google Scholar] [CrossRef]
- Shi, X.; Zou, D.; Hu, S.; Mai, G.; Ma, Z.; Li, G. Photosynthetic characteristics of three cohabitated macroalgae in the Daya Bay, and their responses to temperature rises. Plants 2021, 10, 2441. [Google Scholar] [CrossRef]
- Wan, M.; Wang, Z.; Mai, G.; Ma, Z.; Xia, X.; Tan, Y.; Li, G. Photosynthetic characteristics of macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with special reference to the effects of light quality. Sustainability 2022, 14, 8063. [Google Scholar] [CrossRef]
- Desmond, M.J.; Pritchard, D.W.; Hurd, C.L.; Richards, D.K.; Schweikert, K.; Wing, S.; Hepburn, C.D. Superior photosynthetic performance of the invasive kelp Undaria pinnatifida may contribute to continued range expansion in a wave-exposed kelp forest community. Mar. Biol. 2019, 166, 1–11. [Google Scholar] [CrossRef]
- Wu, W.; Yan, J.; Song, D. Study on the tidal dynamics in Daya Bay, China-Part I. Observation and numerical simulation of tidal dynamic system. J. Trop. Oceanogr. 2017, 36, 34–45. [Google Scholar]
- Liu, L.; Liu, Z.; He, Q.; Yang, Y.; Zou, D. Distribution patterns of intertidal macroalgal diversity and biomass in the intertidal zone of Guangdong-Hong Kong-Macao Greater Bay Area. Chin. J. Ecol. 2023, 42, 677–684. [Google Scholar]
- Wernberg, T.; Goldberg, N. Short-term temporal dynamics of algal species in a subtidal kelp bed in relation to changes in environmental conditions and canopy biomass. Estuar. Coast. Shelf Sci. 2008, 76, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Spector, M.; Edwards, M.S. Species-specific biomass drives macroalgal benthic primary production on temperate rocky reefs. Algae 2020, 35, 237–252. [Google Scholar] [CrossRef]
- Zeng, C. Chinese Seaweed Flora; Science Press: Beijing, China, 2000. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Henley, W.J. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 1993, 29, 729–739. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Santos, D.Y.A.C. Growth and photosynthetic pigments of Gracilariopsis tenuifrons (Rhodophyta, Gracilariaceae) under high light in vitro culture. J. Appl. Phycol. 2015, 27, 1243–1251. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; He, P. Effects of solar radiation on photosynthesis and pigmentation in the red alga Pyropia yezoensis Ueda (Bangiales, Rhodophyta). Indian J. Geo-Mar. Sci. 2014, 43, 473–480. [Google Scholar]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Chaves, P.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Physiological and biochemical responses driven by different UV-visible radiation in Osmundea pinnatifida (Hudson) Stackhouse (Rhodophyta). Photochem. Photobiol. Sci. 2020, 19, 1650–1664. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.; Proctor, M.C.F. Are Bryophytes Shade Plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann. Bot. 2004, 94, 593–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, I.; López-Figueroa, F.; Ulloa, N.; Morales, V.; Lovengreen, C.; Huovinen, P.; Hess, S. Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar. Ecol. Prog. Ser. 2004, 270, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Sant, N.; Ballesteros, E. Photosynthetic activity of macroalgae along a bathymetric gradient: Interspecific and seasonal variability. Sci. Mar. 2020, 84, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Gong, J.; Lou, W.; Zou, D. Photosynthetic behaviors in response to intertidal zone and algal mat density in Ulva lactuca (Chlorophyta) along the coast of Nan’ao Island, Shantou, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 13346–13353. [Google Scholar] [CrossRef]
- Reed, D.C.; Rassweiler, A.; Arkema, K.K. Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology 2008, 89, 2493–2505. [Google Scholar] [CrossRef]
- Tait, L.W.; Schiel, D.R. Dynamics of productivity in naturally structured macroalgal assemblages: Importance of canopy structure on light-use efficiency. Mar. Ecol. Prog. Ser. 2011, 421, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Park, S.R.; Kim, S.; Kim, Y.K.; Kang, C.K.; Lee, K.S. Photoacclimatory responses of Zostera marina in the intertidal and subtidal zones. PLoS One 2016, 11, e0156214. [Google Scholar] [CrossRef] [Green Version]
- Desmond, M.J.; Pritchard, D.W.; Hepburn, C.D. Light dose versus rate of delivery: Implications for macroalgal productivity. Photosynth. Res. 2017, 132, 257–264. [Google Scholar] [CrossRef]
- Abidizadegan, M.; Khalesi, M.K.; Ajdari, D. Depth-dependent pigment fluctuations in the agarophyte Gracilaria corticata at intertidal waters. Thalassas 2018, 34, 247–253. [Google Scholar] [CrossRef]





| Species | Phylum | Morphology | Location | Biomass (g FW m−2) |
|---|---|---|---|---|
| Daya Bay | ||||
| Ulva fasciata (UF) | Green algae | Sheet-like (S-L) | Upper intertidal area | 1328 |
| Ulva linza (UL) | Green algae | Sheet-like (S-L) | Lower intertidal area | 2144 |
| Ulva conglobata (UC) | Green algae | Sheet-like (S-L) | Upper intertidal area | 1216 |
| Amphiroa ephedraea (AE) | Red algae | Finely branched (FB) | Upper intertidal area | 171 |
| Scinaia boergesenii (SB) | Red algae | Finely branched (FB) | Lower intertidal area | 747 |
| Sargassum hemiphyllum (SHM) | Brown algae | Canopy-forming (C-F) | Lower intertidal area | 7111 |
| Sargassum graminifolium (SGM) | Brown algae | Canopy-forming (C-F) | Lower intertidal area | 1636 |
| Sargassum glaucescens (SGS) | Brown algae | Canopy-forming (C-F) | Lower intertidal area | 5600 |
| Sargassum henslowianum (SHN) | Brown algae | Canopy-forming (C-F) | Lower intertidal area | 3733 |
| Wanshan Islands | ||||
| Ulva conglobata (UC) | Green algae | Sheet-like (S-L) | Upper intertidal area | 2080 |
| Ulva fasciata (UF) | Green algae | Sheet-like (S-L) | Upper intertidal area | 1467 |
| Pterocladiella capillacea (PC) | Red algae | Finely branched (FB) | Lower intertidal area | 5813 |
| Gelidium pacificum (GP) | Red algae | Finely branched (FB) | Lower intertidal area | 725 |
| Laurencia okamurae (LO) | Red algae | Coarsely branched (CB) | Upper intertidal area | 320 |
| Sargassum hemiphyllum (SHM) | Brown algae | Canopy-forming (C-F) | Lower intertidal area | 4245 |
| Chuanshan Islands | ||||
| Ulva conglobata (UC) | Green algae | Sheet-like (S-L) | Upper intertidal area | 1227 |
| Corallina officinalis (CO) | Red algae | Sheet-like (S-L) | Lower intertidal area | 667 |
| Hypnea chordacea (HC) | Red algae | Coarsely branched (CB) | Upper intertidal area | 187 |
| Grateloupia livida (GL) | Red algae | Sheet-like (S-L) | Upper intertidal area | 171 |
| Gracilaria biodgettii (GB) | Red algae | Coarsely branched (CB) | Upper intertidal area | 149 |
| Chondrus ocellatus (CO) | Red algae | Finely branched (FB) | Upper intertidal area | 480 |
| Chondracanthus intermedius (CI) | Red algae | Coarsely branched (CB) | Upper intertidal area | 187 |
| Species | Chl a | Car | Chl a/Car | α | Pmax | EK | EC | Rd | Rd/Pmax |
|---|---|---|---|---|---|---|---|---|---|
| Green algae | |||||||||
| U. linza (D) | 0.24 ± 0.02 | 0.17 ± 0.02 | 1.42 ± 0.01 | 0.36 ± 0.03 | 65.55 ± 3.54 | 230.92 ± 24.94 | 46.00 ± 6.77 | 16.30 ± 1.83 | 0.25 ± 0.04 |
| U. fasciata (D) | 0.23 ± 0.02 | 0.17 ± 0.01 | 1.40 ± 0.01 | 0.41 ± 0.03 | 91.31 ± 6.05 | 274.67 ± 9.98 | 53.71 ± 9.95 | 22.04 ± 3.36 | 0.24 ± 0.05 |
| U. conglobata (D) | 0.41 ± 0.02 | 0.28 ± 0.03 | 1.46 ± 0.08 | 0.61 ± 0.04 | 184.00 ± 6.47 | 329.99 ± 36.10 | 26.17 ± 2.85 | 15.86 ± 0.80 | 0.09 ± 0.01 |
| U. conglobata (W) | 0.49 ± 0.02 | 0.39 ± 0.01 | 1.25 ± 0.02 | 0.65 ± 0.01 | 181.86 ± 8.31 | 294.57 ± 11.47 | 16.31 ± 2.80 | 10.68 ± 1.92 | 0.06 ± 0.01 |
| U. fasciata (W) | 0.38 ± 0.01 | 0.27 ± 0.01 | 1.40 ± 0.01 | 0.80 ± 0.02 | 265.30 ± 5.46 | 351.31 ± 14.25 | 20.17 ± 3.45 | 16.14 ± 2.65 | 0.06 ± 0.01 |
| U. conglobata (C) | 1.10 ± 0.08 | 0.75 ± 0.05 | 1.47 ± 0.01 | 0.61 ± 0.03 | 235.47 ± 8.01 | 384.07 ± 11.12 | 12.10 ± 1.24 | 7.44 ± 1.00 | 0.03 ± 0.01 |
| Red algae | |||||||||
| A. ephedraea (D) | 0.14 ± 0.02 | 0.05 ± 0.01 | 2.94 ± 0.09 | 0.10 ± 0.01 | 45.27 ± 1.25 | 500.20 ± 54.58 | 42.36 ± 10.72 | 4.15 ± 0.75 | 0.09 ± 0.01 |
| S. boergesenii (D) | 0.03 ± 0.01 | 0.01 ± 0.01 | 2.25 ± 0.13 | 0.05 ± 0.01 | 11.24 ± 0.57 | 270.33 ± 11.05 | 28.98 ± 7.02 | 1.33 ± 0.26 | 0.12 ± 0.03 |
| G. pacificum (W) | 0.49 ± 0.05 | 0.18 ± 0.01 | 2.72 ± 0.10 | 0.25 ± 0.03 | 82.60 ± 2.52 | 371.05 ± 52.36 | 33.65 ± 11.07 | 8.06 ± 1.69 | 0.10 ± 0.02 |
| L. okamurae (W) | 0.15 ± 0.02 | 0.07 ± 0.01 | 2.33 ± 0.08 | 0.22 ± 0.05 | 54.23 ± 1.72 | 289.06 ± 45.51 | 29.98 ± 6.04 | 6.48 ± 1.82 | 0.12 ± 0.03 |
| P. capillacea (W) | 0.44 ± 0.04 | 0.19 ± 0.01 | 2.36 ± 0.01 | 0.28 ± 0.03 | 86.15 ± 5.14 | 348.60 ± 25.67 | 36.43 ± 2.13 | 10.18 ± 1.57 | 0.12 ± 0.02 |
| C. intermedius (C) | 0.20 ± 0.01 | 0.08 ± 0.01 | 2.44 ± 0.10 | 0.14 ± 0.02 | 42.50 ± 4.89 | 336.24 ± 19.56 | 28.34 ± 8.76 | 3.77 ± 0.69 | 0.09 ± 0.02 |
| C. ocellatus (C) | 0.27 ± 0.02 | 0.11 ± 0.01 | 2.36 ± 0.07 | 0.33 ± 0.02 | 74.15 ± 6.74 | 239.45 ± 7.39 | 14.40 ± 1.17 | 4.72 ± 0.31 | 0.06 ± 0.01 |
| C. officinalis (C) | 0.33 ± 0.01 | 0.15 ± 0.01 | 2.26 ± 0.01 | 0.09 ± 0.02 | 46.18 ± 4.20 | 647.72 ± 168.61 | 88.63 ± 15.43 | 7.50 ± 0.99 | 0.16 ± 0.02 |
| G. biodgettii (C) | 0.30 ± 0.01 | 0.12 ± 0.01 | 2.53 ± 0.02 | 0.21 ± 0.01 | 64.15 ± 3.73 | 334.88 ± 27.53 | 22.32 ± 1.06 | 4.59 ± 0.13 | 0.07 ± 0.01 |
| G. livida (C) | 0.33 ± 0.02 | 0.11 ± 0.01 | 2.87 ± 0.22 | 0.20 ± 0.01 | 44.72 ± 0.64 | 293.69 ± 12.68 | 72.40 ± 2.92 | 14.73 ± 1.57 | 0.33 ± 0.04 |
| H. chordacea (C) | 0.27 ± 0.01 | 0.13 ± 0.01 | 2.13 ± 0.08 | 0.13 ± 0.01 | 30.88 ± 3.85 | 282.14 ± 62.71 | 47.07 ± 13.32 | 6.15 ± 1.22 | 0.20 ± 0.01 |
| Brown algae | |||||||||
| S. glaucescens (D) | 0.70 ± 0.05 | 0.40 ± 0.03 | 1.75 ± 0.02 | 0.26 ± 0.02 | 75.95 ± 0.72 | 309.11 ± 26.27 | 20.33 ± 1.58 | 5.33 ± 0.01 | 0.07 ± 0.01 |
| S. graminifolium (D) | 0.66 ± 0.05 | 0.23 ± 0.01 | 2.89 ± 0.01 | 0.20 ± 0.01 | 55.25 ± 4.32 | 307.96 ± 13.58 | 28.33 ± 4.92 | 5.58 ± 0.94 | 0.10 ± 0.02 |
| S. hemiphyllum (D) | 0.62 ± 0.02 | 0.19 ± 0.01 | 3.29 ± 0.03 | 0.25 ± 0.01 | 83.05 ± 4.03 | 364.10 ± 16.41 | 28.67 ± 6.48 | 7.11 ± 1.65 | 0.09 ± 0.02 |
| S. henslowianum (D) | 0.59 ± 0.02 | 0.32 ± 0.02 | 1.84 ± 0.02 | 0.22 ± 0.01 | 79.55 ± 10.78 | 400.33 ± 44.45 | 46.27 ± 7.88 | 10.41 ± 1.81 | 0.14 ± 0.04 |
| S. hemiphyllum (W) | 0.47 ± 0.03 | 0.22 ± 0.02 | 2.18 ± 0.05 | 0.20 ± 0.02 | 77.72 ± 7.00 | 434.42 ± 23.65 | 44.11 ± 4.89 | 8.78 ± 1.22 | 0.11 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, L.; Qin, Y.; Lin, Q.; Ni, G.; Chen, B.; Ma, Z.; Zou, D.; Li, G. Taxonomic and Morpho-Functional Photosynthetic Patterns of 18 Intertidal Macroalgal Species in the Guangdong–Hong Kong–Macao Greater Bay Area, China. J. Mar. Sci. Eng. 2023, 11, 1409. https://doi.org/10.3390/jmse11071409
He Q, Liu L, Qin Y, Lin Q, Ni G, Chen B, Ma Z, Zou D, Li G. Taxonomic and Morpho-Functional Photosynthetic Patterns of 18 Intertidal Macroalgal Species in the Guangdong–Hong Kong–Macao Greater Bay Area, China. Journal of Marine Science and Engineering. 2023; 11(7):1409. https://doi.org/10.3390/jmse11071409
Chicago/Turabian StyleHe, Quan, Linqing Liu, Yujie Qin, Qiang Lin, Guangyan Ni, Binbin Chen, Zengling Ma, Dinghui Zou, and Gang Li. 2023. "Taxonomic and Morpho-Functional Photosynthetic Patterns of 18 Intertidal Macroalgal Species in the Guangdong–Hong Kong–Macao Greater Bay Area, China" Journal of Marine Science and Engineering 11, no. 7: 1409. https://doi.org/10.3390/jmse11071409






