Elemental Distribution in Tissues of Shorthorn Sculpins (Myoxocephalus scorpius) from Kongsfjorden in Svalbard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Pretreatment, and Storage
2.2. Chemicals and Reagents
2.3. Instrumentation
2.4. Sample Treatment and Analysis
2.5. Quality Control
2.6. Data Processing
3. Results
3.1. Measured Concentrations in Tissues
3.2. Size-Related Elemental Pattern
3.3. Elemental Distribution in Male and Female Specimens
4. Discussion
4.1. Distribution in Tissues
4.2. Relationship with Size
4.3. Sex-Related Differences
4.4. Mercury Speciation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paglia, E. A Higher Level of Civilisation? The Transformation of Ny-Ålesund from Arctic Coalmining Settlement in Svalbard to Global Environmental Knowledge Center at 79° North. Polar Rec. 2020, 56, e15. [Google Scholar] [CrossRef]
- Eckhardt, S.; Hermansen, O.; Grythe, H.; Fiebig, M.; Stebel, K.; Cassiani, M.; Baecklund, A.; Stohl, A. The Influence of Cruise Ship Emissions on Air Pollution in Svalbard—A Harbinger of a More Polluted Arctic? Atmos. Chem. Phys. 2013, 13, 8401–8409. [Google Scholar] [CrossRef]
- Pedersen, Å.Ø.; Convey, P.; Newsham, K.K.; Mosbacher, J.B.; Fuglei, E.; Ravolainen, V.; Hansen, B.B.; Jensen, T.C.; Augusti, A.; Biersma, E.M.; et al. Five Decades of Terrestrial and Freshwater Research at Ny-Ålesund, Svalbard. Polar Res. 2022, 41, 6310. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ianni, C.; Magi, E.; Udisti, R. Bioavailability of Trace Elements in Surface Sediments from Kongsfjorden, Svalbard. Mar. Pollut. Bull. 2013, 77, 367–374. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ardini, F.; Bazzano, A.; Moroni, B.; Vivani, R.; Cappelletti, D.; Misic, C. Trace Elements in Surface Sediments from Kongsfjorden, Svalbard: Occurrence, Sources and Bioavailability. Int. J. Environ. Anal. Chem. 2017, 97, 401–418. [Google Scholar] [CrossRef]
- Rajaram, R.; Ganeshkumar, A.; Emmanuel Charles, P. Ecological Risk Assessment of Metals in the Arctic Environment with Emphasis on Kongsfjorden Fjord and Freshwater Lakes of Ny-Ålesund, Svalbard. Chemosphere 2023, 310, 136737. [Google Scholar] [CrossRef]
- Bazzano, A.; Rivaro, P.; Soggia, F.; Ardini, F.; Grotti, M. Anthropogenic and Natural Sources of Particulate Trace Elements in the Coastal Marine Environment of Kongsfjorden, Svalbard. Mar. Chem. 2014, 163, 28–35. [Google Scholar] [CrossRef]
- Bazzano, A.; Ardini, F.; Terol, A.; Rivaro, P.; Soggia, F.; Grotti, M. Effects of the Atlantic Water and Glacial Run-off on the Spatial Distribution of Particulate Trace Elements in the Kongsfjorden. Mar. Chem. 2017, 191, 16–23. [Google Scholar] [CrossRef]
- Øverjordet, I.B.; Kongsrud, M.B.; Gabrielsen, G.W.; Berg, T.; Ruus, A.; Evenset, A.; Borgå, K.; Christensen, G.; Jenssen, B.M. Toxic and Essential Elements Changed in Black-Legged Kittiwakes (Rissa Tridactyla) during Their Stay in an Arctic Breeding Area. Sci. Total Environ. 2015, 502, 548–556. [Google Scholar] [CrossRef]
- Øverjordet, I.B.; Gabrielsen, G.W.; Berg, T.; Ruus, A.; Evenset, A.; Borgå, K.; Christensen, G.; Lierhagen, S.; Jenssen, B.M. Effect of Diet, Location and Sampling Year on Bioaccumulation of Mercury, Selenium and Cadmium in Pelagic Feeding Seabirds in Svalbard. Chemosphere 2015, 122, 14–22. [Google Scholar] [CrossRef]
- Singh, S.M.; Tsuji, M.; Singh, P.; Mulik, R.U. Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates. Sustainability 2022, 14, 11727. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, L.; Xie, Z.; Wang, J.; Li, Z.; Tu, L.; Sun, L. Historical Records and Contamination Assessment of Potential Toxic Elements (PTEs) over the Past 100 Years in Ny-Ålesund, Svalbard. Environ. Pollut. 2020, 266, 115205. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Sreelakshmi, U.; Vishnu Sagar, M.K.; Gopikrishna, V.G.; Pandit, G.G.; Sahu, S.K.; Tiwari, M.; Ajmal, P.Y.; Kannan, V.M.; Abdul Shukkur, M.; et al. Rate of Sediment Accumulation and Historic Metal Contamination in a Tidewater Glacier Fjord, Svalbard. Mar. Pollut. Bull. 2018, 131, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Prearo, M.; Brizio, P.; Gavinelli, S.; Pellegrino, M.; Scanzio, T.; Guarise, S.; Benedetto, A.; Abete, M.C. Heavy Metals Distribution in Muscle, Liver, Kidney and Gill of European Catfish (Silurus Glanis) from Italian Rivers. Chemosphere 2013, 90, 358–365. [Google Scholar] [CrossRef]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. (Eds.) Homeostasis and Toxicology of Essential Metals; Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 31A, ISBN 978-0-12-378636-4. [Google Scholar]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. (Eds.) Homeostasis and Toxicology of Non-Essential Metals; Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 31B, ISBN 978-0-12-378634-0. [Google Scholar]
- Mecklenburg, C.W.; Lynghammar, A.; Johannesen, E.; Byrkjedal, I.; Christiansen, J.S.; Dolgov, A.V.; Karamushko, O.V.; Mecklenburg, T.A.; Møller, P.R.; Steinke, D.; et al. Marine Fishes of the Arctic Region Volume 1; CAFF Monitoring Series Report 28; Conservation of Arctic Flora and Fauna: Akureyri, Iceland, 2018. [Google Scholar]
- Brand, M.; Fischer, P. Species Composition and Abundance of the Shallow Water Fish Community of Kongsfjorden, Svalbard. Polar Biol. 2016, 39, 2155–2167. [Google Scholar] [CrossRef]
- Cardinale, M. Ontogenetic Diet Shifts of Bull-Rout, Myoxocephalus Scorpius (L.), in the South-Western Baltic Sea. J. Appl. Ichthyol. 2000, 16, 231–239. [Google Scholar] [CrossRef]
- Gray, B.P.; Norcross, B.L.; Beaudreau, A.H.; Blanchard, A.L.; Seitz, A.C. Food Habits of Arctic Staghorn Sculpin (Gymnocanthus Tricuspis) and Shorthorn Sculpin (Myoxocephalus Scorpius) in the Northeastern Chukchi and Western Beaufort Seas. Deep-Sea Res. Pt. II 2017, 135, 111–123. [Google Scholar] [CrossRef]
- Landry, J.J.; Fisk, A.T.; Yurkowski, D.J.; Hussey, N.E.; Dick, T.; Crawford, R.E.; Kessel, S.T. Feeding Ecology of a Common Benthic Fish, Shorthorn Sculpin (Myoxocephalus Scorpius) in the High Arctic. Polar Biol. 2018, 41, 2091–2102. [Google Scholar] [CrossRef]
- Luksenburg, J.A.; Pedersen, T. Sexual and Geographical Variation in Life History Parameters of the Shorthorn Sculpin. J. Fish. Biol. 2002, 61, 1453–1464. [Google Scholar] [CrossRef]
- Datsky, A.V. Biological Features of the Common Fish Species in Olyutorsky-Navarin Region and the Adjacent Waters of the Bering Sea: 4. Family Sculpins (Cottidae). J. Ichthyol. 2017, 57, 341–353. [Google Scholar] [CrossRef]
- Nørregaard, R.D.; Bach, L.; Geertz-Hansen, O.; Nabe-Nielsen, J.; Nowak, B.; Jantawongsri, K.; Dang, M.; Søndergaard, J.; Leifsson, P.S.; Jenssen, B.M.; et al. Element Concentrations, Histology and Serum Biochemistry of Arctic Char (Salvelinus Alpinus) and Shorthorn Sculpins (Myoxocephalus Scorpius) in Northwest Greenland. Environ. Res. 2022, 208, 112742. [Google Scholar] [CrossRef] [PubMed]
- Datsky, A.V.; Vedishcheva, E.V.; Trofimova, A.O. Features of the Biology of Mass Fish Species in Russian Waters of the Chukchi Sea. 2. Families Pleuronectidae and Cottidae. J. Ichthyol. 2022, 62, 863–884. [Google Scholar] [CrossRef]
- Madenjian, C.P.; Rediske, R.R.; Krabbenhoft, D.P.; Stapanian, M.A.; Chernyak, S.M.; O’Keefe, J.P. Sex Differences in Contaminant Concentrations of Fish: A Synthesis. Biol. Sex. Differ. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.; Pearson, T.; Hegseth, E.N.; Kovacs, K.M.; Wiencke, C.; Kwasniewski, S.; Eiane, K.; Mehlum, F.; Gulliksen, B.; Wlodarska-Kowalczuk, M.; et al. The Marine Ecosystem of Kongsfjorden, Svalbard. Polar Res. 2002, 21, 167–208. [Google Scholar] [CrossRef]
- AMAP AMAP Assessment 2021: Mercury in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Tromsø, Norway, 2021; ISBN 978-82-7971-106-3.
- Hansson, S.V.; Desforges, J.-P.; van Beest, F.M.; Bach, L.; Halden, N.M.; Sonne, C.; Mosbech, A.; Søndergaard, J. Bioaccumulation of Mining Derived Metals in Blood, Liver, Muscle and Otoliths of Two Arctic Predatory Fish Species (Gadus Ogac and Myoxocephalus Scorpius). Environ. Res. 2020, 183, 109194. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, E.; Svavarsson, J.; Sturve, J.; Ericson, G.; Adolfsson-Erici, M.; Förlin, L. Biochemical Indicators of Pollution Exposure in Shorthorn Sculpin (Myoxocephalus Scorpius), Caught in Four Harbours on the Southwest Coast of Iceland. Aquat. Toxicol. 2000, 48, 431–442. [Google Scholar] [CrossRef]
- Bohn, A. Arsenic in Marine Organisms from West Greenland. Mar. Pollut. Bull. 1975, 6, 87–89. [Google Scholar] [CrossRef]
- Dang, M.; Nørregaard, R.; Bach, L.; Sonne, C.; Søndergaard, J.; Gustavson, K.; Aastrup, P.; Nowak, B. Metal Residues, Histopathology and Presence of Parasites in the Liver and Gills of Fourhorn Sculpin (Myoxocephalus Quadricornis) and Shorthorn Sculpin (Myoxocephalus Scorpius) near a Former Lead-Zinc Mine in East Greenland. Environ. Res. 2017, 153, 171–180. [Google Scholar] [CrossRef]
- Dang, M.; Pittman, K.; Bach, L.; Sonne, C.; Hansson, S.V.; Søndergaard, J.; Stride, M.; Nowak, B. Mucous Cell Responses to Contaminants and Parasites in Shorthorn Sculpins (Myoxocephalus Scorpius) from a Former Lead-zinc Mine in West Greenland. Sci. Total Environ. 2019, 678, 207–216. [Google Scholar] [CrossRef]
- Dang, M.; Pittman, K.; Sonne, C.; Hansson, S.; Bach, L.; Søndergaard, J.; Stride, M.; Nowak, B. Histological Mucous Cell Quantification and Mucosal Mapping Reveal Different Aspects of Mucous Cell Responses in Gills and Skin of Shorthorn Sculpins (Myoxocephalus Scorpius). Fish. Shellfish. Immun. 2020, 100, 334–344. [Google Scholar] [CrossRef]
- Dietz, R.; Riget, F.; Johansen, P. Lead, Cadmium, Mercury and Selenium in Greenland Marine Animals. Sci. Total Environ. 1996, 186, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Kaarsholm, H.M.; Verland, N.; Nørregaard, R.D.; Bach, L.; Søndergaard, J.; Rigét, F.F.; Dietz, R.; Hansen, M.; Eulaers, I.; Desforges, J.-P.; et al. Histology of Sculpin Spp. in East Greenland. II. Histopathology and Trace Element Concentrations. Toxicol. Environ. Chem. 2018, 100, 769–784. [Google Scholar] [CrossRef]
- Nørregaard, R.D.; Dang, M.; Bach, L.; Geertz-Hansen, O.; Gustavson, K.; Aastrup, P.; Leifsson, P.S.; Søndergaard, J.; Nowak, B.; Sonne, C. Comparison of Heavy Metals, Parasites and Histopathology in Sculpins (Myoxocephalus Spp.) from Two Sites at a Lead-Zinc Mine in North East Greenland. Environ. Res. 2018, 165, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, J. Dispersion and Bioaccumulation of Elements from an Open-Pit Olivine Mine in Southwest Greenland Assessed Using Lichens, Seaweeds, Mussels and Fish. Environ. Monit. Assess. 2013, 185, 7025–7035. [Google Scholar] [CrossRef]
- Sonne, C.; Bach, L.; Søndergaard, J.; Rigét, F.F.; Dietz, R.; Mosbech, A.; Leifsson, P.S.; Gustavson, K. Evaluation of the Use of Common Sculpin (Myoxocephalus Scorpius) Organ Histology as Bioindicator for Element Exposure in the Fjord of the Mining Area Maarmorilik, West Greenland. Environ. Res. 2014, 133, 304–311. [Google Scholar] [CrossRef]
- Bohn, A.; Fallis, B.W. Metal Concentrations (As, Cd, Cu, Pb and Zn) in Shorthorn Sculpins, Myoxocephalus Scorpius (Linnaeus), and Arctic Char, Salvelinus Alpinus (Linnaeus), from the Vicinity of Strathcona Sound, Northwest Territories. Water Res. 1978, 12, 659–663. [Google Scholar] [CrossRef]
- Harley, J.; Lieske, C.; Bhojwani, S.; Castellini, J.M.; López, J.A.; O’Hara, T.M. Mercury and Methylmercury Distribution in Tissues of Sculpins from the Bering Sea. Polar Biol. 2015, 38, 1535–1543. [Google Scholar] [CrossRef]
- Jantawongsri, K.; Nørregaard, R.D.; Bach, L.; Dietz, R.; Sonne, C.; Jørgensen, K.; Lierhagen, S.; Ciesielski, T.M.; Jenssen, B.M.; Haddy, J.; et al. Histopathological Effects of Short-Term Aqueous Exposure to Environmentally Relevant Concentration of Lead (Pb) in Shorthorn Sculpin (Myoxocephalus Scorpius) under Laboratory Conditions. Environ. Sci. Pollut. Res. 2021, 28, 61423–61440. [Google Scholar] [CrossRef]
- Søndergaard, J.; Halden, N.; Bach, L.; Gustavson, K.; Sonne, C.; Mosbech, A. Otolith Chemistry of Common Sculpins (Myoxocephalus Scorpius) in a Mining Polluted Greenlandic Fiord (Black Angel Lead-Zinc Mine, West Greenland). Water Air Soil Pollut. 2015, 226, 336. [Google Scholar] [CrossRef]
- Svendsen, H.; Beszczynska-Møller, A.; Hagen, J.O.; Lefauconnier, B.; Tverberg, V.; Gerland, S.; Ørbøk, J.B.; Bischof, K.; Papucci, C.; Zajaczkowski, M.; et al. The Physical Environment of Kongsfjorden-Krossfjorden, and Arctic Fjord System in Svalbard. Polar Res. 2002, 21, 133–166. [Google Scholar]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative Weight (Wr) Status and Current Use in Fisheries Assessment and Management. Rev. Fish. Sci. 2000, 8, 1–44. [Google Scholar] [CrossRef]
- Rivaro, P.; Ianni, C.; Soggia, F.; Frache, R. Mercury Speciation in Environmental Samples by Cold Vapour Atomic Absorption Spectrometry with in Situ Preconcentration on a Gold Trap. Microchim. Acta 2007, 158, 345–352. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 27 March 2023).
- Usero, J.; González-Regalado, E.; Gracia, I. Trace Metals in the Bivalve Mollusc Chamelea Gallina from the Atlantic Coast of Southern Spain. Mar. Pollut. Bull. 1996, 32, 305–310. [Google Scholar] [CrossRef]
- AMAP. AMAP Assessment Report: Arctic Pollution Issues; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 1998. [Google Scholar]
- Zhang, J.; Tan, Q.-G.; Huang, L.; Ye, Z.; Wang, X.; Xiao, T.; Wu, Y.; Zhang, W.; Yan, B. Intestinal Uptake and Low Transformation Increase the Bioaccumulation of Inorganic Arsenic in Freshwater Zebrafish. J. Hazard. Mater. 2022, 434, 128904. [Google Scholar] [CrossRef]
- Daglish, R.W.; Nowak, B.F. Rainbow Trout Gills Are a Sensitive Biomarker of Short-Term Exposure to Waterborne Copper. Arch. Environ. Contam. Toxicol. 2002, 43, 98–102. [Google Scholar] [CrossRef]
- Łokas, E.; Zaborska, A.; Kolicka, M.; Różycki, M.; Zawierucha, K. Accumulation of Atmospheric Radionuclides and Heavy Metals in Cryoconite Holes on an Arctic Glacier. Chemosphere 2016, 160, 162–172. [Google Scholar] [CrossRef]
- Witters, H.E.; VanPuymbroeck, S.; Stouthart, A.J.H.X.; Bonga, S.E.W. Physicochemical Changes of Aluminium in Mixing Zones: Mortality and Physiological Disturbances in Brown Trout (Salmo Trutta L.). Environ. Toxicol. Chem. 1996, 15, 986–996. [Google Scholar] [CrossRef]
- Ploetz, D.M.; Fitts, B.E.; Rice, T.M. Differential Accumulation of Heavy Metals in Muscle and Liver of a Marine Fish, (King Mackerel, Scomberomorus Cavalla Cuvier) from the Northern Gulf of Mexico, USA. Bull. Environ. Contam. Toxicol. 2007, 78, 134–137. [Google Scholar] [CrossRef]
- Jovičić, K.; Nikolić, D.M.; Višnjić-Jeftić, Ž.; Đikanović, V.; Skorić, S.; Stefanović, S.M.; Lenhardt, M.; Hegediš, A.; Krpo-Ćetković, J.; Jarić, I. Mapping Differential Elemental Accumulation in Fish Tissues: Assessment of Metal and Trace Element Concentrations in Wels Catfish (Silurus Glanis) from the Danube River by ICP-MS. Environ. Sci. Pollut. Res. 2015, 22, 3820–3827. [Google Scholar] [CrossRef]
- Monferrán, M.V.; Garnero, P.; de los Angeles Bistoni, M.; Anbar, A.A.; Gordon, G.W.; Wunderlin, D.A. From Water to Edible Fish. Transfer of Metals and Metalloids in the San Roque Reservoir (Córdoba, Argentina). Implications Associated with Fish Consumption. Ecol. Indic. 2016, 63, 48–60. [Google Scholar] [CrossRef]
- European Union Commission Regulation (EC). No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- MacKinnon, J.C. Summer Storage of Energy and Its Use for Winter Metabolism and Gonad Maturation in American Plaice (Hippoglossoides Platessoides). J. Fish. Res. Board Can. 1972, 29, 1749–1759. [Google Scholar] [CrossRef]
- Ennis, G.P. Reproduction and Associated Behaviour in the Shorthorn Sculpin, Myoxocephalus Scorpius in Newfoundland Waters. J. Fish. Res. Board Can. 1970, 27, 2037–2045. [Google Scholar] [CrossRef]
- Luksenburg, J.A.; Pedersen, T.; Falk-Petersen, I.B. Reproduction of the Shorthorn Sculpin Myoxocephalus Scorpius in Northern Norway. J. Sea Res. 2004, 51, 157–166. [Google Scholar] [CrossRef]
- Ahilan, B.; Jeyaseelan, M.J.P. Effect of Cobalt Chloride and Vitamin B12 on the Growth and Gonadal Maturation of Goldfish Carassius Auratus. Indian J. Fish. 2001, 48, 369–374. [Google Scholar]
- Farkas, A.; Salánki, J.; Specziár, A. Age- and Size-Specific Patterns of Heavy Metals in the Organs of Freshwater Fish Abramis Brama L. Populating a Low-Contaminated Site. Water Res. 2003, 37, 959–964. [Google Scholar] [CrossRef]
- Authman, M.M.; Zaki, M.S.; Khallaf, E.A.; Abbas, H.H. Use of Fish as Bio-Indicator of the Effects of Heavy Metals Pollution. J. Aquac. Res. Dev. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Singh, S.M.; Naik, S.; Mulik, R.U.; Sharma, J.; Upadhyay, A.K. Elemental Composition and Bacterial Occurrence in Sediment Samples on Two Sides of Brøggerhalvøya, Svalbard. Polar Rec. 2015, 51, 680–691. [Google Scholar] [CrossRef]
- Singh, S.M.; Avinash, K.; Sharma, P.; Mulik, R.U.; Upadhyay, A.K.; Ravindra, R. Elemental Variations in Glacier Cryoconites of Indian Himalaya and Spitsbergen, Arctic. Geosci. Front. 2017, 8, 1339–1347. [Google Scholar] [CrossRef]
- Lorenzana, R.M.; Yeow, A.Y.; Colman, J.T.; Chappell, L.L.; Choudhury, H. Arsenic in Seafood: Speciation Issues for Human Health Risk Assessment. Hum. Ecol. Risk Assess. 2009, 15, 185–200. [Google Scholar] [CrossRef]
- Liu, G.; Cai, Y.; O’Driscoll, N. Environmental Chemistry and Toxicology of Mercury; John Wiley and Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-57872-8. [Google Scholar]
- Wagemann, R.; Trebacz, E.; Boila, G.; Lockhart, W.L. Methylmercury and Total Mercury in Tissues of Arctic Marine Mammals. Sci. Total Environ. 1998, 218, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wagemann, R.; Trebacz, E.; Boila, G.; Lockhart, W.L. Mercury Species in the Liver of Ringed Seals. Sci. Total Environ. 2000, 261, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Nieboer, E.; Varakina, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and Non-Essential Trace Elements in Fish Consumed by Indigenous Peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Sonne, C.; Basu, N.; Braune, B.; O’Hara, T.; Letcher, R.J.; Scheuhammer, T.; Andersen, M.; Andreasen, C.; Andriashek, D.; et al. What Are the Toxicological Effects of Mercury in Arctic Biota? Sci. Total Environ. 2013, 443, 775–790. [Google Scholar] [CrossRef]
- Raymond, L.J.; Ralston, N.V.C. Selenium’s Importance in Regulatory Issues Regarding Mercury. Fuel Process Technol. 2009, 90, 1333–1338. [Google Scholar] [CrossRef]
- Peterson, S.A.; Ralston, N.V.C.; Whanger, P.D.; Oldfield, J.E.; Mosher, W.D. Selenium and Mercury Interactions with Emphasis on Fish Tissue. Environ. Bioindic. 2009, 4, 318–334. [Google Scholar] [CrossRef]
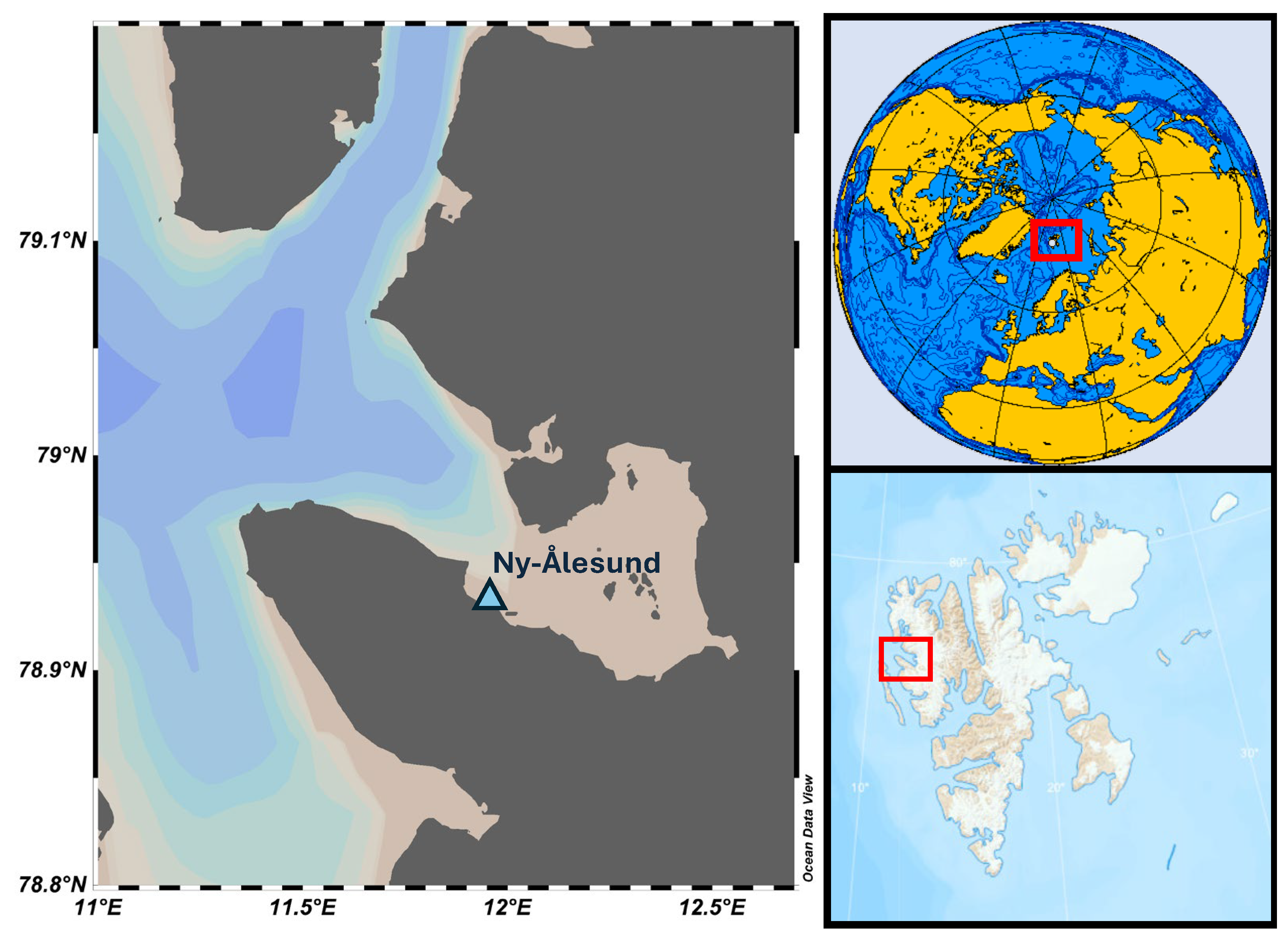
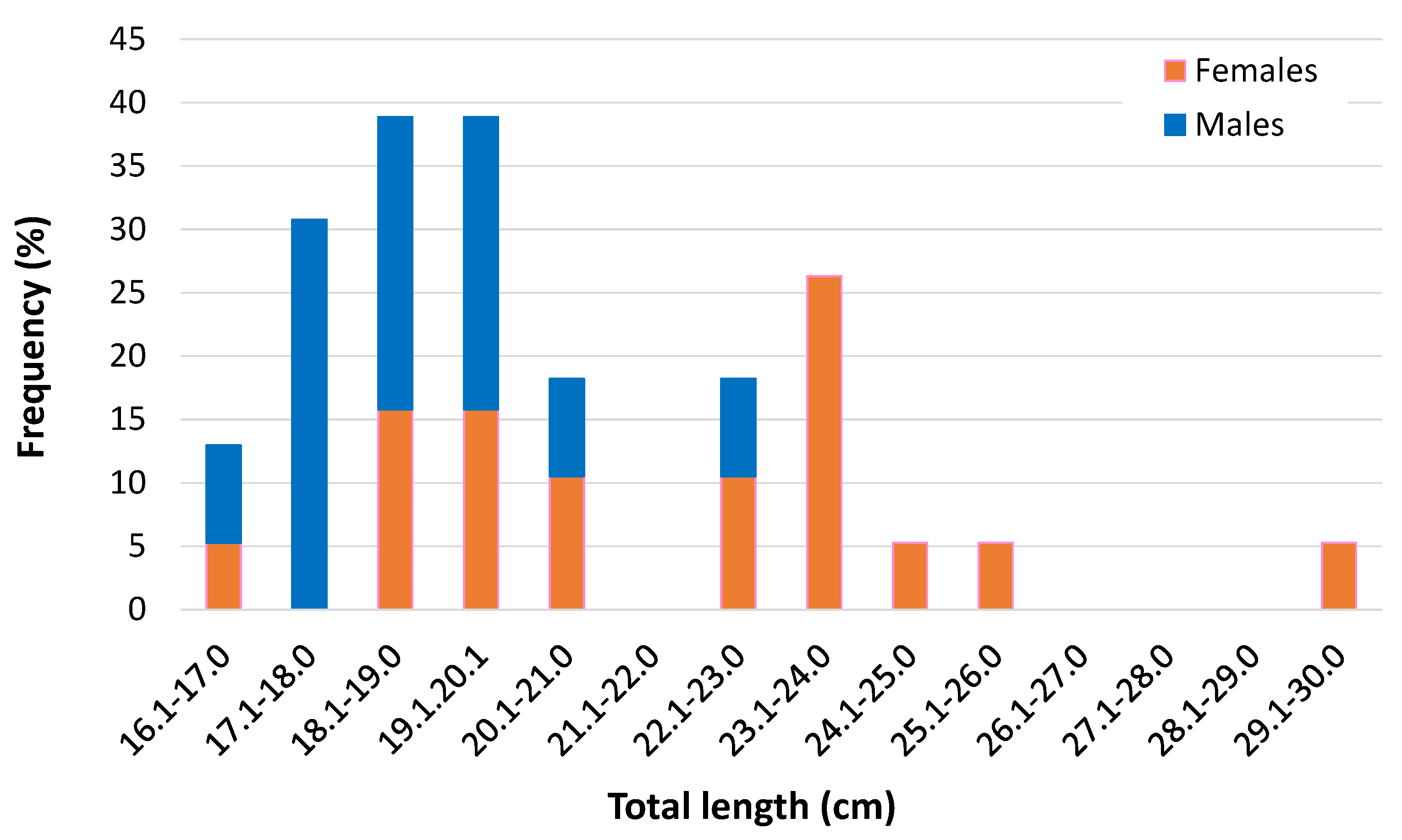
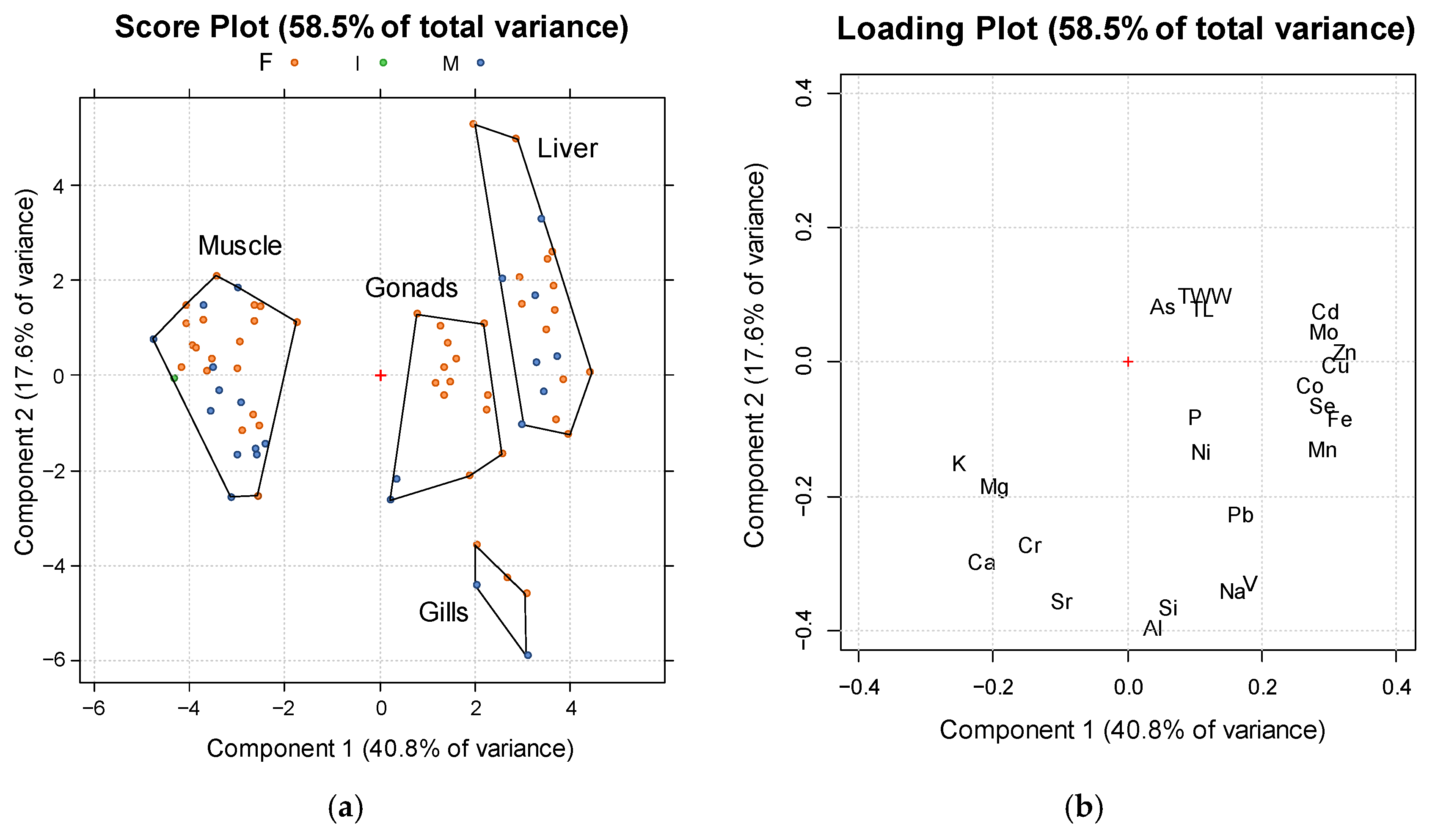
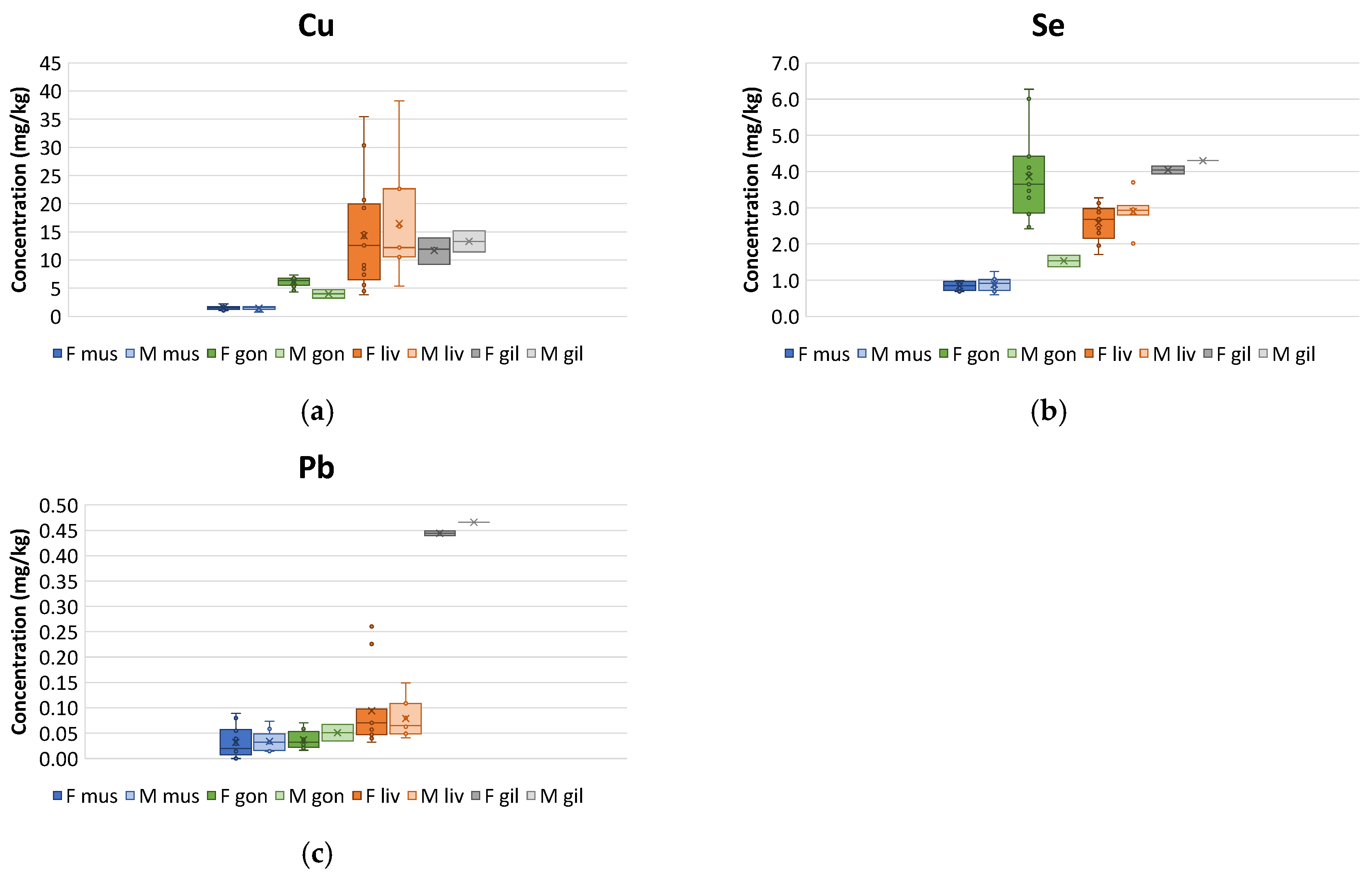


| Sex | n | TL (cm) | TWW (g) | Fulton’s CI |
|---|---|---|---|---|
| Female | 19 | 22.3 ± 3.1 (17–30) | 142.1 ± 64.4 (46–328) | 1.21 ± 0.12 (0.94–1.42) |
| Male | 13 | 19.1 ± 1.7 (16.5–23) | 84.9 ± 22.2 (54–138) | 1.21 ± 0.19 (0.93–1.74) |
| Immature | 1 | 14 | 38 | 1.38 |
| Tissue | Al | As | Ba | Ca | Cd | Co | Cr | Cu | Fe | Hg | K | Mg | Mn | |
| mg/kg | mg/kg | µg/kg | mg/kg | µg/kg | mg/kg | mg/kg | mg/kg | vmg/kg | mg/kg | mg/kg | mg/kg | mg/kg | ||
| Muscle | Min | <1.20 | 4.7 | <16 | 493 | <25 | 0.033 | 0.150 | 0.8 | 9 | 0.081 | 11,001 | 1021 | 0.75 |
| Mean | 8 | 9.7 | 64 | 1904 | <25 | 0.073 | 0.886 | 1.5 | 26 | 0.276 | 12,444 | 1149 | 1.27 | |
| Median | 4 | 8.3 | 32 | 1601 | <25 | 0.069 | 0.612 | 1.5 | 24 | 0.210 | 12,486 | 1151 | 1.22 | |
| Max | 47 | 25.9 | 236 | 5817 | <25 | 0.136 | 4.436 | 2.2 | 55 | 0.923 | 13,360 | 1389 | 2.24 | |
| Liver | Min | <1.20 | 5.4 | <16 | 61 | 199 | 0.137 | <0.101 | 3.8 | 99 | - | 6192 | 463 | 1.90 |
| Mean | 6 | 12.7 | 32 | 312 | 848 | 0.294 | 0.284 | 15.1 | 356 | - | 8641 | 754 | 3.64 | |
| Median | 4 | 9.8 | 32 | 318 | 613 | 0.267 | 0.167 | 12.4 | 400 | - | 8766 | 741 | 3.50 | |
| Max | 20 | 31.0 | 35 | 664 | 2467 | 0.446 | 0.990 | 38.2 | 739 | - | 10,913 | 1415 | 6.28 | |
| Gonads | Min | <1.20 | 3.5 | <16 | 210 | <25 | 0.186 | <0.101 | 3.2 | 60 | - | 9806 | 696 | 1.48 |
| Mean | 18 | 6.5 | 441 | 460 | 57 | 0.558 | 0.506 | 5.9 | 106 | - | 11,498 | 1124 | 3.41 | |
| Median | 6 | 6.0 | 441 | 445 | 52 | 0.624 | 0.208 | 6.0 | 108 | - | 11,512 | 1051 | 3.22 | |
| Max | 130 | 10.8 | 441 | 896 | 95 | 0.762 | 2.072 | 7.4 | 148 | - | 13,590 | 1714 | 5.12 | |
| Gills | Min | 27 | 6.0 | 147 | 1420 | 72 | 0.179 | 1.715 | 9.2 | 493 | 0.087 | 9316 | 831 | 4.98 |
| Mean | 42 | 7.4 | 325 | 1862 | 99 | 0.217 | 3.015 | 12.3 | 570 | 0.124 | 9779 | 886 | 6.12 | |
| Median | 38 | 6.7 | 258 | 1868 | 97 | 0.197 | 2.359 | 11.9 | 552 | 0.134 | 9697 | 894 | 5.79 | |
| Max | 66 | 9.7 | 568 | 2233 | 136 | 0.273 | 4.970 | 15.2 | 644 | 0.143 | 10,294 | 942 | 7.75 | |
| Tissue | Mo | Na | Ni | P | Pb | Sb | Se | Si | Sn | Sr | V | Zn | MeHg | |
| mg/kg | mg/kg | µg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | µg/kg | mg/kg | mg/kg | ||
| Muscle | Min | <0.005 | 2590 | <102 | 6708 | <0.014 | <0.007 | 0.60 | 13 | <0.053 | 0.9 | <40 | 21 | 0.037 |
| Mean | 0.022 | 4076 | 217 | 8045 | 0.037 | 0.024 | 0.85 | 28 | 0.827 | 9.0 | 143 | 35 | 0.164 | |
| Median | 0.013 | 3822 | 197 | 7995 | 0.031 | 0.022 | 0.85 | 27 | 0.386 | 6.9 | 119 | 34 | 0.117 | |
| Max | 0.080 | 6220 | 488 | 9756 | 0.089 | 0.041 | 1.24 | 55 | 3.609 | 38.3 | 407 | 52 | 0.679 | |
| Liver | Min | 0.225 | 2858 | 138 | 5865 | <0.014 | <0.007 | 1.71 | 12 | <0.053 | <0.033 | <40 | 81 | - |
| Mean | 0.355 | 4414 | 254 | 8752 | 0.088 | <0.007 | 2.69 | 28 | 0.121 | 2.6 | 267 | 143 | - | |
| Median | 0.356 | 4474 | 248 | 9102 | 0.067 | <0.007 | 2.82 | 24 | 0.121 | 2.1 | 147 | 141 | - | |
| Max | 0.548 | 5788 | 397 | 11,282 | 0.260 | <0.007 | 3.70 | 77 | 0.121 | 6.4 | 739 | 223 | - | |
| Gonads | Min | 0.057 | 4769 | <102 | 8436 | <0.014 | <0.007 | 1.37 | 16 | <0.053 | 1.4 | <40 | 91 | - |
| Mean | 0.083 | 6163 | 248 | 10,893 | 0.040 | 0.014 | 3.55 | 32 | 0.150 | 4.7 | 211 | 115 | - | |
| Median | 0.080 | 6012 | 224 | 10,686 | 0.036 | 0.014 | 3.47 | 30 | 0.150 | 4.7 | 158 | 119 | - | |
| Max | 0.110 | 8040 | 511 | 14,726 | 0.070 | 0.016 | 6.27 | 59 | 0.150 | 8.8 | 806 | 129 | - | |
| Gills | Min | 0.082 | 8529 | 181 | 7532 | 0.440 | 0.007 | 3.93 | 60 | 0.325 | 11.0 | 1985 | 75 | <0.016 |
| Mean | 0.086 | 9107 | 310 | 7693 | 0.452 | 0.032 | 4.13 | 120 | 0.790 | 17.1 | 2380 | 84 | 0.030 | |
| Median | 0.085 | 8672 | 331 | 7770 | 0.449 | 0.012 | 4.15 | 111 | 0.675 | 17.2 | 2376 | 85 | 0.030 | |
| Max | 0.091 | 10500 | 405 | 7780 | 0.466 | 0.077 | 4.30 | 190 | 1.370 | 23.4 | 2710 | 87 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardini, F.; Moggia, F.; Di Blasi, D.; Rivaro, P.; Grotti, M.; Ghigliotti, L. Elemental Distribution in Tissues of Shorthorn Sculpins (Myoxocephalus scorpius) from Kongsfjorden in Svalbard. J. Mar. Sci. Eng. 2024, 12, 2245. https://doi.org/10.3390/jmse12122245
Ardini F, Moggia F, Di Blasi D, Rivaro P, Grotti M, Ghigliotti L. Elemental Distribution in Tissues of Shorthorn Sculpins (Myoxocephalus scorpius) from Kongsfjorden in Svalbard. Journal of Marine Science and Engineering. 2024; 12(12):2245. https://doi.org/10.3390/jmse12122245
Chicago/Turabian StyleArdini, Francisco, Federico Moggia, Davide Di Blasi, Paola Rivaro, Marco Grotti, and Laura Ghigliotti. 2024. "Elemental Distribution in Tissues of Shorthorn Sculpins (Myoxocephalus scorpius) from Kongsfjorden in Svalbard" Journal of Marine Science and Engineering 12, no. 12: 2245. https://doi.org/10.3390/jmse12122245
APA StyleArdini, F., Moggia, F., Di Blasi, D., Rivaro, P., Grotti, M., & Ghigliotti, L. (2024). Elemental Distribution in Tissues of Shorthorn Sculpins (Myoxocephalus scorpius) from Kongsfjorden in Svalbard. Journal of Marine Science and Engineering, 12(12), 2245. https://doi.org/10.3390/jmse12122245







