Length–Weight and Body Condition Relationships of the Exploited Sea Cucumber Pearsonothuria graeffei
Abstract
:1. Introduction
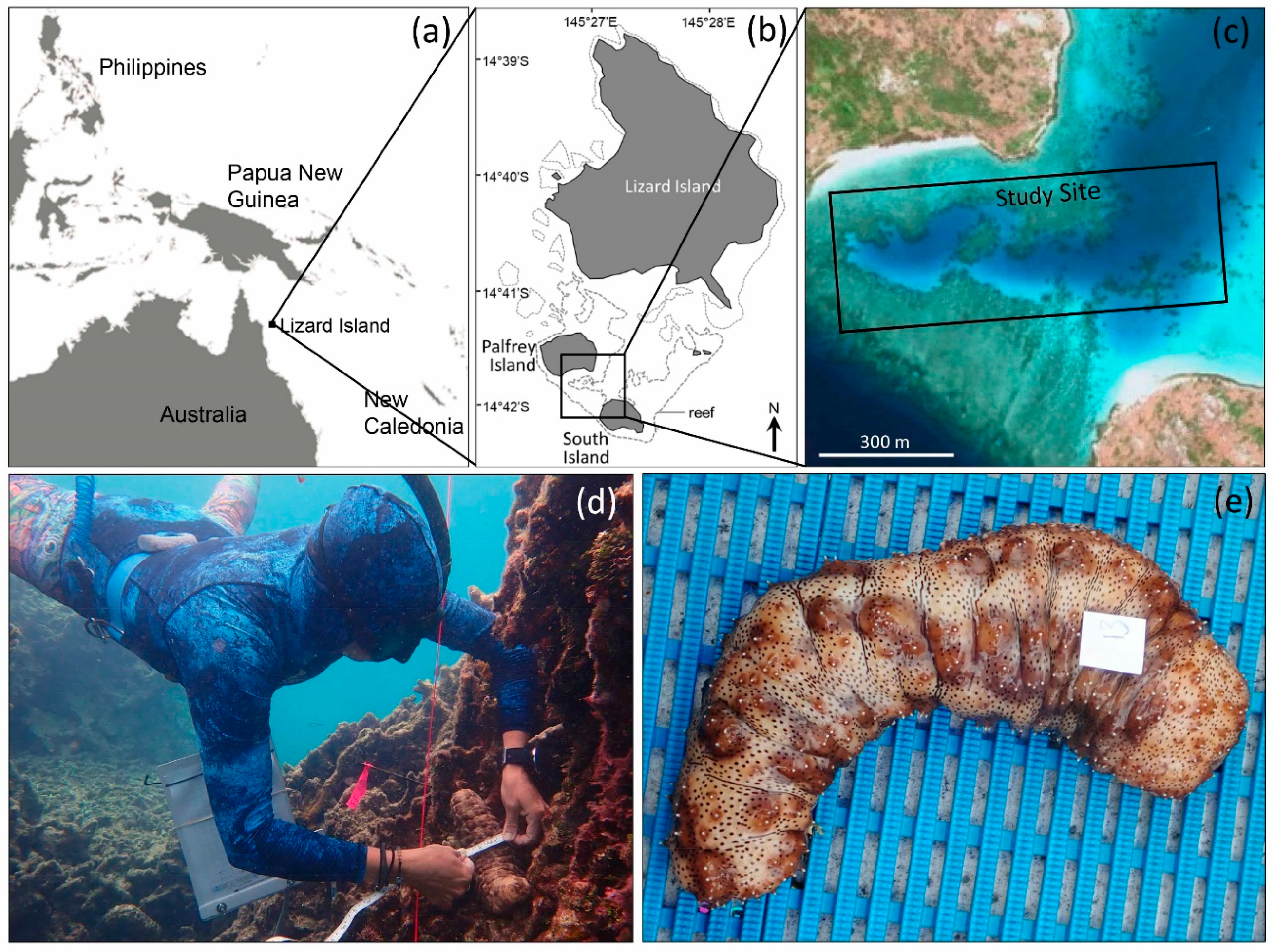
2. Materials and Methods
2.1. Field Methods
2.2. Analytical Procedures
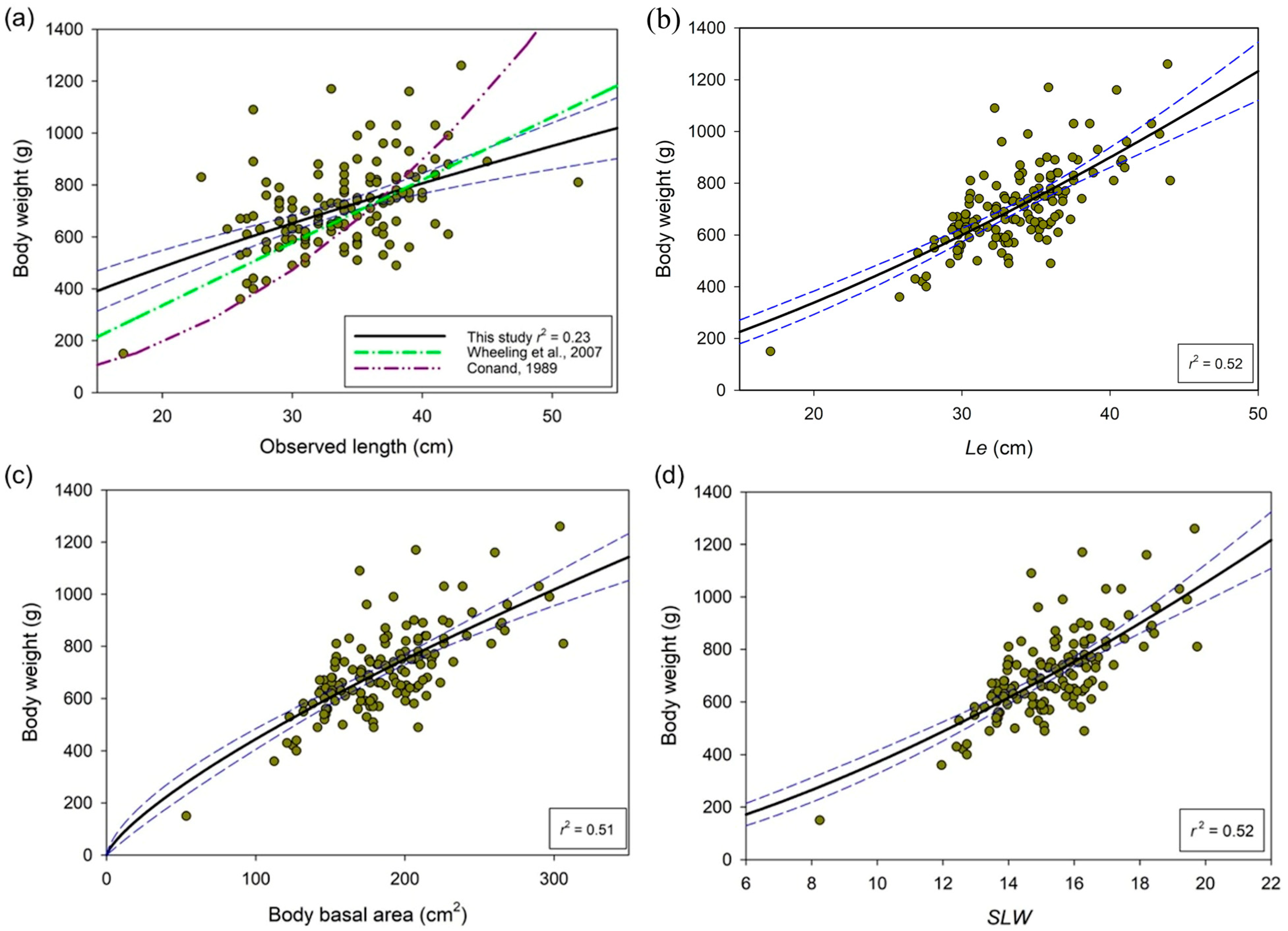
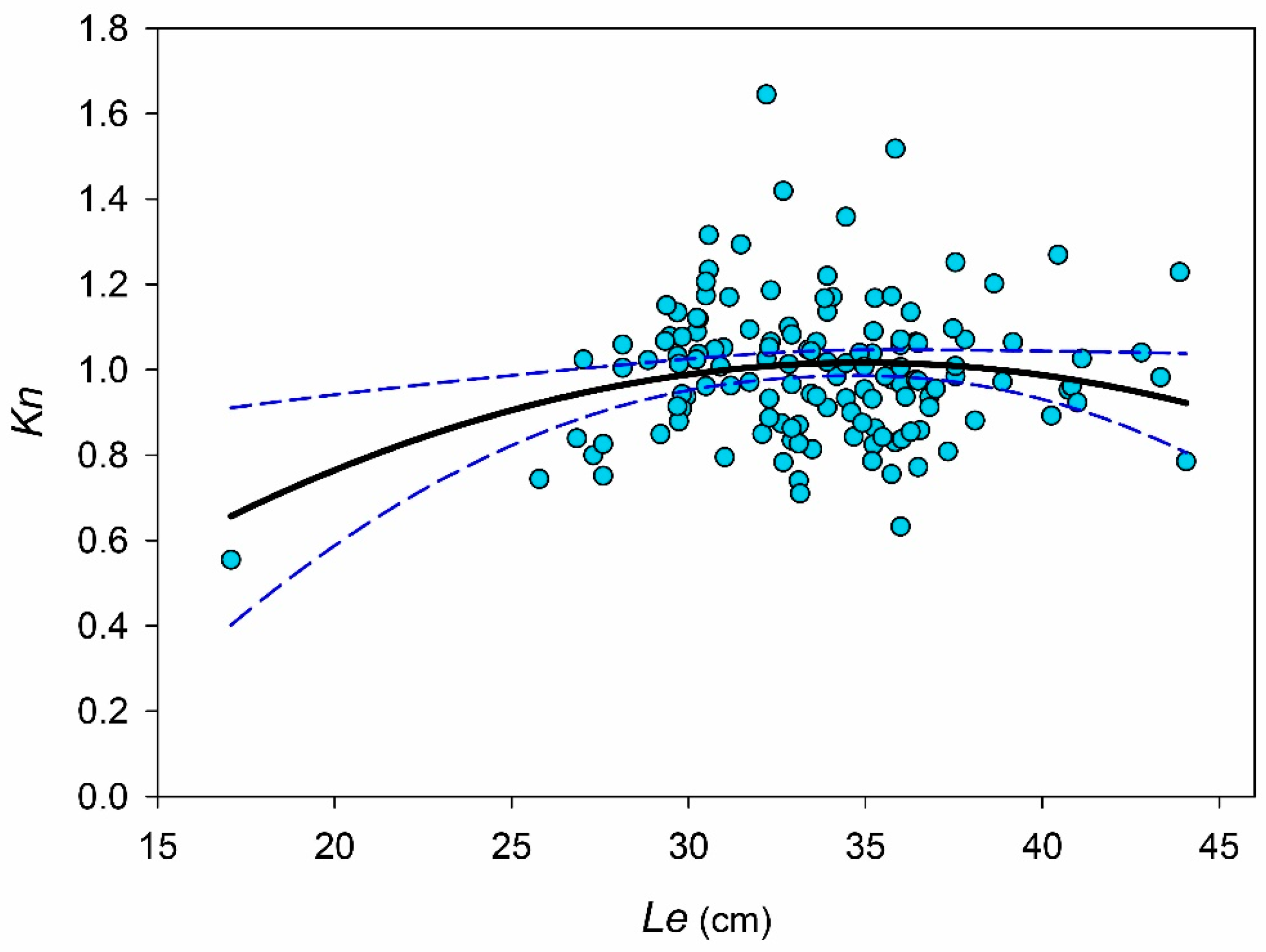
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zale, A.V.; Parrish, D.L.; Sutton, T.M. Fisheries Techniques; American Fisheries Society Bethesda: Bethesda, MD, USA, 2012. [Google Scholar]
- Purcell, S.W. Managing Sea Cucumber Fisheries with an Ecosystem Approach; Lovatelli, A., Vasconcellos, M., Yimin, Y., Eds.; FAO Fisheries and Aquaculture Technical Paper No. 520; FAO: Rome, Italy, 2010. [Google Scholar]
- Gilbert, A.; Georget, S.; Guillemot, N.; Ton, C.; Léopold, M.; Purcell, S.; Van Wynsberge, S.; Andréfouët, S. État des Lieux des Stocks D’holothuries Commerciales en Nouvelle-Calédonie (2021–2022); Rapport ADECAL Technopole—Projet PROTEGE; ADECAL Technopole: Noumea, New Caledonia, 2022; p. 65. [Google Scholar]
- Léopold, M.; Cornuet, N.; Andréfouët, S.; Moenteapo, Z.; Duvauchelle, C.; Raubani, J.; Ham, J.; Dumas, P. Comanaging small-scale sea cucumber fisheries in New Caledonia and Vanuatu using stock biomass estimates to set spatial catch quotas. Environ. Conserv. 2013, 40, 367–379. [Google Scholar] [CrossRef]
- Prescott, J.; Zhou, S.; Prasetyo, A.P. Soft bodies make estimation hard: Correlations among body dimensions and weights of multiple species of sea cucumbers. Mar. Freshwater Res. 2015, 66, 857–865. [Google Scholar] [CrossRef]
- Feary, D.A.; Hamilton, R.; Matawai, M.; Molai, C.; Karo, M.; Almany, G. Assessing Sandfish Population Stocks within the South Coast of Manus, and a Summary Report of Sandfish Connectivity Field Research; The Nature Conservancy Asia Pacific Division: Queensland, Australia, 2014. [Google Scholar]
- González-Wangüemert, M.; Valente, S.; Henriques, F.; Domínguez-Godino, J.A.; Serrão, E.A. Setting preliminary biometric baselines for new target sea cucumbers species of the NE Atlantic and Mediterranean fisheries. Fish. Res. 2016, 179, 57–66. [Google Scholar] [CrossRef]
- Helidoniotis, F. Stock Assessment of Black Teatfish (Holothuria whitmaei) in Queensland, Australia; Department of Agriculture and Fisheries Queensland: Brisbane, Australia, 2021.
- Cone, R.S. The need to reconsider the use of condition indices in fishery science. Trans. Am. Fish. Soc. 1989, 118, 510–514. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- McShane, P.E.; Schiel, D.R.; Mercer, S.F.; Murray, T. Morphometric variation in Haliotis iris (Mollusca: Gastropoda): Analysis of 61 populations. N. Z. J. Mar. Fresh. 1994, 28, 357–364. [Google Scholar] [CrossRef]
- Defeo, O.; Cardoso, R.S. Macroecology of population dynamics and life history traits of the mole crab Emerita brasiliensis in Atlantic sandy beaches of South America. Mar. Ecol. Prog. Ser. 2002, 239, 169–179. [Google Scholar] [CrossRef]
- Ferreri, G.A.B. Length–weight relationships and condition factors of the Humboldt Squid (Dosidicus gigas) from the Gulf of California and the Pacific Ocean. J. Shellfish Res. 2014, 33, 769–780. [Google Scholar] [CrossRef]
- Powell, E.N.; Mann, R.; Ashton-Alcox, K.A.; Kim, Y.; Bushek, D. The allometry of oysters: Spatial and temporal variation in the length–biomass relationships for Crassostrea virginica. J. Mar. Biol. Assoc. UK 2016, 96, 1127–1144. [Google Scholar] [CrossRef]
- Purcell, S.W.; Ceccarelli, D.M. Population colonization of introduced trochus (Gastropoda) on coral reefs in Samoa. Restor. Ecol. 2021, 29, e13312. [Google Scholar] [CrossRef]
- Asha, P.; Ranjith, L.; Vivekanandan, E.; Johnson, B.; Subin, C.; Sheik Mohamed, M. Spatial variations in the population characteristics of sea cucumber resources in Gulf of Mannar and Palk Bay, south-east coast of India. Indian J. Fish. 2019, 66, 1–11. [Google Scholar] [CrossRef]
- Azevedo e Silva, F.; Brito, A.; Simões, T.; Pombo, A.; Marques, T.; Rocha, C.; Sousa, J.; Venâncio, E.; Félix, P. Allometric relationships to assess ontogenetic adaptative changes in three NE Atlantic commercial sea cucumbers (Echinodermata, Holothuroidea). Aquat. Ecol. 2021, 55, 711–720. [Google Scholar] [CrossRef]
- Conand, C. Les Holothuries Aspidochirotes du Lagon de Nouvelle-Calédonie: Biologie, Ecologie et Exploitation; ORSTOM: Paris, France, 1989; p. 393. [Google Scholar]
- Purcell, S.W.; Gossuin, H.; Agudo, N.S. Status and Management of the Sea Cucumber Fishery of La Grande Terre, New Caledonia; WorldFish Centre Studies and Reviews No. 1901; WorldFish Centre: Penang, Malaysia, 2009. [Google Scholar]
- Anibeze, C. Length-weight relationship and relative condition of Heterobranchus longifilis (Valenciennes) from Idodo River, Nigeria. Naga ICLARM Q. 2000, 23, 34–35. [Google Scholar]
- Herrero-Pérezrul, M.D.; Reyes-Bonilla, H. Weight-Length relationship and relative condition of the holothurian Isostichopus fuscus at Espíritu Santo Island, Gulf of California, México. Rev. Biol. Trop. 2008, 56, 273–280. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Sewell, M.A. Aspects of the ecology of Stichopus mollis (Echinodermata: Holothuroidea) in north-eastern New Zealand. N. Z. J. Mar. Fresh. 1990, 24, 97–103. [Google Scholar] [CrossRef]
- Wheeling, R.J.; Verde, E.A.; Nestler, J.R. Diel cycles of activity, metabolism, and ammonium concentration in tropical holothurians. Mar. Biol. 2007, 152, 297–305. [Google Scholar] [CrossRef]
- Skewes, T.; Smith, L.; Dennis, D.; Rawlinson, N.; Donovan, A.; Ellis, N. Conversion Ratios for Commercial Beche-de-Mer Species in Torres Strait; Australian Fisheries Management Authority, Torres Strait Research Program: Canberra, Australia, 2004.
- Laboy-Nieves, E.N.; Conde, J.E. A new approach for measuring Holothuria mexicana and Isostichopus badionotus for stock assessments. SPC Beche-de-mer Inf. Bull. 2006, 24, 39–44. [Google Scholar]
- Poot-Salazar, A.; Hernández-Flores, A.; Ardisson, P.-L. Use of the SLW index to calculate growth function in the sea cucumber Isostichopus badionotus. Sci. Rep. 2014, 4, 5151. [Google Scholar] [CrossRef]
- Gray, B.C.; Byrne, M.; Clements, M.; Foo, S.A.; Purcell, S.W. Length-weight relationship for the dragonfish, Stichopus cf. monotuberculatus (Holothuroidea). Fish. Res. 2023, 268, 106851. [Google Scholar] [CrossRef]
- Purcell, S.W.; Lovatelli, A.; González-Wangüemert, M.; Solís-Marín, F.A.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World, 2nd ed.; FAO Species Catalogue for Fishery Purposes No. 6, Rev. 1; FAO: Rome, Italy, 2023. [Google Scholar]
- Gao, X.; Endo, H.; Taniguchi, K.; Agatsuma, Y. Combined effects of seawater temperature and nutrient condition on growth and survival of juvenile sporophytes of the kelp Undaria pinnatifida (Laminariales; Phaeophyta) cultivated in northern Honshu, Japan. J. Appl. Phycol. 2013, 25, 269–275. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018, 9, 5371–5380. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, M.; Guo, R.; Zhao, T.; Gao, X.; Li, K.; Guo, X.; Li, J.; Li, D. Fucoidans from Pearsonothuria graeffei prevent obesity by regulating intestinal lipid metabolism and inflammation related signalling pathways. Food Funct. 2022, 13, 12234–12245. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cong, P.; Xu, J.; Li, G.; Liu, X.; Li, Z.; Xue, C.; Xue, Y.; Wang, Y. Absorption and pharmacokinetic study of two sulphated triterpenoid saponins in rat after oral and intravenous administration of saponin extracts of Pearsonothuria graeffei by HPLC-MS. J. Funct. Foods 2016, 25, 62–69. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Wang, J.F.; Li, H.; Long, T.T.; Li, Z.; Wang, Y.M.; Dong, P.; Xue, C.H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012, 92, 965–974. [Google Scholar] [CrossRef]
- Hammond, A.R.; Purcell, S.W. Limited long-term movement and slow growth of the sea cucumber Pearsonothuria graeffei. Mar. Ecol. Prog. Ser. 2023, 704, 1–14. [Google Scholar] [CrossRef]
- Conand, C.; Gamboa, R.; Purcell, S. Pearsonothuria graeffei. The IUCN Red List of Threatened Species. 2013. Available online: https://www.researchgate.net/publication/295547237_Holothuria_scabra_The_IUCN_Red_List_of_Threatened_Species_2013_eT180257A1606648 (accessed on 15 March 2013).
- Mangubhai, S.; Lalavanua, W.; Purcell, S. Fiji’s Sea Cucumber Fishery: Advances in Science; Report No. 01/17; Wildlife Conservation Society: Suva, Fiji, 2017. [Google Scholar]
- Toral-Granda, V.; Lovatelli, A.; Vasconcellos, M. Sea Cucumbers. A Global Review on Fishery and Trade; FAO Fisheries Technical Paper No. 516; FAO: Rome, Italy, 2008. [Google Scholar]
- Pakoa, K.; Saladrau, W.; Lalavanua, W.; Valotu, D.; Tuinasavusavu, I.; Sharp, M.; Bertram, I. Status of Sea Cucumber Resources and Fisheries Management in Fiji; Secretariat of the Pacific Community: Noumea, New Caledonia, 2013. [Google Scholar]
- Carleton, C.; Hambrey, J.; Govan, H.; Medley, P.; Kinch, J. Effective management of sea cucumber fisheries and the beche-de-mer trade in Melanesia. SPC Fish. Newsl. 2013, 140, 24–42. [Google Scholar]
- Czechura, G. The Great Barrier Reef: A Queensland Museum Discovery Guide; Queensland Museum: Brisbane, Australia, 2013.
- Waterson, P.; Waghorn, A.; Swartz, J.; Brown, R. What’s in a name? Beyond the Mary Watson stories to a historical archaeology of Lizard Island. Int. J. Hist. Archaeol. 2013, 17, 590–612. [Google Scholar] [CrossRef]
- Purcell, S.W.; Piddocke, T.P.; Dalton, S.J.; Wang, Y.-G. Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas. Mar. Ecol. Prog. Ser. 2016, 551, 201–214. [Google Scholar] [CrossRef]
- Siddique, S.; Ayub, Z. To estimate growth function by the use of SLW index in the sea cucumber Holothuria arenicola (Holothuroidea: Echinodermata) of Pakistan (Northern Arabian Sea). Thalassas 2019, 35, 123–132. [Google Scholar] [CrossRef]
- Froese, R.; Tsikliras, A.C.; Stergiou, K.I. Editorial note on weight–length relations of fishes. Acta Ichthyol. Piscat. 2011, 41, 261–263. [Google Scholar] [CrossRef]
- Mustagfirin, M.; Wijayanti, D.P.; Subagiyo, S. Reproductive activity and morphometric assessment of three commercial species of sea cucumber (Echinodermata) from Karimunjawa National Park, Indonesia. Biodiversitas 2021, 22, 29. [Google Scholar] [CrossRef]
- Kinch, J.; Purcell, S.; Uthicke, S.; Friedman, K. Population status, fisheries and trade of sea cucumbers in the Western Central Pacific. In Sea Cucumbers: A Global Review of Fisheries and Trade; FAO Fisheries and Aquaculture Technical Paper No. 516; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; FAO: Rome, Italy, 2008; pp. 7–55. [Google Scholar]
- Yang, J.-F.; Gao, R.-C.; Wu, H.-T.; Li, P.-F.; Hu, X.-S.; Zhou, D.-Y.; Zhu, B.-W.; Su, Y.-C. Analysis of apoptosis in ultraviolet-induced sea cucumber (Stichopus japonicus) melting using terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling assay and cleaved caspase-3 immunohistochemistry. J. Agr. Food Chem. 2015, 63, 9601–9608. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.; Alicia, P.-S.; Ali, Q.M.; Levent, B. Seasonal variation in the length-weight relationships and condition factor of four commercially important sea cucumbers species from Karachi Coast-Northern Arabian Sea. NESciences 2018, 3, 265–281. [Google Scholar] [CrossRef]
- Siddique, S.; Ayub, Z.; Siddiqui, G. Length-weight relationship and condition factor in Holothuria arenicola (Holothuroidea: Echinodermata) found on two rocky coasts of Karachi, Pakistan. Pak. J. Mar. Sci. 2014, 23, 51–63. [Google Scholar]
- Uthicke, S. Spawning observations from the Lizard Island Area. SPC Beche-de-Mer Inf. Bull. 1994, 6, 12–14. [Google Scholar]
- Vail, L.; Hoggett, A. Pearsonothuria graeffei. Available online: http://lifg.australianmuseum.net.au/Group.html?groupId=JRWUPmov (accessed on 20 September 2022).

| Relationship with Body Weight | ||||||
|---|---|---|---|---|---|---|
| Body Measurement | Mean (±SD) | CV | a | b | p | r2 |
| Basal area (cm2) | 186.8 (40.0) | 21.4 | 13.9 | 0.75 | <0.001 | 0.51 |
| SLW | 15.3 (1.6) | 10.7 | 11.6 | 1.51 | <0.001 | 0.52 |
| Observed length (cm) | 33.7 (4.9) | 14.7 | 53.3 | 0.74 | <0.001 | 0.23 |
| Le from SLW (cm) | 33.7 (3.9) | 11.6 | 4.9 | 1.41 | <0.001 | 0.52 |
| Body weight (g) | 709 (162) | 22.9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, A.R.; Purcell, S.W. Length–Weight and Body Condition Relationships of the Exploited Sea Cucumber Pearsonothuria graeffei. J. Mar. Sci. Eng. 2024, 12, 371. https://doi.org/10.3390/jmse12030371
Hammond AR, Purcell SW. Length–Weight and Body Condition Relationships of the Exploited Sea Cucumber Pearsonothuria graeffei. Journal of Marine Science and Engineering. 2024; 12(3):371. https://doi.org/10.3390/jmse12030371
Chicago/Turabian StyleHammond, Alison R., and Steven W. Purcell. 2024. "Length–Weight and Body Condition Relationships of the Exploited Sea Cucumber Pearsonothuria graeffei" Journal of Marine Science and Engineering 12, no. 3: 371. https://doi.org/10.3390/jmse12030371





