Simulated Testing of the Characteristics and Environmental Impacts of Disinfection By-Products Generated by Ballast Water Management Systems in Ports during Phytoplankton Blooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Method
2.2. Environmental Analysis
2.3. Analysis and Risk Assessment of Disinfection By-Products
2.4. Environmental Risk Assessment
3. Results
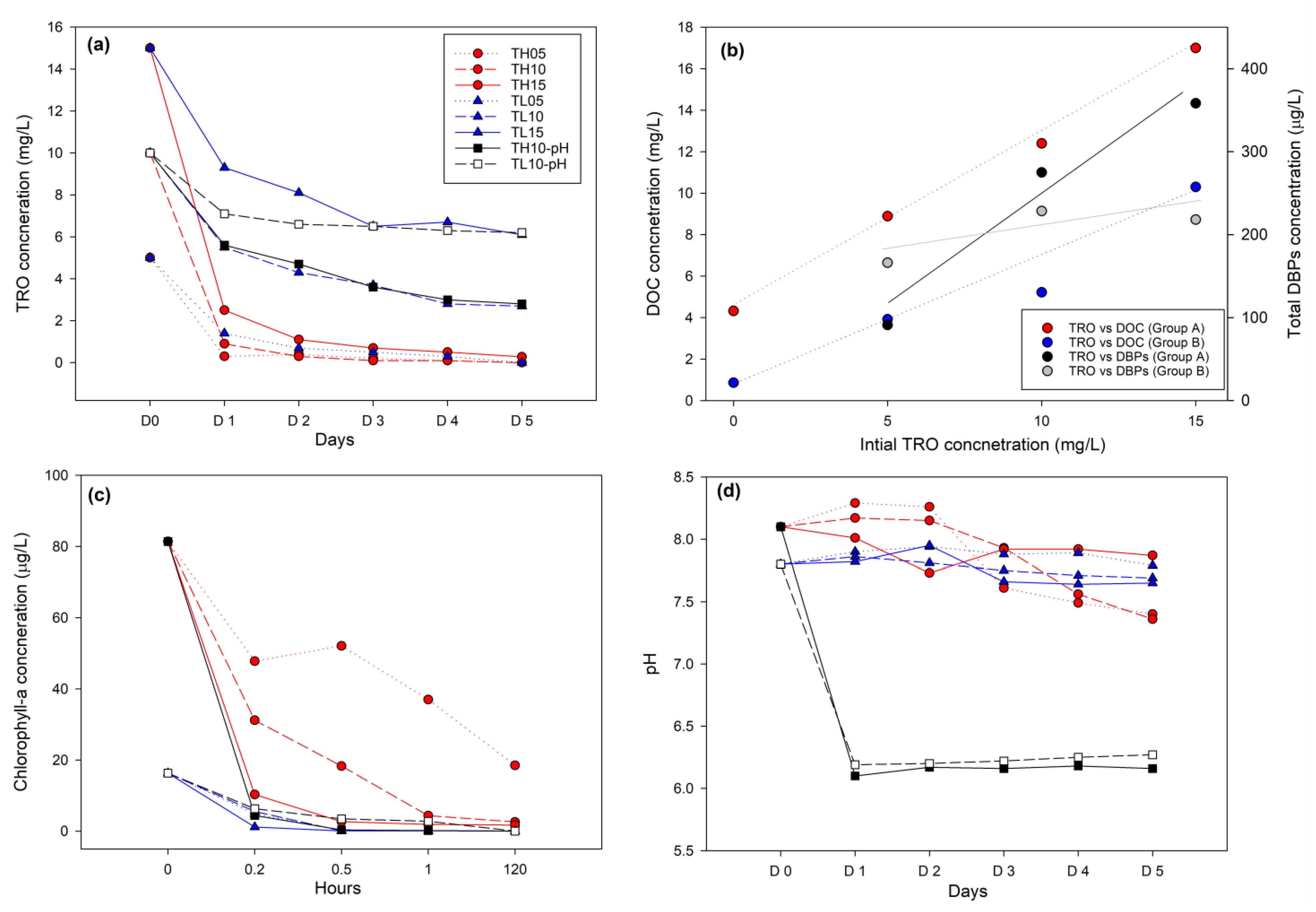
3.1. Environmental Parameters
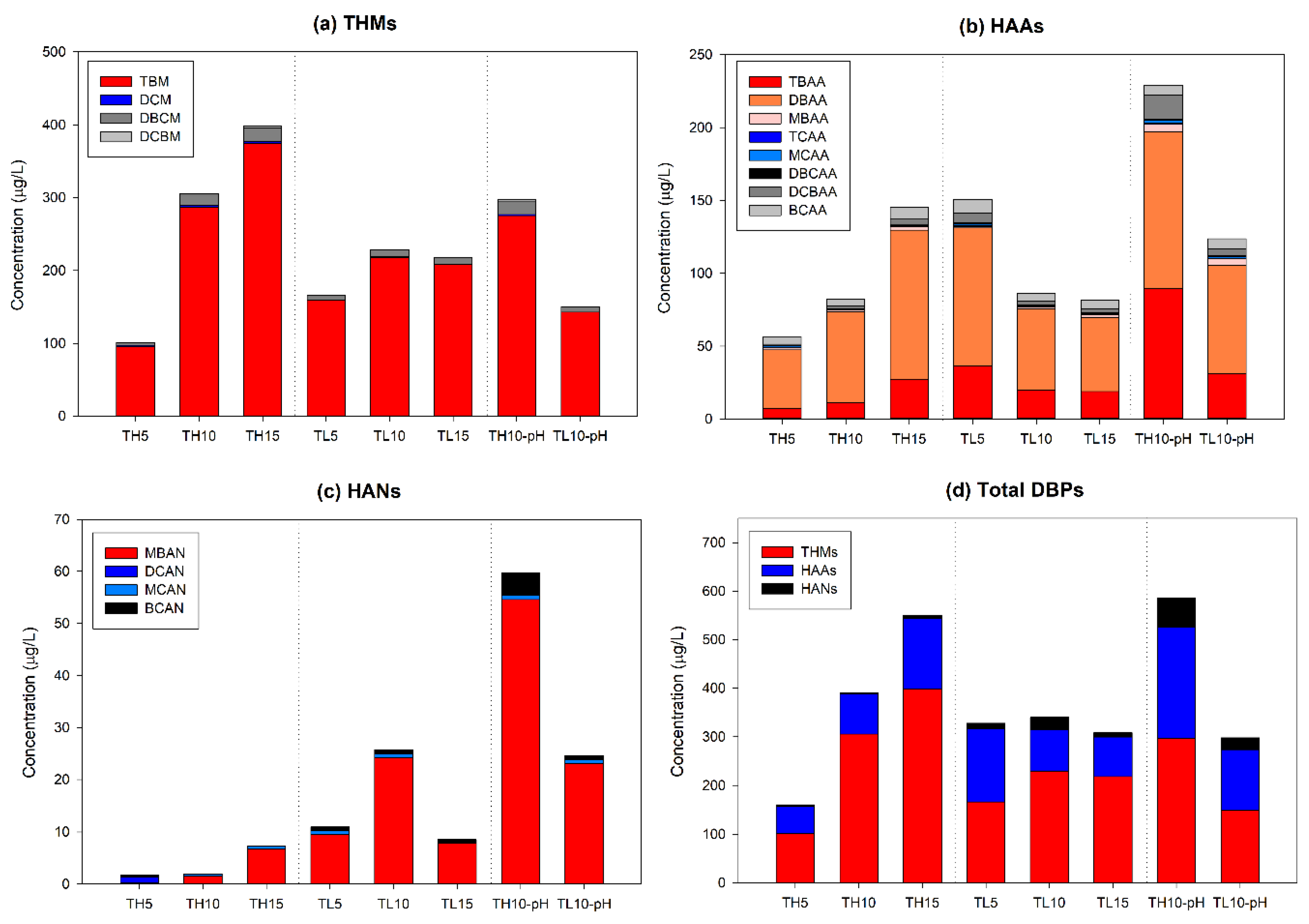
3.2. Changes of the DBP Concentration on Day 1 and Day 5
3.3. MEPC Results of Type Approved BWMSs by Applying 2008 G8 Guideline
4. Discussion
4.1. Changes of Organic Matter Concentration According to Environmental Conditions
4.2. Changes in the DBP Characteristics According to the Organic Matter Concentration
4.3. Comparison with MEPC Results
4.4. Environmental Risk Assessment on DBPs among Groups
5. Conclusions
- When ballasting with BWMS using AS during massive phytoplankton blooms, the concentration of DBPs can increase, potentially raising the environmental risk in ports.
- The environmental risk assessment of IMO G9 performed with cultured species may substitute the outcome with a natural plankton community.
- DBAN, which can be problematic because of its high toxicity in land-based tests, might be affected by the addition of glucose to artificially increase the dissolved organic matter.
- According to the results of the environmental risk assessment, MCAA may cause toxicity in ports, thus necessitating port monitoring.
- The initial TRO concentration and final TRO concentration play a crucial role in determining the concentration and composition ratio of DBPs.
- By obtaining information on the biological quantity and DOM concentration using a calibrated sensor in ports before ballasting, it is possible to treat ballast water with an appropriate TRO concentration to increase the energy efficiency of BWMS operations and reduce the environmental risk of DBPs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, P.G.; Cha, H.G. Long-term changes of disinfection byproducts in treatment of simulated ballast water. Ocean Sci. J. 2020, 55, 265–277. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef]
- Jang, P.G.; Cha, H.G.; Jang, M.C.; Hyun, B.; Choi, T.S.; Kang, Y.; Shin, K. Characteristic and relative environmental risk of disinfection by products associated with simple glucose or naturally occurring algal organic matter as tested in ballast water treatment system. J. Mar. Sci. Eng. 2022, 10, 1928. [Google Scholar] [CrossRef]
- Lakshmi, E.; Priya, M.; Achari, V.S. An overview on the treatment of ballast water in ships. Ocean Coast. Manag. 2021, 199, 105296. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO). Methodology for Information Gathering and Conduct of Work of GESAMP-BWWG (BWM.2/Circ.13/Rev.5). 8 September 2022. Available online: https://docs.imo.org/Shared/Download.aspx?did=138301 (accessed on 18 January 2024).
- International Maritime Organization (IMO). List of Ballast Water Management Systems That Make Use of Active Substances Which Received Basic and Final Approval (BWM.2/Circ.34/Rev.10). 14 December 2021. Available online: https://docs.imo.org/Search.aspx?keywords=BWM.2%2FCirc.34%2FRev.10 (accessed on 12 December 2023).
- Delacroix, S.; Vogelsang, C.; Tobiesen, A.; Liltved, H. Disinfection by-products and ecotoxicity of ballast water after oxidative treatment–results and experiences from seven years of full-scale testing of ballast water management systems. Mar. Pollut. Bull. 2013, 73, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Liu, Z.Q.; Salhi, E.; Hoefer, T.; Werschkun, B.; Von Gunten, U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: Influence of water quality parameters. Environ. Sci. Water Res. Technol. 2015, 1, 465–480. [Google Scholar] [CrossRef]
- Yu, H.W.; Oh, S.G.; Kim, I.S.; Pepper, I.; Snyder, S.; Jang, A. Formation and speciation of haloacetic acids in seawater desalination using chlorine dioxide as disinfectant. J. Ind. Eng. Chem. 2015, 26, 193–201. [Google Scholar] [CrossRef]
- Cha, H.G.; Seo, M.H.; Lee, H.Y.; Lee, J.H.; Lee, D.S.; Shin, K.; Choi, K.H. Enhancing the efficacy of electrolytic chlorination for ballast water treatment by adding carbon dioxide. Mar. Pollut. Bull. 2015, 95, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, J.; Wang, Q.; Yuan, L.; Wu, H. Formation of halogenated disinfection by-products during ballast water chlorination. Environ. Sci. Water Res. Technol. 2022, 8, 648–656. [Google Scholar] [CrossRef]
- Yu, Y.; Reckhow, D.A. Kinetic analysis of haloacetonitrile stability in drinking waters. Environ. Sci. Technol. 2015, 49, 11028–11036. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.T.; Lai, D.; McLain, J.L.; Manibusan, M.K.; Dellarco, V. Use of mechanism-based structure-activity relationships analysis in carcinogenic potential ranking for drinking water disinfection by-products. Environ. Health Perspect. 2002, 110, 75–87. [Google Scholar] [CrossRef]
- Gonsior, M.; Mitchelmore, C.; Heyes, A.; Harir, M.; Richardson, S.D.; Petty, W.T.; Wright, D.A.; Schmitt-Kopplin, P. Bromination of marine dissolved organic matter following full scale electrochemical ballast water disinfection. Environ. Sci. Technol. 2015, 49, 9048–9055. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Peperzak, L. Operational and environmental factors affecting disinfection byproducts formation in ballast water treatment systems. Chemosphere 2019, 232, 496–505. [Google Scholar] [CrossRef]
- Yang, X.; Guo, W.; Zhang, X.; Chen, F.; Ye, T.; Liu, W. Formation of disinfection by-products after pre-oxidation with chlorine dioxide or ferrate. Water Res. 2013, 47, 5856–5864. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, K.H.; Yang, J.H. Effects of physicochemical parameters of water on byproduct formation during electrolysis of ballast water. Front. Environ. Sci. 2023, 11, 1114353. [Google Scholar] [CrossRef]
- Jung, S.W.; Kang, J.; Park, J.S.; Joo, H.M.; Suh, S.S.; Kang, D.; Lee, T.K.; Kim, H.J. Dynamic bacterial community response to Akashiwo sanguinea (Dinophyceae) bloom in indoor marine microcosms. Sci. Rep. 2021, 11, 6983. [Google Scholar] [CrossRef] [PubMed]
- Biddanda, B.; Benner, R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnol. Oceanogr. 1997, 42, 506–518. [Google Scholar] [CrossRef]
- Chrost, R.H.; Faust, M.A. Organic carbon release by phytoplankton: Its composition and utilization by bacterioplankton. J. Plankton Res. 1983, 5, 477–493. [Google Scholar] [CrossRef]
- Thornton, D.C. Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 2014, 49, 20–46. [Google Scholar] [CrossRef]
- Myklestad, S.M. Dissolved organic carbon from phytoplankton. Mar. Chem. 2000, 5, 111–148. [Google Scholar] [CrossRef]
- Liu, C.; Ersan, M.S.; Plewa, M.J.; Amy, G.; Karanfil, T. Formation of regulated and unregulated disinfection byproducts during chlorination of algal organic matter extracted from freshwater and marine algae. Water Res. 2018, 142, 313–324. [Google Scholar] [CrossRef]
- Huang, J.; Graham, N.; Templeton, M.R.; Zhang, Y.; Collins, C.; Nieuwenhuijsen, M. A comparison of the role of two blue-green algae in THM and HAA formation. Water Res. 2009, 43, 3009–3018. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO). Procedure for Approval of Ballast Water Management System that Make Use of Active Substance (G9), MEPC 57/21. 2008. Available online: https://www.imorules.com/MEPCRES_169.57.html (accessed on 15 December 2023).
- International Maritime Organization (IMO). Guidelines for Approval of Ballast Water Management Systems (G8), MEPC 58/23. 2008. Available online: https://docs.imo.org/Shared/Download.aspx?did=51243 (accessed on 15 December 2023).
- Marine Environmental Protection Committee (MEPC). Code for Approval of Ballast Water Management System (BWMS CODE). MEPC 72/17/Add.1/Annex 5. 2018. Available online: https://docs.imo.org/Shared/Download.aspx?did=110918 (accessed on 15 December 2023).
- Marine Environmental Protection Committee (MEPC). Information on the GESAMP-BWWG Database of Chemicals Most Commonly Associated with Treated Ballast Water, MEPC 67/INF17. 2014. Available online: https://docs.imo.org/Documents/Detail.aspx?did=88063 (accessed on 15 December 2023).
- Jin, X.; Zha, J.; Xu, Y.; Giesy, J.P.; Richardson, K.L.; Wang, Z. Derivation of predicted no effect concentrations (PNEC) for 2,4,6trichlorophenol based on Chines resident species. Chemosphere 2012, 86, 17–23. [Google Scholar] [CrossRef]
- Jang, P.G.; Hyun, B.; Shin, K. Ballast water treatment performance evaluation under real changing conditions. J. Mar. Sci. Eng. 2020, 8, 817. [Google Scholar] [CrossRef]
- Biswas, H.; Jie, J.; Li, Y.; Zhang, G.; Zhu, Z.Y.; Wu, Y.; Zhang, G.L.; Li, Y.W.; Lil, S.M.; Zhang, J. Response of a natural phytoplankton community from the Qingdao coast (Yellow Sea, China) to variable CO2 levels over a short-term incubation experiment. Curr. Sci. 2015, 108, 1901–1909. [Google Scholar]
- Smith Jr, W.O.; Barber, R.T.; Huntsman, S.A. Primary production off the coast of northwest Africa: Excretion of dissolved organic matter and its heterotrophic uptake. Deep-Sea Res. 1977, 24, 35–47. [Google Scholar] [CrossRef]
- Lee, W.; Westerhoff, P.; Croué, J.P. Dissolved organic nitrogen as a precursor for chloroform, dichloroacetonitrile, N-nitrosodimethylamine, and trichloronitromethane. Environ. Sci. Technol. 2007, 41, 5485–5490. [Google Scholar] [CrossRef]
- Kali, S.; Khan, M.; Ghaffar, M.S.; Rasheed, S.; Waseem, A.; Iqbal, M.M.; Niazi, M.B.K.; Zafar, M.I. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: A comprehensive review. Environ. Pollut. 2021, 281, 116950. [Google Scholar] [CrossRef] [PubMed]
- International Maritime Organization (IMO). Guidance for the Commissioning Testing of Ballast Water Management Systems, BWM.2/Circ.70. 2018. Available online: https://docs.imo.org/Shared/Download.aspx?did=113378 (accessed on 15 December 2023).
- Hansen, K.M.; Willach, S.; Antoniou, M.G.; Mosbæk, H.; Albrechtsen, H.J.; Andersen, H.R. Effect of pH on the formation of disinfection byproducts in swimming pool water–is less THM better? Water Res. 2012, 46, 6399–6409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, B.; Li, J.; Zhang, Z. Factors affecting formation of chemical by-products during ballast water treatment based on an advanced oxidation process. Chem. Eng. J. 2013, 231, 427–433. [Google Scholar] [CrossRef]
- Han, K.H.; Li, Z.; Youn, J.Y.; Kang, B.J.; Kim, H.J.; Seo, M.H.; Soh, H.Y.; Shin, H.H. Bloom-forming dinoflagellate Akashiwo sanguinea (Dinophyceae) in Jangmok Harbour of Geoje Island, Korea: Morphology, phylogeny and effects of temperature and salinity on growth. Korean J. Environ. Biol. 2019, 37, 119–128. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current perspective on wastewater treatment using photobioreactor for Tetraselmis sp.: An emerging and foreseeable sustainable approach. Environ. Sci. Pollut. Res. 2022, 29, 61905–61937. [Google Scholar] [CrossRef] [PubMed]
- Summerson, R.; Bloomfield, N.; Arthur, T. Treated ballast water and its impact on port water quality. ABARES Canberra 2019, 1–84. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, X.Y. Biodegradation and biotransformation of wastewater organics as precursors of disinfection byproducts in water. Chemosphere 2010, 81, 1075–1083. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, X.Y.; Xie, Y.F.; Tang, H. Characterization of soluble microbial products as precursors of disinfection byproducts in drinking water supply. Sci. Total Environ. 2014, 472, 818–824. [Google Scholar] [CrossRef]
- Hua, L.C.; Chao, S.J.; Huang, K.; Huang, C. Characteristics of low and high SUVA precursors: Relationships among molecular weight, fluorescence, and chemical composition with DBP formation. Sci. Total Environ. 2020, 727, 138638. [Google Scholar] [CrossRef]


| Raw Water (Cells/mL) | Diluted Water (Cells/mL) | |
|---|---|---|
| Bacillariophyceae | ||
| Pseudo-nitzschia spp. | 20 | 0 |
| Rhizosolenia spp. | 10 | 0 |
| Thalassiosira spp. | 10 | 0 |
| Dinophyceae | ||
| Akashiwo sanguinea | 2160 | 420 |
| Alexandrium spp. | 50 | 0 |
| Unidentified dinoflagellate | 10 | 10 |
| Euglenophyceae | ||
| Eutreptiella gymnastica | 30 | 10 |
| Small flagellates (<5 µm) | 720 | 70 |
| <20 µm Ciliates | 30 | 10 |
| Compounds | Abbreviations | Compounds | Abbreviations |
|---|---|---|---|
| Active substance | AS | Tribromoacetic acid | TBAA |
| Total residual oxidants | TRO | Dibromoacetic acid | DBAA |
| Dissolved organic matter | DOM | Monobromoacetic acid | MBAA |
| Dissolved organic carbon | DOC | Trichloroacetic acid | TCAA |
| Dissolved organic nitrogen | DON | Dichloroacetic acid | DCAA |
| Trihalomethanes | THMs | Monochloroacetic acid | MCAA |
| Haloacetic acids | HAAs | Dibromochloroacetic acid | DBCAA |
| Haloacetonitriles | HANs | Dichlorobromoacetic acid | DCBAA |
| Bromoform | TBM | Bromochloroacetic acid | BCAA |
| Dibromomethane | DBM | Dibromoacetonitrile | DBAN |
| Chloroform | TCM | Monobromoacetonitrile | MBAN |
| Dichloromethane | DCM | Dichloroacetonitrile | DCAN |
| Dibromochloromethane | DBCM | Monochloroacetonitrile | MCAN |
| Dichlorobromomethane | DCBM | Tribromoacetic acid | BCAN |
| Bromodichloromethane | BDCM |
| Conditions of the Test Water on Day 0 | Conditions of the Treated Water on Day 1 | Conditions of the Treated Water on Day 5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Biomass | TRO | pH | A. Chl a (1) | DOC | TRO | pH | DOC | DON | TRO | pH | DOC | DON | |
| (cells/mL) | (mg/L) | (μg/L) | (mg/L) | (mg/L) | (mg/L) | (μM) | (mg/L) | (mg/L) | (μM) | |||||
| A | TH05 | 3100 | 5 | 8.1 | 30.2 | 4.32 | 0.30 | 8.29 | 8.89 | 27.94 | 0.00 | 7.40 | 7.12 | 27.27 |
| TH10 | 10 | 0.90 | 8.17 | 12.4 | 30.77 | 0.00 | 7.36 | 9.17 | 21.75 | |||||
| TH15 | 15 | 2.50 | 8.01 | 17.0 | 36.42 | 0.28 | 7.87 | 15.4 | 36.64 | |||||
| B | TL5 | 510 | 5 | 7.8 | 6.1 | 0.86 | 1.40 | 7.90 | 3.92 | 12.92 | 0.00 | 7.79 | 5.05 | 15.9 |
| TL10 | 10 | 5.50 | 7.86 | 5.22 | 18.68 | 2.70 | 7.69 | 7.59 | 15.3 | |||||
| TL15 | 15 | 9.30 | 7.82 | 10.3 | 9.63 | 6.10 | 7.64 | 9.31 | 22.7 | |||||
| C | TH10-pH (2) | 3100 | 10 | 8.1 | 30.2 | 4.32 | 5.60 | 6.10 | 12.7 | 21.31 | 2.80 | 6.16 | 17.5 | 46.7 |
| TL10-pH | 500 | 10 | 7.8 | 6.1 | 0.86 | 7.10 | 6.19 | 8.44 | 16.53 | 6.20 | 6.27 | 11.2 | 21.3 | |
| Type of BWMS | Temp. | TRO | TRO on Day 5 | Predominant Species (2) | Abundance | DOM | DOM Type | Number of DBPs (D1/D5) | |
|---|---|---|---|---|---|---|---|---|---|
| C | This study (1) | 20 | 10 | 0.00 | Akashiwo sanguinea | 3010 | 4.31 | Natural | 13/15 |
| C2 (1) | N.I. | 10 | N.I. | Tetraselmis sp. (3) | N.I. | N.I. | Methylcellulose | N.I/13 | |
| C3 (1) | 16 | 9.5 | 1.12 | Tetraselmis sp. | 2393 | 3.34 | Glucose | 9/9 | |
| D | D1 | 18.6 | 11 | 4.23 | Tetraselmis sp. | 1195 | 2.81 | Glucose | 11/12 |
| D2 | 20 | 10 | 3.40 | Tetraselmis sp. | 1550 | 3.04 | Glucose | 15/12 | |
| S | S1 | 19.4 | 10 | 3.15 | N.I. (4) | N.I. | 2.42 | N.I. | 16/17 |
| S2 | 14.1 | 9.5 | 1.88 | Tetraselmis sp. | 2691 | 2.87 | Glucose | 13/11 | |
| S3 | 14.8 | 9 | 4.33 | Tetraselmis sp. | 1727 | 1.99 | Glucose | 15/15 | |
| TH5 | TH10 | TH15 | TL5 | TL10 | TL15 | TH10-pH | TL10-pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBPs | General | NS | General | NS | General | NS | General | NS | General | NS | General | NS | General | NS | General | NS |
| DCBM | 2.6 × 10−4 | 5.3 × 10−³ | 2.7 × 10−4 | 5.4 × 10−³ | 2.6 × 10−4 | 5.3 × 10−³ | ||||||||||
| DBCM | 6.9 × 10−³ | 3.0 × 10−³ | 2.4 × 10−2 | 1.0 × 10−2 | 3.4 × 10−2 | 1.5 × 10−2 | 1.3 × 10−2 | 5.5 × 10−³ | 1.6 × 10−2 | 6.9 × 10−³ | 1.7 × 10−2 | 7.5 × 10−³ | 3.2 × 10−2 | 1.4 × 10−2 | 1.3 × 10−2 | 5.5 × 10−³ |
| TBM | 1.2 × 10−2 | 2.0 × 10−1 | 3.6 × 10−2 | 6.1 × 10−1 | 4.7 × 10−2 | 8.0 × 10−1 | 2.0 × 10−2 | 3.4 × 10−1 | 2.7 × 10−2 | 4.6 × 10−1 | 2.6 × 10−2 | 4.4 × 10−1 | 3.4 × 10−2 | 5.9 × 10−1 | 1.8 × 10−2 | 3.0 × 10−1 |
| MCAA | 3.0 × 10−2 | 3.7 × 10−1 | 3.2 × 10−2 | 3.9 × 10−1 | 5.1 × 10−2 | 6.2 × 10−1 | 2.8 × 10−2 | 3.5 × 10−1 | ||||||||
| MBAA | 1.5 × 10−³ | 1.8 × 10−2 | 1.6 × 10−³ | 1.9 × 10−2 | 2.6 × 10−³ | 3.2 × 10−2 | 1.2 × 10−3 | 1.4 × 10−2 | 1.7 × 10−³ | 2.1 × 10−2 | 2.3 × 10−³ | 2.8 × 10−2 | 5.2 × 10−³ | 6.4 × 10−2 | 4.6 × 10−³ | 5.6 × 10−2 |
| TCAA | 9.6 × 10−6 | 1.2 × 10−4 | 2.5 × 10−5 | 3.1 × 10−4 | 2.7 × 10−5 | 3.3 × 10−4 | 2.2 × 10−5 | 2.7 × 10−4 | 2.1 × 10−5 | 2.6 × 10−4 | 1.7 × 10−5 | 2.1 × 10−4 | 4.4 × 10−5 | 5.4 × 10−4 | 2.3 × 10−5 | 2.8 × 10−4 |
| BCAA | 5.0 × 10−³ | 6.2 × 10d2 | 4.9 × 10−³ | 6.0 × 10−2 | 8.1 × 10−³ | 9.9 × 10−2 | 9.6 × 10−³ | 1.2 × 10−1 | 5.7 × 10−³ | 6.9 × 10−2 | 6.4 × 10−³ | 7.9 × 10−2 | 7.3 × 10−³ | 9.0 × 10−2 | 7.1 × 10−³ | 8.7 × 10−2 |
| DBCAA | 2.1 × 10−5 | 2.5 × 10−4 | 2.7 × 10−5 | 3.3 × 10−4 | 3.0 × 10−5 | 3.7 × 10d4 | 7.8 × 10−5 | 9.5 × 10−4 | 4.9 × 10−5 | 6.0 × 10d4 | 5.4 × 10−5 | 6.6 × 10−4 | 5.8 × 10−5 | 7.1 × 10−4 | 4.5 × 10−5 | 5.5 × 10−4 |
| DBAA | 9.8 × 10−5 | 1.2 × 10−³ | 1.5 × 10−4 | 1.9 × 10−³ | 2.5 × 10−4 | 3.1 × 10−³ | 2.3 × 10−4 | 2.8 × 10−³ | 1.4 × 10−4 | 1.7 × 10−³ | 1.2 × 10−4 | 1.5 × 10−³ | 2.6 × 10−4 | 3.2 × 10−³ | 1.8 × 10−4 | 2.2 × 10−³ |
| DCBAA | 3.0 × 10−4 | 2.2 × 10−³ | 5.3 × 10−4 | 3.9 × 10−³ | 1.3 × 10−³ | 9.4 × 10−³ | 1.8 × 10−³ | 1.3 × 10−2 | 7.0 × 10−4 | 5.1 × 10−³ | 7.4 × 10−4 | 5.5 × 10−³ | 4.6 × 10−³ | 3.4 × 10−2 | 1.4 × 10−³ | 1.0 × 10−2 |
| TBAA | 9.1 × 10−6 | 7.1 × 10−5 | 1.4 × 10−5 | 1.1 × 10−4 | 3.3 × 10−5 | 2.6 × 10−4 | 4.4 × 10−5 | 3.4 × 10−4 | 2.4 × 10−5 | 1.9 × 10−4 | 2.3 × 10−5 | 1.8 × 10−4 | 1.1 × 10−4 | 8.4 × 10−4 | 3.7 × 10−5 | 2.9 × 10−4 |
| MCAN | 1.7 × 10−2 | 3.4 × 10−2 | 2.8 × 10−2 | 5.5 × 10−2 | 3.7 × 10−2 | 7.3 × 10−2 | 4.0 × 10−2 | 7.8 × 10−2 | 8.1 × 10−³ | 1.6 × 10−2 | 4.3 × 10−2 | 8.4 × 10−2 | 4.0 × 10−2 | 7.9 × 10−2 | ||
| DCAN | 7.3 × 10−4 | 8.9 × 10−4 | ||||||||||||||
| MBAN | 1.6 × 10−4 | 2.0 × 10−4 | 1.1 × 10−³ | 1.4 × 10−³ | 4.9 × 10−³ | 6.1 × 10−³ | 6.9 × 10−³ | 8.5 × 10−³ | 1.8 × 10−2 | 2.2 × 10−2 | 5.7 × 10−³ | 7.1 × 10−³ | 4.0 × 10−2 | 4.9 × 10−2 | 1.7 × 10−2 | 2.1 × 10−2 |
| BCAN | 5.1 × 10−³ | 1.4 × 10−2 | 8.2 × 10−³ | 2.2 × 10−2 | 8.3 × 10−³ | 2.3 × 10−2 | 5.6 × 10−³ | 1.5 × 10−2 | 4.6 × 10−2 | 1.3 × 10−1 | 7.8 × 10−³ | 2.1 × 10−2 | ||||
| DCM | 1.1 × 10−5 | 1.6 × 10−³ | 1.4 × 10−5 | 2.2 × 10−³ | 1.2 × 10−5 | 1.8 × 10−³ | 8.2 × 10−6 | 1.3 × 10−³ | 7.7 × 10−6 | 1.2 × 10−³ | ||||||
| SUM | 0.06 | 0.67 | 0.09 | 0.75 | 0.13 | 1.02 | 0.13 | 0.99 | 0.12 | 0.69 | 0.07 | 0.60 | 0.26 | 1.69 | 0.14 | 0.93 |
| TH5 | TH10 | TH15 | ||||
|---|---|---|---|---|---|---|
| DBPs | General | NS | General | NS | General | NS |
| DCBM | 3.6 × 10−4 | 5.5 × 10−3 | 2.9 × 10−4 | 5.9 × 10−3 | 3.6 × 10−4 | 7.1 × 10−3 |
| DBCM | 7.4 × 10−3 | 3.2 × 10−3 | 2.6 × 10−2 | 1.1 × 10−2 | 5.4 × 10−2 | 2.3 × 10−2 |
| TBM | 1.4 × 10−2 | 2.3 × 10−1 | 3.6 × 10−2 | 6.2 × 10−1 | 5.6 × 10−2 | 9.6 × 10−1 |
| MCAA | 3.0 × 10−2 | 3.7 × 10−1 | 7.9 × 10−2 | 9.7 × 10−1 | 3.6 × 10−2 | 4.3 × 10−1 |
| MBAA | 1.5 × 10−3 | 1.8 × 10−2 | 8.8 × 10−3 | 1.1 × 10−1 | 6.6 × 10−3 | 8.1 × 10−2 |
| TCAA | 9.6 × 10−6 | 1.2 × 10−4 | 2.5 × 10−5 | 3.1 × 10−4 | 2.7 × 10−5 | 3.3 × 10−4 |
| BCAA | 5.6 × 10−3 | 6.8 × 10−2 | 1.1 × 10−2 | 1.4 × 10−1 | 1.6 × 10−2 | 1.9 × 10−1 |
| DBCAA | 5.0 × 10−5 | 6.2 × 10−4 | 2.3 × 10−3 | 2.9 × 10−2 | 6.9 × 10−5 | 8.5 × 10−4 |
| DBAA | 9.8 × 10−5 | 1.2 × 10−3 | 2.6 × 10−4 | 3.2 × 10−3 | 3.3 × 10−4 | 4.1 × 10−3 |
| DCBAA | 4.3 × 10−5 | 3.1 × 10−3 | 2.2 × 10−4 | 1.6 × 10−2 | 2.2 × 10−4 | 1.6 × 10−2 |
| TBAA | 1.0 × 10−5 | 8.0 × 10−5 | 5.9 × 10−5 | 4.6 × 10−4 | 6.2 × 10−5 | 4.9 × 10−4 |
| MCAN | 0.0 | 0.0 | 6.0 × 10−2 | 1.2 × 10−1 | 9.8 × 10−2 | 1.9 × 10−1 |
| DCAN | 1.9 × 10−5 | 8.6 × 10−4 | 9.5 × 10−6 | 4.4 × 10−4 | 1.6 × 10−5 | 7.2 × 10−4 |
| MBAN | 1.6 × 10−4 | 2.0 × 10−4 | 6.7 × 10−3 | 8.3 × 10−3 | 4.9 × 10−3 | 6.1 × 10−3 |
| BCAN | 5.6 × 10−3 | 1.5 × 10−2 | 1.0 × 10−2 | 2.8 × 10−2 | 0.0 | 0.0 |
| DCM | 1.1 × 10−5 | 1.6 × 10−3 | 1.4 × 10−5 | 2.2 × 10−3 | 1.2 × 10−5 | 1.8 × 10−3 |
| SUM | 0.06 | 0.72 | 0.24 | 2.06 | 0.27 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.-G.; Hyun, B.; Jang, M.-C.; Choi, K.-H.; Shin, K.; Seo, J.-Y.; Jang, P.-G. Simulated Testing of the Characteristics and Environmental Impacts of Disinfection By-Products Generated by Ballast Water Management Systems in Ports during Phytoplankton Blooms. J. Mar. Sci. Eng. 2024, 12, 238. https://doi.org/10.3390/jmse12020238
Cha H-G, Hyun B, Jang M-C, Choi K-H, Shin K, Seo J-Y, Jang P-G. Simulated Testing of the Characteristics and Environmental Impacts of Disinfection By-Products Generated by Ballast Water Management Systems in Ports during Phytoplankton Blooms. Journal of Marine Science and Engineering. 2024; 12(2):238. https://doi.org/10.3390/jmse12020238
Chicago/Turabian StyleCha, Hyung-Gon, Bonggil Hyun, Min-Chul Jang, Keun-Hyung Choi, Kyoungsoon Shin, Jin-Young Seo, and Pung-Guk Jang. 2024. "Simulated Testing of the Characteristics and Environmental Impacts of Disinfection By-Products Generated by Ballast Water Management Systems in Ports during Phytoplankton Blooms" Journal of Marine Science and Engineering 12, no. 2: 238. https://doi.org/10.3390/jmse12020238
APA StyleCha, H.-G., Hyun, B., Jang, M.-C., Choi, K.-H., Shin, K., Seo, J.-Y., & Jang, P.-G. (2024). Simulated Testing of the Characteristics and Environmental Impacts of Disinfection By-Products Generated by Ballast Water Management Systems in Ports during Phytoplankton Blooms. Journal of Marine Science and Engineering, 12(2), 238. https://doi.org/10.3390/jmse12020238





