Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment
Abstract
:1. Introduction
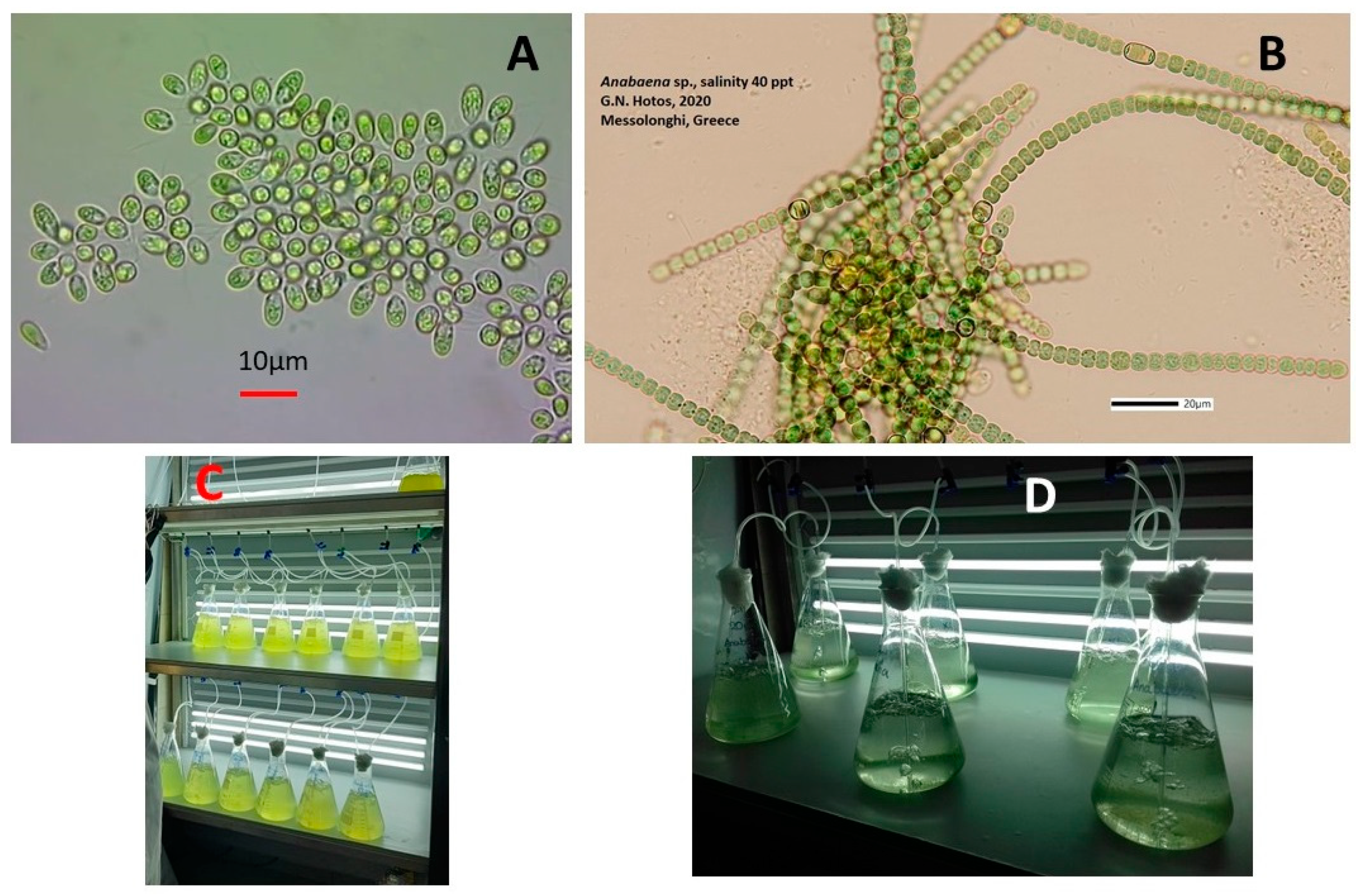
2. Materials and Methods
- PCyield = mg of phycocyanin per g algal dry weight
- V = volume of solvent used (mL)
- D.W. = grams of dry weight of the algal mass used
3. Results
3.1. Dunaliella sp.
3.1.1. Effect of Low (2000 lux) and High (8000 lux) White Light Illumination
3.1.2. Effect of Colored (Green, Blue and Red) Light Illumination
3.2. Anabaena sp.
3.2.1. Effect of Low (2000 lux) and High (8000 lux) White Light Illumination
3.2.2. Effect of Colored (Green, Blue and red) Light Illumination
4. Discussion
- Enough absorption spectra should be recorded along the course of the culture of a certain microalga at a more or less certain set of culture conditions.
- On the same day of recording, a certain absorption spectrum and biomass density should be properly calculated, and various pigments’ concentration analyses must be meticulously performed. Additionally, the absorbance values of 750 nm and of the peaks for each pigment should be recorded.
- The above procedure should be repeated for each spectrum recorded along the culture course. After getting at regular intervals a number of absorption spectra and data on biomass density and pigment concentrations for a particular culture, the data should be processed.
- Regression equations for the pairs of biomass density and absorbance at 750 nm should be constructed using the above bulk of data in order to see if there is a strong correlation (i.e., R2 > 0.85). If so, then the equation can be used for predicting biomass using the OD value of 750 nm. The same procedure should be executed for the pairs of each pigment concentration and OD at 750 nm in order to predict in the future each pigment’s concentration based on the constructed equations.
- The above painstaking procedure should be performed once in the beginning in order to construct the calibration equations for the certain culture. Then, for future cultures of the same species in the same conditions, the grower can use the constructed equations to get fairly accurate estimates for biomass and pigments.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae Biomass Production for a Biorefinery System: Recent Advances and the Way towards Sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from Microalgae: The Potential of Domestication towards Sustainable Biofactories. Microb. Cell Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef]
- Subramanian, S.; Sayre, R.T. The Right Stuff; Realizing the Potential for Enhanced Biomass Production in Microalgae. Front. Energy Res. 2022, 10, 979747. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 Bio-Mitigation Using Microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-Mitigation of Carbon Dioxide Using Microalgal Systems: Advances and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Awal, S.; Christie, A. Suitability of Inland Saline Ground Water for the Growth of Marine Microalgae for Industrial Purposes. J. Aquac. Mar. Biol. 2015, 3, 00063. [Google Scholar] [CrossRef]
- Muhammad, G.; Alam, M.A.; Xiong, W.; Lv, Y.; Xu, J.-L. Microalgae Biomass Production: An Overview of Dynamic Operational Methods. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 415–432. [Google Scholar]
- Farooq, W. Sustainable Production of Microalgae Biomass for Biofuel and Chemicals through Recycling of Water and Nutrient within the Biorefinery Context: A Review. GCB Bioenergy 2021, 13, 914–940. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An Overview of Microalgae Biomass as a Sustainable Aquaculture Feed Ingredient: Food Security and Circular Economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Sirohi, R.; Udayan, A.; Yadav, P.; Raj, A.; Sim, S.J.; Pandey, A. Sustainable Microalgal Biomass Production in Food Industry Wastewater for Low-Cost Biorefinery Products: A Review. Phytochem. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.L.; Ong, H.C.; Zaman, H.B. Microalgae Biomass as Biofuel and the Green Applications. Energies 2022, 15, 7280. [Google Scholar] [CrossRef]
- Paper, M.; Glemser, M.; Haack, M.; Lorenzen, J.; Mehlmer, N.; Fuchs, T.; Schenk, G.; Garbe, D.; Weuster-Botz, D.; Eisenreich, W.; et al. Efficient Green Light Acclimation of the Green Algae Picochlorum Sp. Triggering Geranylgeranylated Chlorophylls. Front. Bioeng. Biotechnol. 2022, 10, 885977. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Toole, C.M.; Allnutt, F.C.T. Red, Cryptomonad and Glaucocystophyte Algal Phycobiliproteins. In Photosynthesis in Algae; Springer: Dordrecht, The Netherlands, 2003; pp. 305–334. [Google Scholar]
- MacColl, R. Cyanobacterial Phycobilisomes. J. Struct. Biol. 1998, 124, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Maroneze, M.M.; Dias, R.R.; Severo, I.A.; Queiroz, M.I. Microalgae-Based Processes for Pigments Production. In Pigments from Microalgae Handbook; Springer International Publishing: Cham, Switzerland, 2020; pp. 241–264. [Google Scholar]
- Jeevanandam, J.; Choudhary, V.; Selvam, J.D.; Danquah, M.K. The Bioeconomy of Production of Microalgal Pigments. In Pigments from Microalgae Handbook; Springer International Publishing: Cham, Switzerland, 2020; pp. 325–362. [Google Scholar]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors Affecting Production of Beta-Carotene from Dunaliella Salina Microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of Β-carotene with Dunaliella Salina CCAP19/18 at Physically Simulated Outdoor Conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; de la Rosa, J.D.P. Phycocyanin Content and Nutritional Profile of Arthrospira Platensis from Mexico: Efficient Extraction Process and Stability Evaluation of Phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Huang, J.; Su, B.; Wei, L.; Zhang, A.-H.; Zhang, D.-F.; Zhou, Y.; Ma, G. Enhanced Phycocyanin Production of Arthrospira Maxima by Addition of Mineral Elements and Polypeptides Using Response Surface Methodology. Front. Mar. Sci. 2022, 9, 1057201. [Google Scholar] [CrossRef]
- Grant, C.; Louda, J. Microalgal Pigment Ratios in Relation to Light Intensity: Implications for Chemotaxonomy. Aquat. Biol. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Vijaya, V.; Anand, N. Blue Light Enhance the Pigment Synthesis in Cyanobacterium Anabaena Ambigua Rao (Nostacales). J. Agric. Biol. Sci. 2009, 4, 36–43. [Google Scholar]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral Conversion of Light for Enhanced Microalgae Growth Rates and Photosynthetic Pigment Production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of Phycocyanin—A Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781439867334. [Google Scholar]
- Wang, C.-Y.; Fu, C.-C.; Liu, Y.-C. Effects of Using Light-Emitting Diodes on the Cultivation of Spirulina Platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Kaur, S.; Kaushal, S.; Singh, Y.; Singh, D.P.; Rana, S.; Gulati, A. Hyperproduction of Phycobiliproteins by the Cyanobacterium Anabaena Fertilissima PUPCCC 410.5 under Optimized Culture Conditions. Algal Res. 2015, 12, 463–469. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Saavedra, M.; Maeda-Martínez, A.N.; Acosta-Galindo, S. Effect of Different Light Spectra on the Growth and Biochemical Composition of Tisochrysis Lutea. J. Appl. Phycol. 2016, 28, 839–847. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin Accumulation in the Green Microalga Haematococcus Pluvialis: Effect of Initial Phosphate Concentration and Stepwise/Continuous Light Stress. Biotechnol. Rep. 2020, 28, e00538. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.C.; Reinehr, C.O.; Costa, J.A.V. Semicontinuous Cultivation of the Cyanobacterium Spirulina Platensis in a Closed Photobioreactor. Braz. J. Chem. Eng. 2006, 23, 23–28. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Galatanu, A.N.; Nylander, T.; Lindman, B. The Behaviour of Protein Preparations from Blue-Green Algae (Spirulina Platensis Strain Pacifica) at the Air/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 181–192. [Google Scholar] [CrossRef]
- Bergmann, P.; Trösch, W. Repeated Fed-Batch Cultivation of Thermosynechococcus Elongatus BP-1 in Flat-Panel Airlift Photobioreactors with Static Mixers for Improved Light Utilization: Influence of Nitrate, Carbon Supply and Photobioreactor Design. Algal Res. 2016, 17, 79–86. [Google Scholar] [CrossRef]
- Ho, S.-H.; Liao, J.-F.; Chen, C.-Y.; Chang, J.-S. Combining Light Strategies with Recycled Medium to Enhance the Economic Feasibility of Phycocyanin Production with Spirulina Platensis. Bioresour. Technol. 2018, 247, 669–675. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zhang, S.; Zhang, X.; Wu, M.; Chen, X.D.; Ng, I.-S.; Jing, K.; Lu, Y. Autotrophic Cultivation of Spirulina Platensis for CO2 Fixation and Phycocyanin Production. Chem. Eng. J. 2012, 183, 192–197. [Google Scholar] [CrossRef]
- Tamary, E.; Kiss, V.; Nevo, R.; Adam, Z.; Bernát, G.; Rexroth, S.; Rögner, M.; Reich, Z. Structural and Functional Alterations of Cyanobacterial Phycobilisomes Induced by High-Light Stress. Biochim. Et. Biophys. Acta BBA-Bioenerg. 2012, 1817, 319–327. [Google Scholar] [CrossRef]
- Klepacz-Smółka, A.; Pietrzyk, D.; Szeląg, R.; Głuszcz, P.; Daroch, M.; Tang, J.; Ledakowicz, S. Effect of Light Colour and Photoperiod on Biomass Growth and Phycocyanin Production by Synechococcus PCC 6715. Bioresour. Technol. 2020, 313, 123700. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N. A Preliminary Survey on the Planktonic Biota in a Hypersaline Pond of Messolonghi Saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D.; Mastropetros, S.G.; Tsigkou, K.; Kouvara, K.; Makridis, P.; Kornaros, M. Isolation, Identification, and Chemical Composition Analysis of Nine Microalgal and Cyanobacterial Species Isolated in Lagoons of Western Greece. Algal Res. 2023, 69, 102935. [Google Scholar] [CrossRef]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira Platensis Cultivated in Semi-Continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium Sp. in Various Salinity and Light Regimes and Their Influence on Its Phycocyanin and Other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Hotos, G.N.; Antoniadis, T.I. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium Sp. and Cyanothece Sp. in Batch Cultures. Life 2022, 12, 837. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D.; Samara, A. The Effect of Salinity and Light Intensity on the Batch Cultured Cyanobacteria Anabaena sp. and Cyanothece sp. Hydrobiology 2022, 1, 278–287. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by Pigment in the Estimation of Microalgal Biomass Concentration by Optical Density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Morowvat, M.H.; Ghasemi, Y. Culture Medium Optimization for Enhanced β-Carotene and Biomass Production by Dunaliella Salina in Mixotrophic Culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural Pigments from Microalgae Grown in Industrial Wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhou, X.-R.; Moncalian, G.; Su, L.; Chen, W.-C.; Zhu, H.-Z.; Chen, D.; Gong, Y.-M.; Huang, F.-H.; Deng, Q.-C. Reprogramming Microorganisms for the Biosynthesis of Astaxanthin via Metabolic Engineering. Prog. Lipid Res. 2021, 81, 101083. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella Salina Cultivation: From Strain Selection to Open Ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium Carterae, Nephroselmis Sp., Tetraselmis Sp. (Var. Red Pappas), Asteromonas gracilis and Dunaliella Sp.] in Laboratory Batch Cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D.; Bekiari, V. Calibration Curves of Culture Density Assessed by Spectrophotometer for Three Microalgae (Nephroselmis Sp., Amphidinium carterae and Phormidium Sp.). Eur. J. Biol. Biotechnol. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Toennies, G.; Gallant, D.L. The Relation between Photometric Turbidity and Bacterial Concentration. Growth 1949, 13, 7–20. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Nicholls, K.H.; Dillon, P.J. An Evaluation of Phosphorus-Chlorophyll-Phytoplankton Relationships for Lakes. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1978, 63, 141–154. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from Microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–492. [Google Scholar]
- An, J.-Y.; Sim, S.-J.; Lee, J.S.; Kim, B.W. Hydrocarbon Production from Secondarily Treated Piggery Wastewater by the Green Alga Botryococcus Braunii. J. Appl. Phycol. 2003, 15, 185–191. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a High-Density Culture of Chlorella Sp. in a Semicontinuous Photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Wu, W.-T. Cultivation of Microalgae for Oil Production with a Cultivation Strategy of Urea Limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, A.M.; Mioni, C.E.; Hellier, K.L.; Allen, J.J.; Carter, S.A.; Bebout, B.M.; Fleming, E.E.; Corrado, C.; Prufert-Bebout, L.E. Evaluation of Wavelength Selective Photovoltaic Panels on Microalgae Growth and Photosynthetic Efficiency. Algal Res. 2015, 9, 170–177. [Google Scholar] [CrossRef]
- Gao, F.; Sá, M.; Teles, I.; Wijffels, R.H.; Barbosa, M.J. Production and Monitoring of Biomass and Fucoxanthin with Brown Microalgae under Outdoor Conditions. Biotechnol. Bioeng. 2021, 118, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, S.; Tomita, R.; Fujiwara, T.; Ohnuma, M.; Kuroiwa, H.; Kuroiwa, T.; Miyagishima, S. Efficient Open Cultivation of Cyanidialean Red Algae in Acidified Seawater. Sci. Rep. 2020, 10, 13794. [Google Scholar] [CrossRef]
- Plöhn, M.; Escudero-Oñate, C.; Funk, C. Biosorption of Cd(II) by Nordic Microalgae: Tolerance, Kinetics and Equilibrium Studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Karita, H.; Mori-Moriyama, N.; Sato, N.; Yoshimoto, K. Reduced Cytotoxicity of Polyethyleneimine by Covalent Modification of Antioxidant and Its Application to Microalgal Transformation. Sci. Technol. Adv. Mater. 2021, 22, 864–874. [Google Scholar] [CrossRef]
- Markina, Z.V.; Maslennikov, S.I.; Botsun, L.A. Application of the Spectrophotometric Method for Determination of the Cell Numbers of Microalgae in the Genus Tetraselmis (Chlorophyta): Calibration Curves and Equations for Calculation. Russ. J. Mar. Biol. 2022, 48, 525–528. [Google Scholar] [CrossRef]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow Cytometry Pulse Width Data Enables Rapid and Sensitive Estimation of Biomass Dry Weight in the Microalgae Chlamydomonas Reinhardtii and Chlorella Vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, R.H.; Ghannoum, M.A.; Sallal, A.-K.; Abu-Elteen, K.H.; Radwan, S.S. Correlative Changes of Growth, Pigmentation and Lipid Composition of Dunaliella Salina in Response to Halostress. J. Gen. Microbiol. 1987, 133, 2607–2616. [Google Scholar] [CrossRef]
- Wagner, I.; Steinweg, C.; Posten, C. Mono- and Dichromatic LED Illumination Leads to Enhanced Growth and Energy Conversion for High-Efficiency Cultivation of Microalgae for Application in Space. Biotechnol. J. 2016, 11, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J. Analysis of Light Absorption and Photosynthetic Activity by Isochrysis galbana under Different Light Qualities. Aquac. Res. 2020, 51, 2893–2902. [Google Scholar] [CrossRef]
- Remias, D.; Lütz-Meindl, U.; Lütz, C. Photosynthesis, Pigments and Ultrastructure of the Alpine Snow Alga Chlamydomonas nivalis. Eur. J. Phycol. 2005, 40, 259–268. [Google Scholar] [CrossRef]
- Mayer, D.; Dubinsky, Z.; Iluz, D. Light as a Limiting Factor for Epilithic Algae in the Supralittoral Zone of Littoral Caves. Front. Mar. Sci. 2016, 3, 18. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella Vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.L.; Campbell, D.A.; Hughes, D.J.; Kuzhiumparambil, U.; Halsey, K.H.; Ralph, P.J.; Suggett, D.J. Divergence of Photosynthetic Strategies amongst Marine Diatoms. PLoS ONE 2020, 15, e0244252. [Google Scholar] [CrossRef] [PubMed]
- Abiusi, F.; Sampietro, G.; Marturano, G.; Biondi, N.; Rodolfi, L.; D’Ottavio, M.; Tredici, M.R. Growth, Photosynthetic Efficiency, and Biochemical Composition of Tetraselmis suecica F&M-M33 Grown with LEDs of Different Colors. Biotechnol. Bioeng. 2014, 111, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing Growth and Lipid Production of Marine Microalgae for Biodiesel Production via the Use of Different LED Wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef]
- Sharma, N.; Fleurent, G.; Awwad, F.; Cheng, M.; Meddeb-Mouelhi, F.; Budge, S.M.; Germain, H.; Desgagné-Penix, I. Red Light Variation an Effective Alternative to Regulate Biomass and Lipid Profiles in Phaeodactylum Tricornutum. Appl. Sci. 2020, 10, 2531. [Google Scholar] [CrossRef]
- Diamantopoulou, C.; Christoforou, E.; Dominoni, D.M.; Kaiserli, E.; Czyzewski, J.; Mirzai, N.; Spatharis, S. Wavelength-Dependent Effects of Artificial Light at Night on Phytoplankton Growth and Community Structure. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210525. [Google Scholar] [CrossRef]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as Source of Commercially Valuable Pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Fan, J.; Zheng, L. Acclimation to NaCl and Light Stress of Heterotrophic Chlamydomonas Reinhardtii for Lipid Accumulation. J. Biosci. Bioeng. 2017, 124, 302–308. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, R.; Lu, J.; Lei, A.; Zhu, H.; Hu, Z.; Wang, J. Effects of Different Abiotic Stresses on Carotenoid and Fatty Acid Metabolism in the Green Microalga Dunaliella Salina Y6. Ann. Microbiol. 2020, 70, 48. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of Light Intensity on Growth and Lipid Production in Microalgae Grown in Wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Cointet, E.; Wielgosz-Collin, G.; Bougaran, G.; Rabesaotra, V.; Gonçalves, O.; Méléder, V. Effects of Light and Nitrogen Availability on Photosynthetic Efficiency and Fatty Acid Content of Three Original Benthic Diatom Strains. PLoS ONE 2019, 14, e0224701. [Google Scholar] [CrossRef]
- Bermejo Román, R.; Alvárez-Pez, J.M.; Acién Fernández, F.G.; Molina Grima, E. Recovery of Pure B-Phycoerythrin from the Microalga Porphyridium Cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Arai, T.; Hirano, M.; Matsunaga, T. Effects of Intensity and Quality of Light on Phycocyanin Production by a Marine Cyanobacterium Synechococcus Sp. NKBG 042902. Appl. Microbiol. Biotechnol. 1995, 43, 1014–1018. [Google Scholar] [CrossRef]
- Hong, S.-J.; Lee, C.-G. Statistical Optimization of Culture Media for Production of Phycobiliprotein by Synechocystis Sp. PCC 6701. Biotechnol. Bioprocess Eng. 2008, 13, 491–498. [Google Scholar] [CrossRef]
- Hemlata; Fatma, T. Screening of Cyanobacteria for Phycobiliproteins and Effect of Different Environmental Stress on Its Yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Parmar, A.; Sonani, R.R.; Madamwar, D. Isolation, Identification and Characterization of Novel Thermotolerant Oscillatoria Sp. N9DM: Change in Pigmentation Profile in Response to Temperature. Process Biochem. 2012, 47, 2472–2479. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid Production by Dunaliella Salina under Red Light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Liu, J.; Qin, R. Light Absorption and Growth Response of Dunaliella under Different Light Qualities. J. Appl. Phycol. 2020, 32, 1041–1052. [Google Scholar] [CrossRef]
- Wu, Z.; Duangmanee, P.; Zhao, P.; Juntawong, N.; Ma, C. The Effects of Light, Temperature, and Nutrition on Growth and Pigment Accumulation of Three Dunaliella Salina Strains Isolated from Saline Soil. Jundishapur J. Microbiol. 2016, 9, e26732. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Jin, E. Comparison of the Responses of Two Dunaliella Strains, Dunaliella Salina CCAP 19/18 and Dunaliella Bardawil to Light Intensity with Special Emphasis on Carotenogenesis. ALGAE 2013, 28, 203–211. [Google Scholar] [CrossRef]
- Vo, T.; Tran, D. Effects of Salinity and Light on Growth of Dunaliella Isolates. J. Appl. Environ. Microbiol. 2014, 2, 208–211. [Google Scholar]
- Gaur, S.; Adholeya, A. Influence of Light Intensity, Photoperiod and Culture Medium on the Dunaliella Tertiolecta and Nannochloropsis Oculata Pigment Production, Lipid Yields and Fatty Acid Composition. SSRN Electron. J. 2022, 9, e12801. [Google Scholar] [CrossRef]
- Ak, I.; Cirik, S.; Goksan, T. Effects of Light Intensity, Salinity and Temperature on Growth in Camalti Strain of Dunaliella Viridis Teodoresco from Turkey. J. Biol. Sci. 2008, 8, 1356–1359. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.; Wosu, C.; Ben-Amotz, A.; Harvey, P. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology 2018, 7, 14. [Google Scholar] [CrossRef]
- Chang, F.H.; Wear, R.G.; Reynolds, J. Effects of Salinity, Temperature, and Light Intensity on the Growth Rates of Two Halophilic Phytoflagellates in Mixed Culture. N. Z. J. Mar. Freshw. Res. 1986, 20, 467–478. [Google Scholar] [CrossRef]
- Hermawan, J.; Masithah, E.D.; Tjahjaningsih, W.; Abdillah, A.A. Increasing β-Carotene Content of Phytoplankton Dunaliella Salina Using Different Salinity Media. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 137, p. 012034. [Google Scholar] [CrossRef]
- Nguyen, A.; Tran, D.; Ho, M.; Louime, C.; Tran, H.; Tran, D. High Light Stress Regimen on Dunaliella Salina Strains For Carotenoids Induction. Integr. Food Nutr. Metab. 2016, 3, 347–350. [Google Scholar] [CrossRef]
- Raqiba, H.; Sibi, G. Light Emitting Diode (LED) Illumination For Enhanced Growth And Cellular Composition In Three Microalgae. Adv. Microbiol. Res. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N. Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment. J. Mar. Sci. Eng. 2023, 11, 1673. https://doi.org/10.3390/jmse11091673
Hotos GN. Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment. Journal of Marine Science and Engineering. 2023; 11(9):1673. https://doi.org/10.3390/jmse11091673
Chicago/Turabian StyleHotos, George N. 2023. "Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment" Journal of Marine Science and Engineering 11, no. 9: 1673. https://doi.org/10.3390/jmse11091673
APA StyleHotos, G. N. (2023). Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment. Journal of Marine Science and Engineering, 11(9), 1673. https://doi.org/10.3390/jmse11091673





