Role of Organic Matter Present in the Water Column on Turbidity Flows
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.1.1. Clay
2.1.2. Flocculant
2.1.3. Saltwater
2.2. Methods
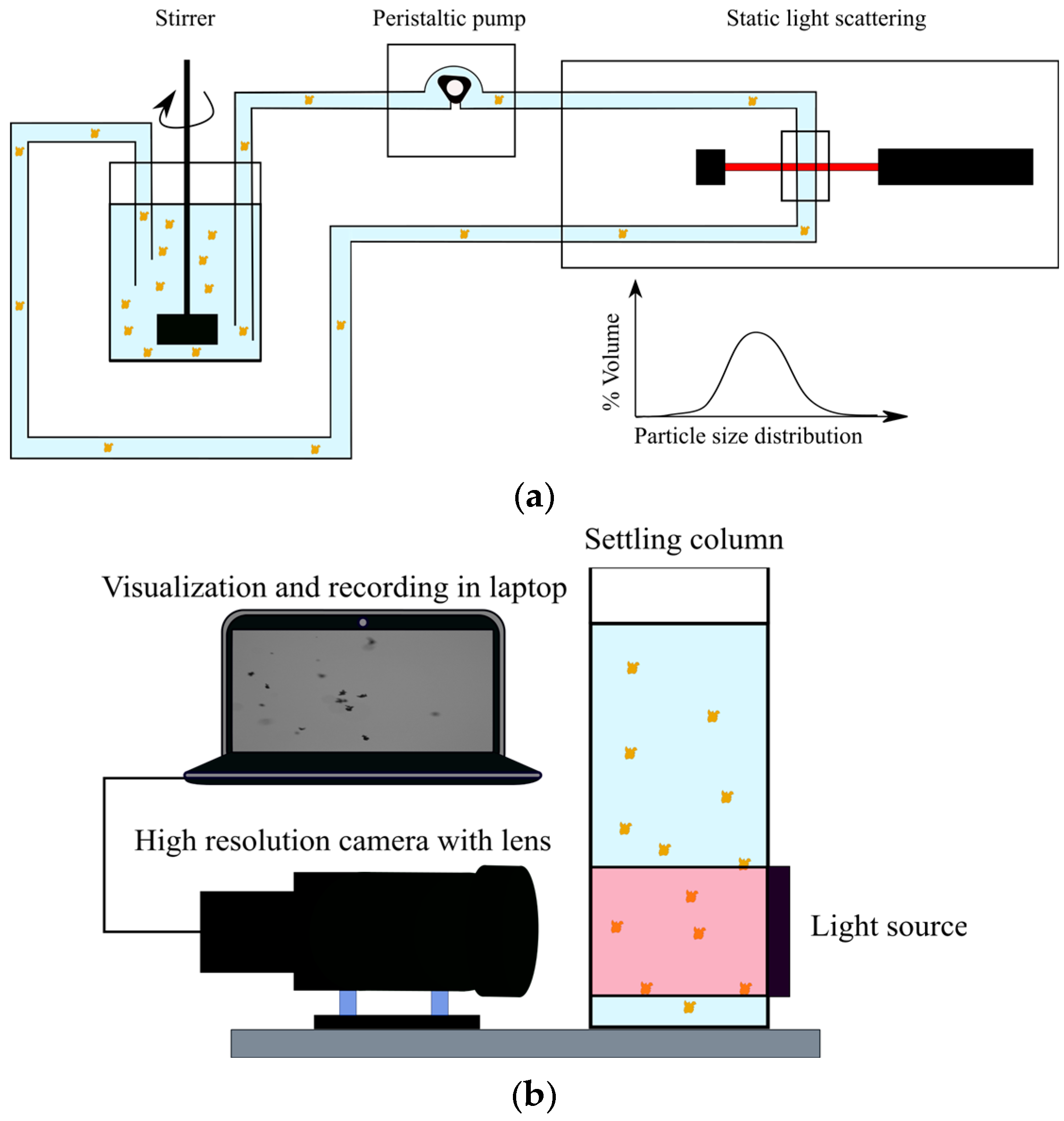
2.2.1. Jar Tests
- Two samples (one for Malvern analysis and the other for FlocCAM analysis) of the chosen concentration (0.5 g/L) of illite and saltwater were prepared using a volumetric flask.
- Each of the illite saltwater suspensions was then mixed separately at 800 rpm using an overhead stirrer for 5 min in order to ensure a homogeneous suspension.
- A stock solution of Zetag 4120 with water was prepared. Four different dosages of Zetag 4120 (milligram per gram of dry mass of clay) (0.35 mg/g, 0.25 mg/g, 0.2 mg/g, and 0.1 mg/g of clay) were chosen for the jar tests.
- The samples (illite and a chosen dosage of Zetag 4120) were then mixed for 30 s at 75 rpm (which corresponds to a 50/s shear rate) using the JLT6 jar test apparatus setup.
- One sample was immediately measured in FlocCAM and videos were recorded.
- For the other sample, a clean jar with saltwater was placed under the JLT6 jar test apparatus and constantly mixed at 75 rpm. The flocs were then scooped from the illite–saltwater suspension containing Zetag 4120 with the help of a pipette and were put into the jar under the JLT6 jar test apparatus.
- The jar was connected through a peristaltic pump to the Malvern Mastersizer 2000. The pumping speed was kept at 88 rpm, which was just sufficient to pump the flocs to and out of Malvern Mastersizer 2000, minimizing the shear that might affect the floc structure.
- The floc size was then obtained from Malvern Mastersizer 2000. The time difference between adding Zetag 4120 and obtaining the first floc size measurement would be at least 90 s.
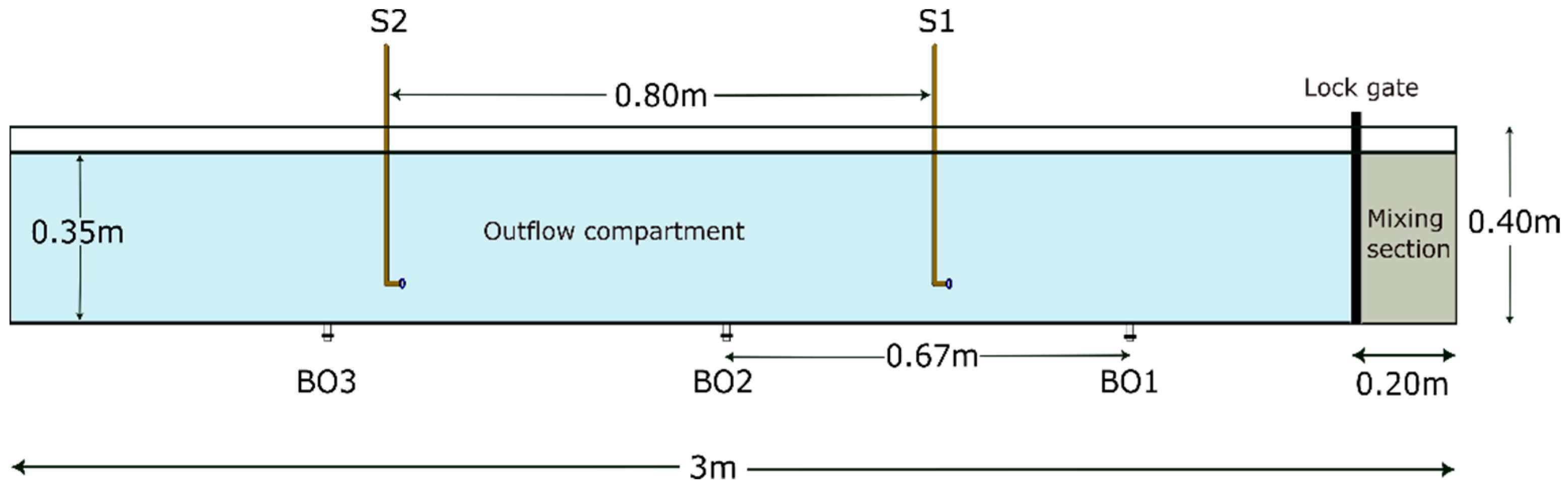
2.2.2. Lock Exchange Setup
- The lock exchange flume was filled with saltwater up to a height of 35 cm, which makes a total of 210 L.
- A weighed mass of illite (according to the concentration) was added to the lock section, and was then mixed for 20 min.
- In case of runs with flocculant, a weighed mass of Zetag 4120 or Zetag 7587 (according to the dosage) was added to the outflow section of the flume and mixed with a hand mixer for 30 s. The lock gate was opened after the water level in the outflow compartment was stable.
- The propagation of turbidity currents was recorded using a GoPro Hero 9 camera for analysis of the front position using Tracker software (Version 6.1.3) [28].
- To collect samples from the turbidity current, a duplicate experiment was performed in each case, following the above-mentioned steps. The samples were taken from two siphons located at the same height but at two different positions in the flume. Bottom outlets were also used to collect samples at three different positions in the flume.
- The collected samples were then analyzed using the particle sizer and FlocCAM setup described in Section 2.2.1.
3. Results
3.1. Jar Tests
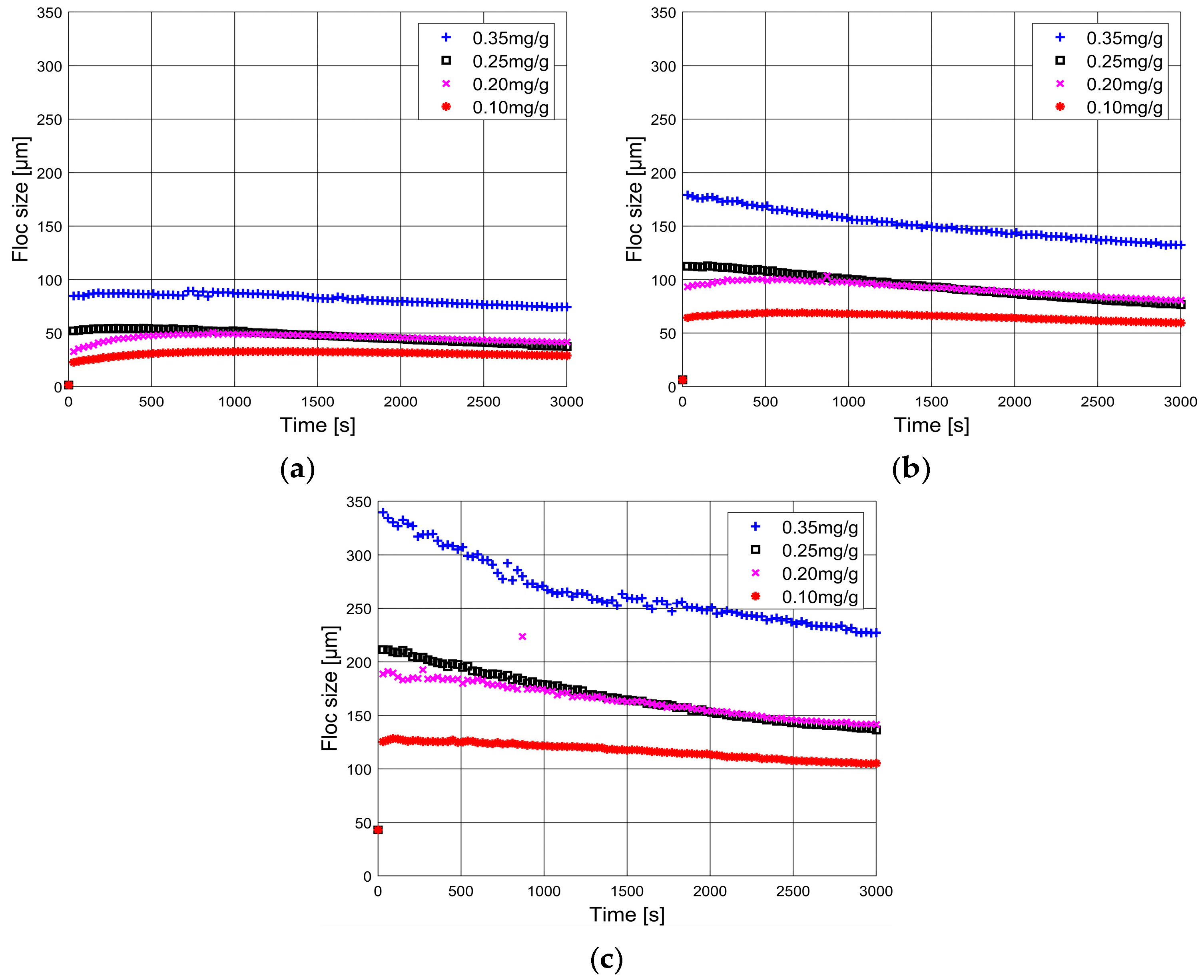
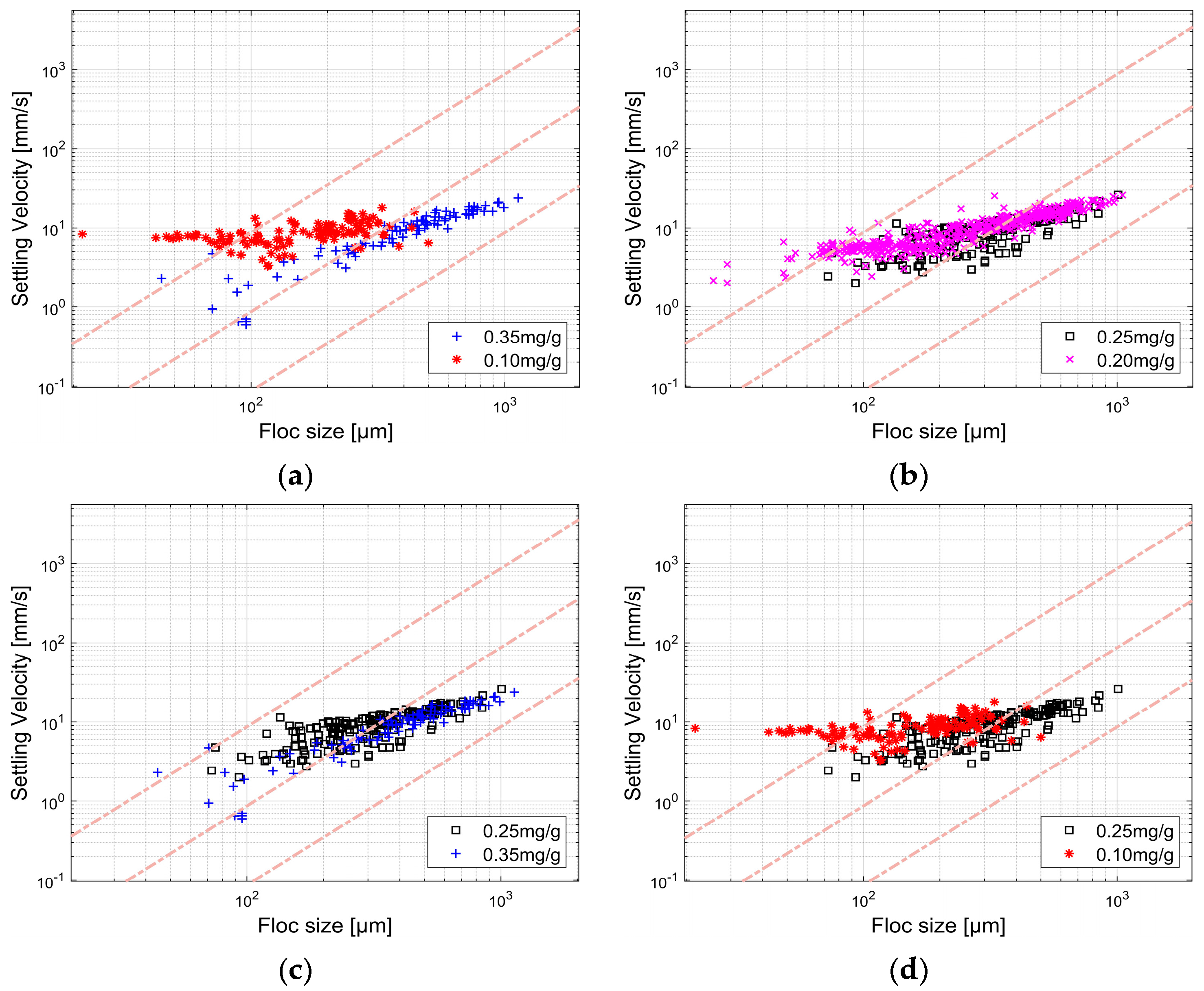
3.1.1. Malvern Mastersizer 2000
3.1.2. FlocCAM
3.1.3. Comparison Between Malvern Mastersizer 2000 and FlocCAM
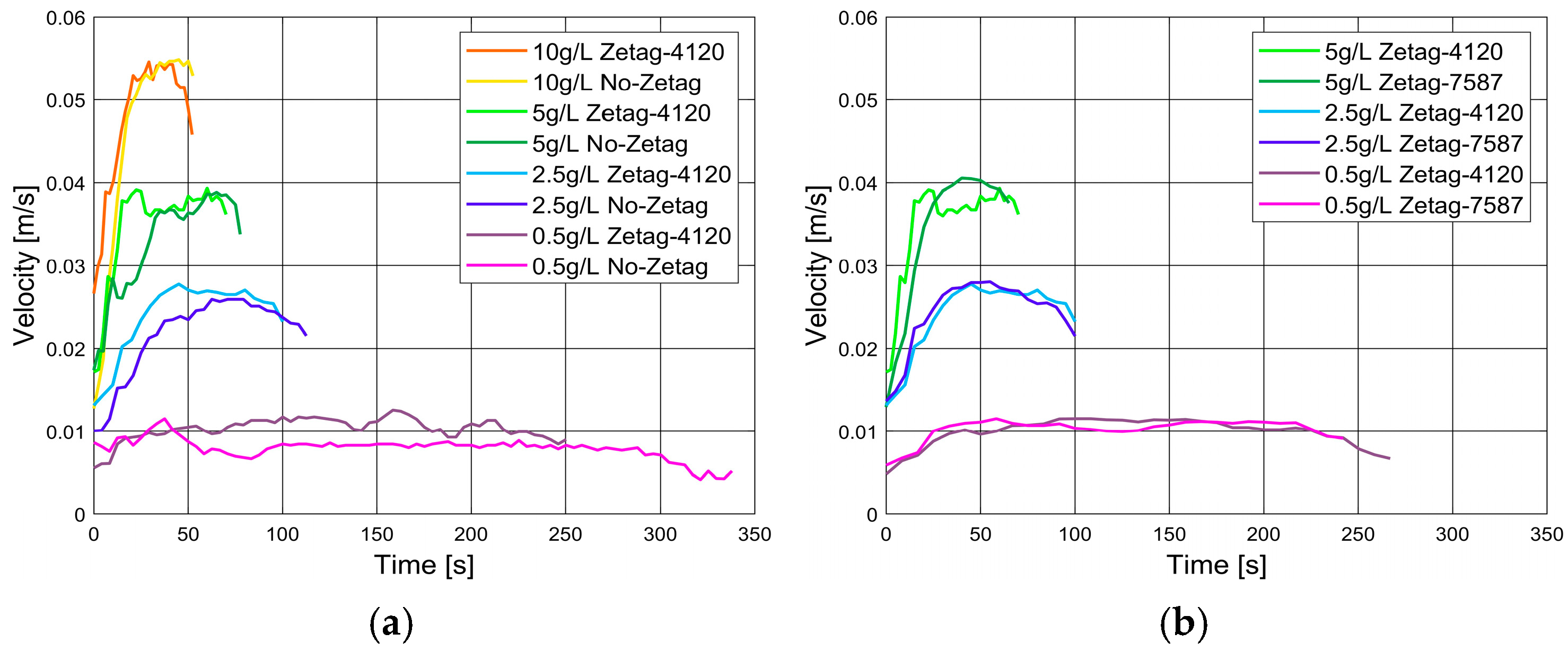
3.2. Lock Exchange Experiments
3.2.1. With Anionic Polyelectrolyte Zetag 4120
3.2.2. With Cationic Polyelectrolyte Zetag 7587
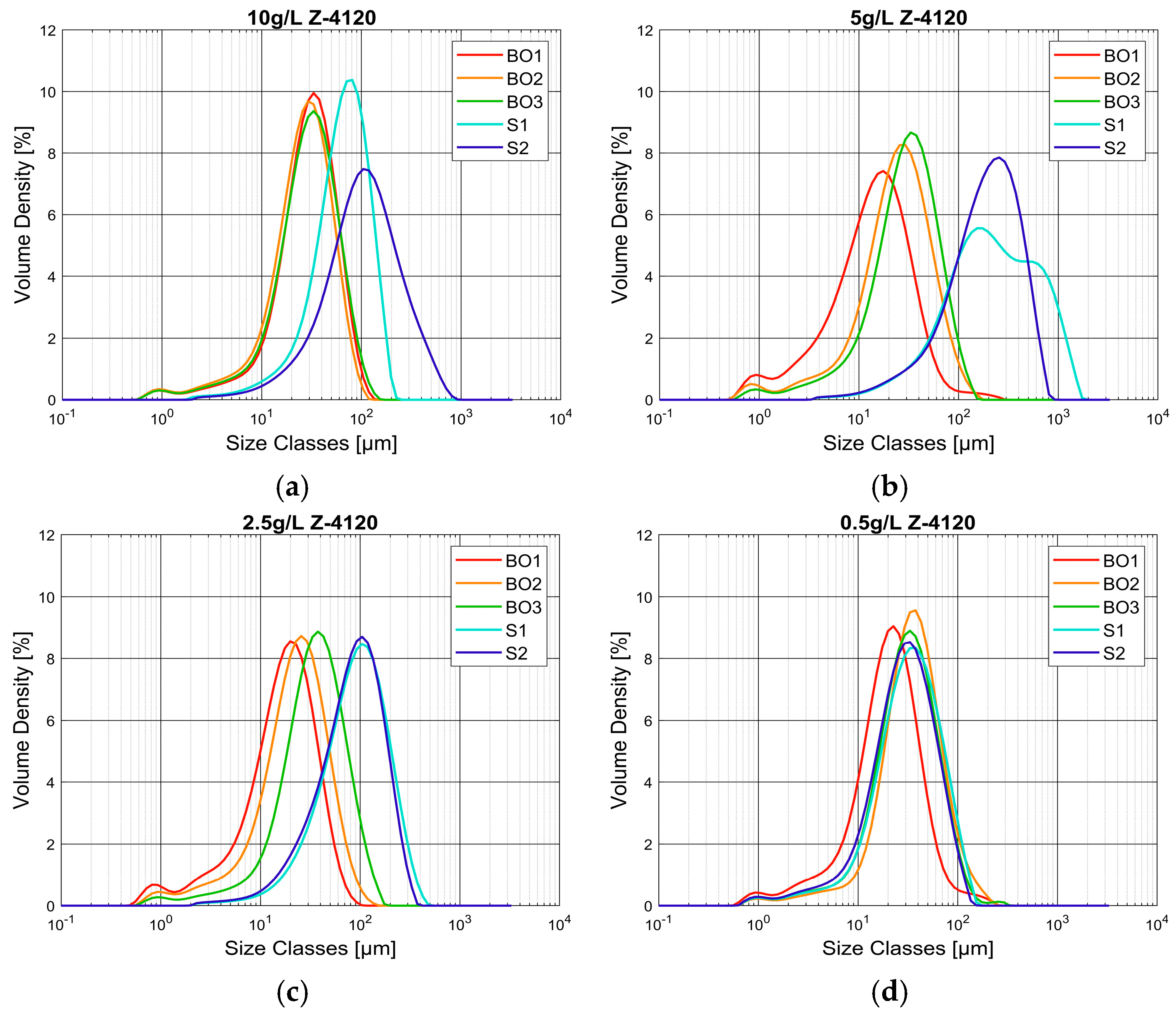
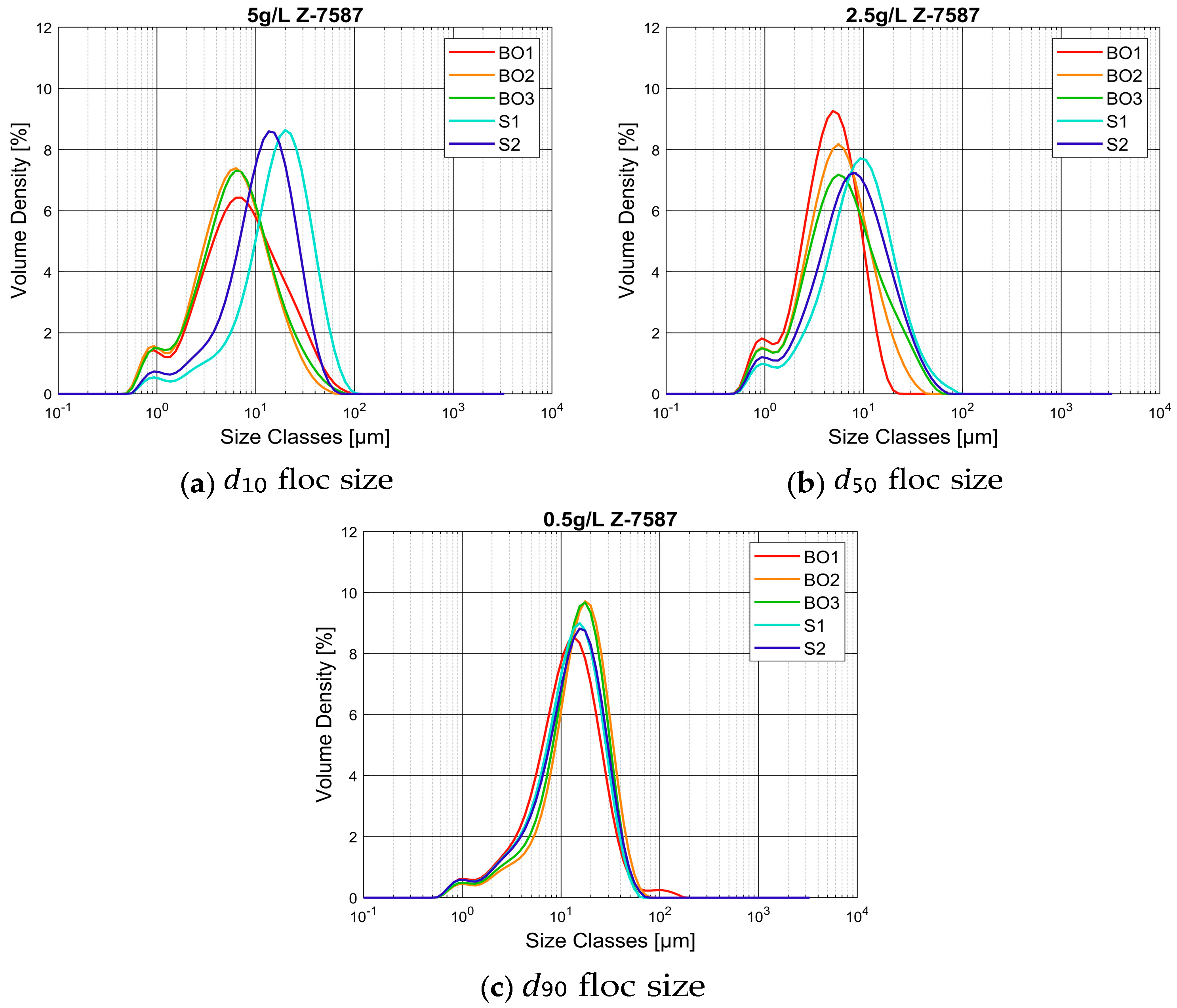
3.2.3. Particle Size Analysis as a Position in the Current
4. Discussion
- Influence of flocculation on turbidity current propagation
- Behavior of flocs obtained from different locations of turbidity currents
- Behavior of flocs analysed with Malvern Mastersizer 2000 and FlocCAM
- Behavior of flocs created with two different types of flocculant
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- CEDA. Ceda Position Paper: Underwater Sound in Relation to Dredging; Terra Et Aqua, International Association of Dredging Companies. December 2011. Available online: https://www.iadc-dredging.com/wp-content/uploads/2017/02/article-ceda-position-paper-underwater-sound-in-relation-to-dredging-125-4.pdf (accessed on 1 November 2023).
- Erftemeijer, P.L.A.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef] [PubMed]
- Victor, O.; Ikenna, O.C.; Ndubuisi, I.G. Environmental effect of dredging and geochemical fractionation of heavy metals in sediments removed from river. Mod. Chem. 2018, 7, 44–49. [Google Scholar]
- Bruun, P.; Gayes, P.; Schwab, W.; Eiser, W. Dredging. In Port and Coastal Engineering Developments in Science and Technology; Journal of Coastal Research, Special Issue No. 46; Coastal Education and Research Foundation, Inc.: Hilton Head Island, SC, USA, 2005; pp. 453–525. [Google Scholar]
- Elerian, M.; Alhaddad, S.; Helmons, R.; van Rhee, C. Near-field analysis of turbidity flows generated by polymetallic nodule mining tools. Mining 2021, 1, 251–278. [Google Scholar] [CrossRef]
- Todd, V.L.; Todd, I.B.; Gardiner, J.C.; Morrin, E.C.; MacPherson, N.A.; DiMarzio, N.A.; Thomsen, F. A review of impacts of marine dredging activities on marine mammals. ICES J. Mar. Sci. 2014, 72, 328–340. [Google Scholar] [CrossRef]
- Gillard, B.; Purkiani, K.; Chatzievangelou, D.; Vink, A.; Iversen, M.H.; Thomsen, L. Physical and hydrodynamic properties of deep sea mining-generated, abyssal sediment plumes in the clarion clipperton fracture zone (eastern-central pacific). Elem. Sci. Anthr. 2019, 7, 5. [Google Scholar] [CrossRef]
- Gausepohl, F.; Hennke, A.; Schoening, T.; Köser, K.; Greinert, J. Scars in the abyss: Reconstructing sequence, location and temporal change of the 78 plough tracks of the 1989 discol deep-sea disturbance experiment in the peru basin. Biogeosciences 2020, 17, 1463–1493. [Google Scholar] [CrossRef]
- van Grunsven, F.; Keetels, G.; van Rhee, C. The initial spreading of turbidity plumes–dedicated laboratory experiments for model validation. In Proceedings of the 48th Underwater Mining Conference (UMC), Bergen, Norway, 10–14 September 2018. [Google Scholar]
- Spearman, J.; Taylor, J.; Crossouard, N.; Cooper, A.; Turnbull, M.; Manning, A.; Lee, M.; Murton, B. Measurement and modelling of deep sea sediment plumes and implications for deep sea mining. Sci. Rep. 2020, 10, 5075. [Google Scholar] [CrossRef]
- Ali, W.; Enthoven, D.; Kirichek, A.; Chassagne, C.; Helmons, R. Effect of flocculation on turbidity currents. Front. Earth Sci. 2022, 10, 1014170. [Google Scholar] [CrossRef]
- Eisma, D. Flocculation and de-flocculation of suspended matter in estuaries. Neth. J. Sea Res. 1986, 20, 183–199. [Google Scholar] [CrossRef]
- Droppo, I.G.; Ongley, E.D. Flocculation of suspended sediment in rivers of southeastern canada. Water Res. 1994, 28, 1799–1809. [Google Scholar] [CrossRef]
- Elerian, M.; Huang, Z.; van Rhee, C.; Helmons, R. Flocculation effect on turbidity flows generated by deep-sea mining: A numerical study. Ocean. Eng. 2023, 277, 114250. [Google Scholar] [CrossRef]
- Nogueira, H.I.; Adduce, C.; Alves, E.; Franca, M.J. Analysis of lock-exchange gravity currents over smooth and rough beds. J. Hydraul. Res. 2013, 51, 417–431. [Google Scholar] [CrossRef]
- Baker, M.L.; Baas, J.H.; Malarkey, J.; Jacinto, R.S.; Craig, M.J.; Kane, I.A.; Barker, S. The effect of clay type on the properties of cohesive sediment gravity flows and their deposits. J. Sediment. Res. 2017, 87, 1176–1195. [Google Scholar] [CrossRef]
- Craig, M.J.; Baas, J.H.; Amos, K.J.; Strachan, L.J.; Manning, A.J.; Paterson, D.M.; Hope, J.A.; Nodder, S.D.; Baker, M.L. Biomediation of submarine sediment gravity flow dynamics. Geology 2019, 48, 72–76. [Google Scholar] [CrossRef]
- Ali, W.; Enthoven, D.; Kirichek, A.; Helmons, R.; Chassagne, C. Can flocculation reduce the dispersion of deep sea sediment plumes? In Proceedings of the WODCON XXIII, Copenhagen, Denmark, 16–20 May 2022. [Google Scholar]
- Shakeel, A.; Safar, Z.; Ibanez, M.; van Paassen, L.; Chassagne, C. Flocculation of clay suspensions by anionic and cationic polyelectrolytes: A systematic analysis. Minerals 2020, 10, 999. [Google Scholar] [CrossRef]
- International Seabed Authority. A Geological Model of Polymetallic Nodule Deposits in the Clarion Clipperton Fracture Zone; Technical Report; International Seabed Authority: Kingston, Jamaica, 2018. [Google Scholar]
- Helmons, R.; de Wit, L.; de Stigter, H.; Spearman, J. Dispersion of benthic plumes in deep-sea mining: What lessons can be learned from dredging? Front. Earth Sci. 2022, 10, 868701. [Google Scholar] [CrossRef]
- Nichols, C.A.M.; Guezennec, J.; Bowman, J.P. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef]
- Deng, Z.; He, Q.; Safar, Z.; Chassagne, C. The role of algae in fine sediment flocculation: In-situ and laboratory measurements. Mar. Geol. 2019, 413, 71–84. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (epss)—Part i: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Mietta, F.; Chassagne, C.; Manning, A.J.; Winterwerp, J.C. Influence of shear rate, organic matter content, pH and salinity on mud flocculation. Ocean. Dyn. 2009, 59, 751–763. [Google Scholar] [CrossRef]
- Manning, A.J.; Friend, P.L.; Prowse, N.; Amos, C.L. Estuarine mud flocculation properties determined using an annular mini-flume and the labsfloc system. Cont. Shelf Res. 2007, 27, 1080–1095. [Google Scholar] [CrossRef]
- Ye, L.; Manning, A.J.; Hsu, T.-J. Oil-mineral flocculation and settling velocity in saline water. Water Res. 2020, 173, 115569. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Christian, W.; Hanson, R. Tracker Video Analysis and Modeling Tool. Available online: https://physlets.org/tracker/ (accessed on 1 August 2023).
- Ali, W.; Kirichek, A.; Chassagne, C. Collective effects on the settling of clay flocs. Appl. Clay Sci. 2024, 254, 107399. [Google Scholar] [CrossRef]
- Sanz, M.I. Flocculation and Consolidation of Cohesive Sediments under the Influence of Coagulant and Flocculant. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2018. [Google Scholar]
- Goodwin, J.W. Colloids and Interfaces with Surfactants and Polymers, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–10. [Google Scholar]
- Barany, S.; Meszaros, R.; Kozakova, I.; Skvarla, I. Kinetics and mechanism of flocculation of bentonite and kaolin suspensions with polyelectrolytes and the strength of floccs. Colloid J. 2009, 71, 285–292. [Google Scholar] [CrossRef]
- Barany, S.; Kozakova, I.; Marcinova, L.; Skvarla, J. Electrokinetic potential of bentonite and kaolin particles in the presence of polymer mixtures. Colloid J. 2010, 72, 595–601. [Google Scholar] [CrossRef]
- Bárány, S.; Meszaros, R.; Marcinova, L.; Skvarla, J. Effect of polyelectrolyte mixtures on the electrokinetic potential and kinetics of flocculation of clay mineral particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 48–55. [Google Scholar] [CrossRef]
- Lee, B.J.; Schlautman, M.A.; Toorman, E.; Fettweis, M. Competition between kaolinite flocculation and stabilization in divalent cation solutions dosed with anionic polyacrylamides. Water Res. 2012, 46, 5696–5706. [Google Scholar] [CrossRef]
- Francesca, M. Evolution of the Floc Size Distribution of Cohesive Sediments. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2010. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, S.A.; Ali, W.; Chassagne, C.; Helmons, R. Role of Organic Matter Present in the Water Column on Turbidity Flows. J. Mar. Sci. Eng. 2024, 12, 1884. https://doi.org/10.3390/jmse12101884
Wahab SA, Ali W, Chassagne C, Helmons R. Role of Organic Matter Present in the Water Column on Turbidity Flows. Journal of Marine Science and Engineering. 2024; 12(10):1884. https://doi.org/10.3390/jmse12101884
Chicago/Turabian StyleWahab, Shaheen Akhtar, Waqas Ali, Claire Chassagne, and Rudy Helmons. 2024. "Role of Organic Matter Present in the Water Column on Turbidity Flows" Journal of Marine Science and Engineering 12, no. 10: 1884. https://doi.org/10.3390/jmse12101884
APA StyleWahab, S. A., Ali, W., Chassagne, C., & Helmons, R. (2024). Role of Organic Matter Present in the Water Column on Turbidity Flows. Journal of Marine Science and Engineering, 12(10), 1884. https://doi.org/10.3390/jmse12101884





