Effect of Biologger Attachment on the Stress and Health State of the Spotted Sea Bass Lateolabrax maculatus
Abstract
:1. Introduction
2. Materials and Methods
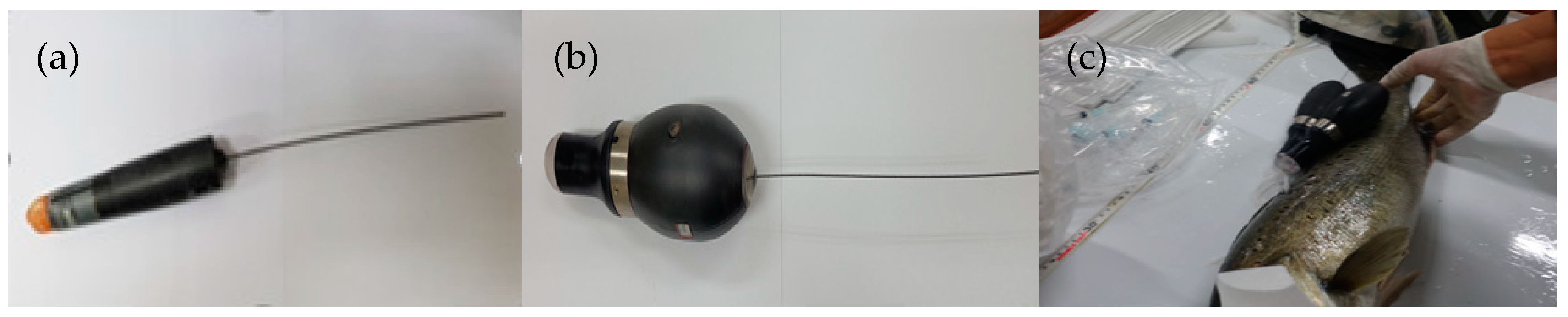
2.1. Experimental Fish and Attachment of the Biologgers
2.2. Blood Biochemistry
2.3. Total RNA Extraction from Liver and Muscle
2.4. cDNA Synthesis Using Reverse Transcriptase
2.5. Assessment of the Expression of Biomarker Genes Using the Real-Time Polymerase Chain Reaction
2.6. Statistical Analyses
3. Results and Discussion
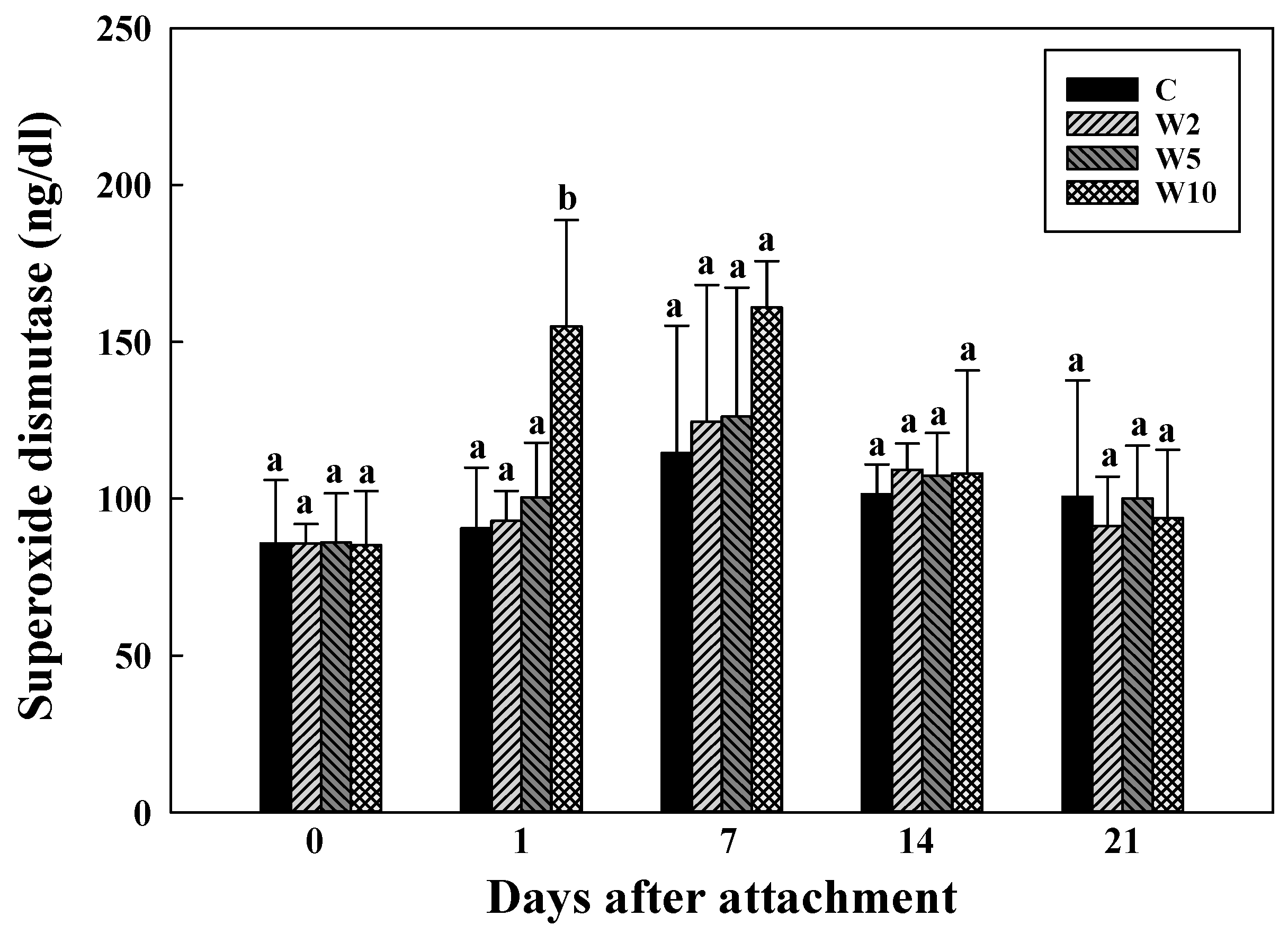
3.1. Blood Biochemistry Analysis for Assessing the Health of Spotted Sea Bass in Response to Stress Induced by the Attached Biologgers at Different Biologger/Fish Body Weight Ratios
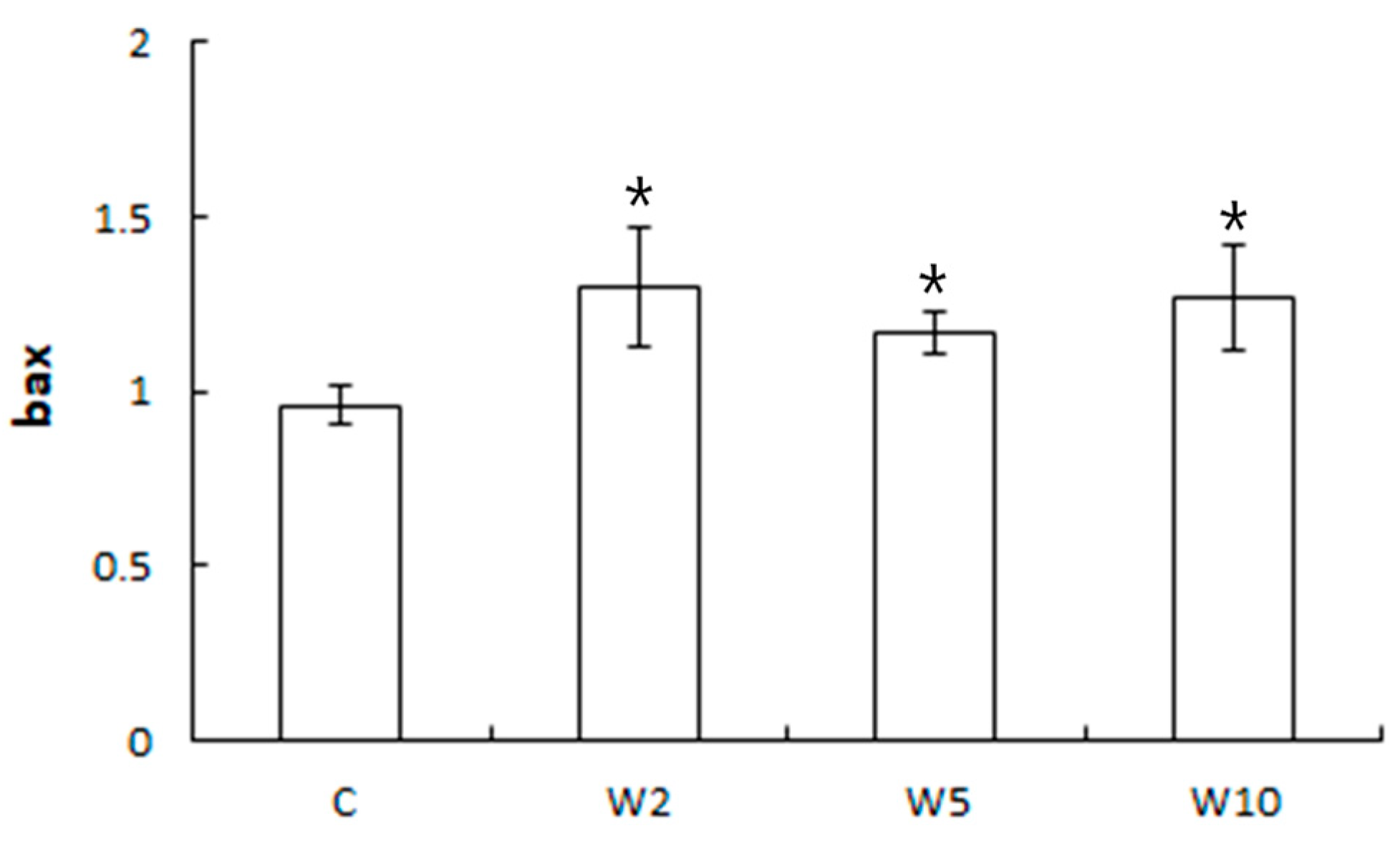
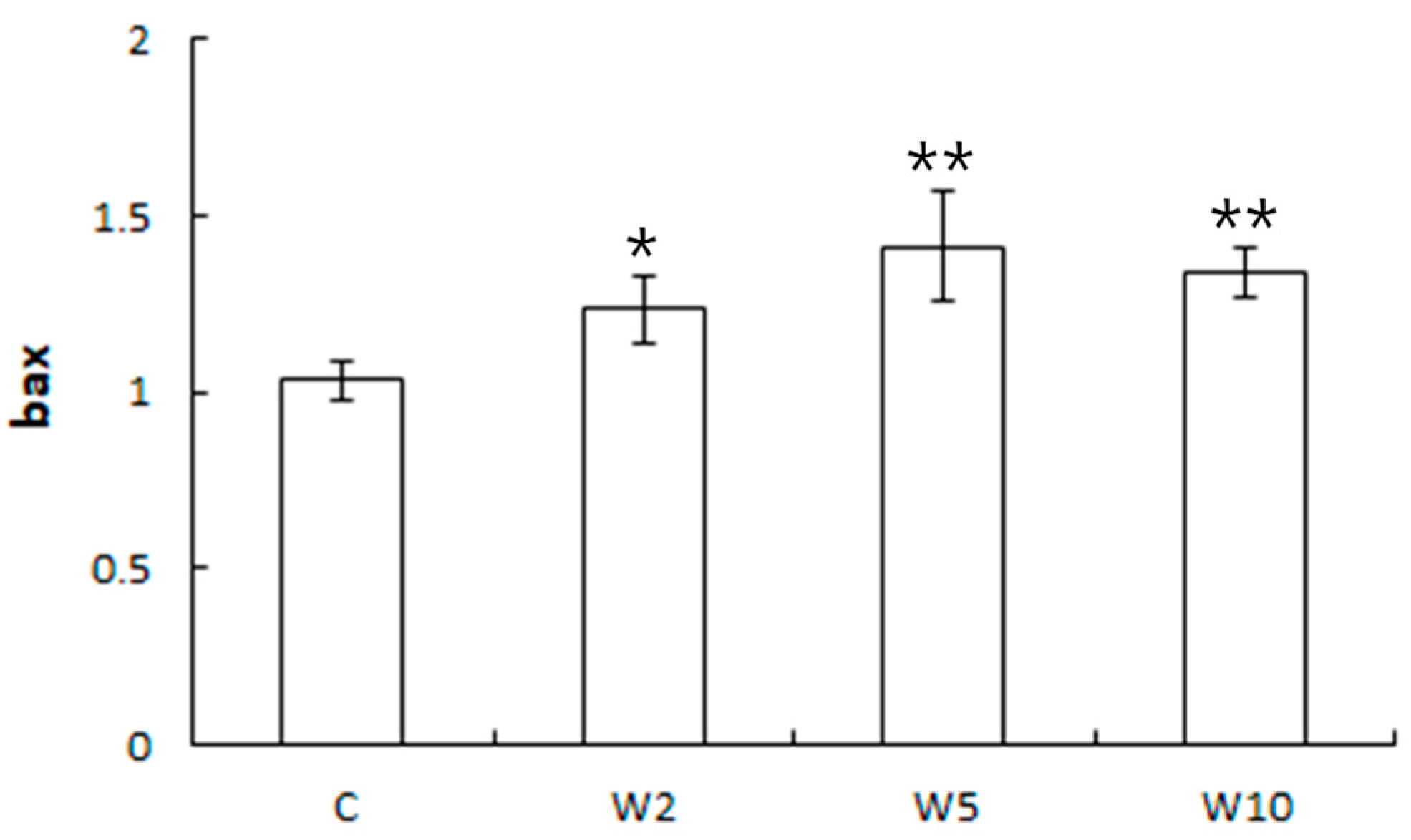
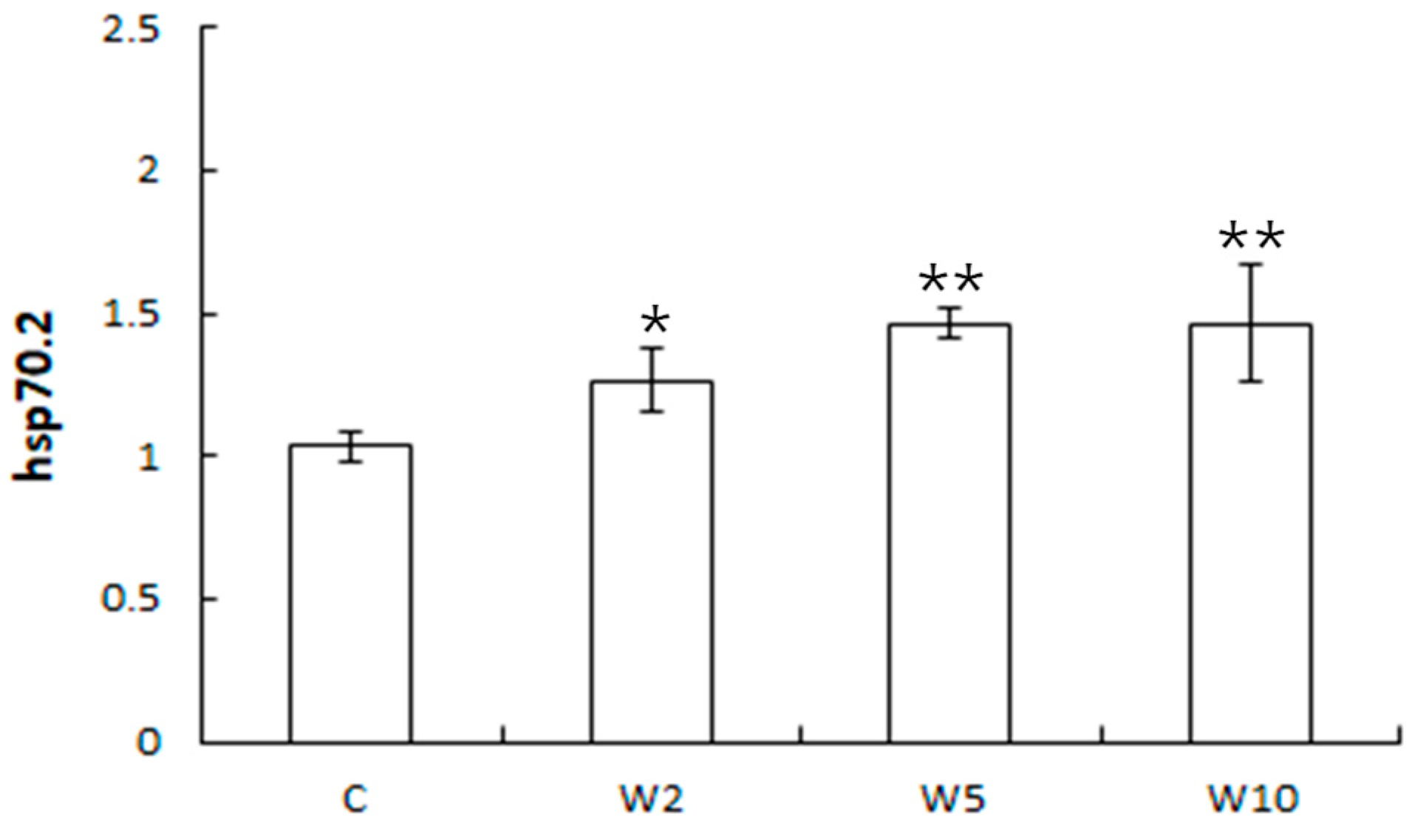
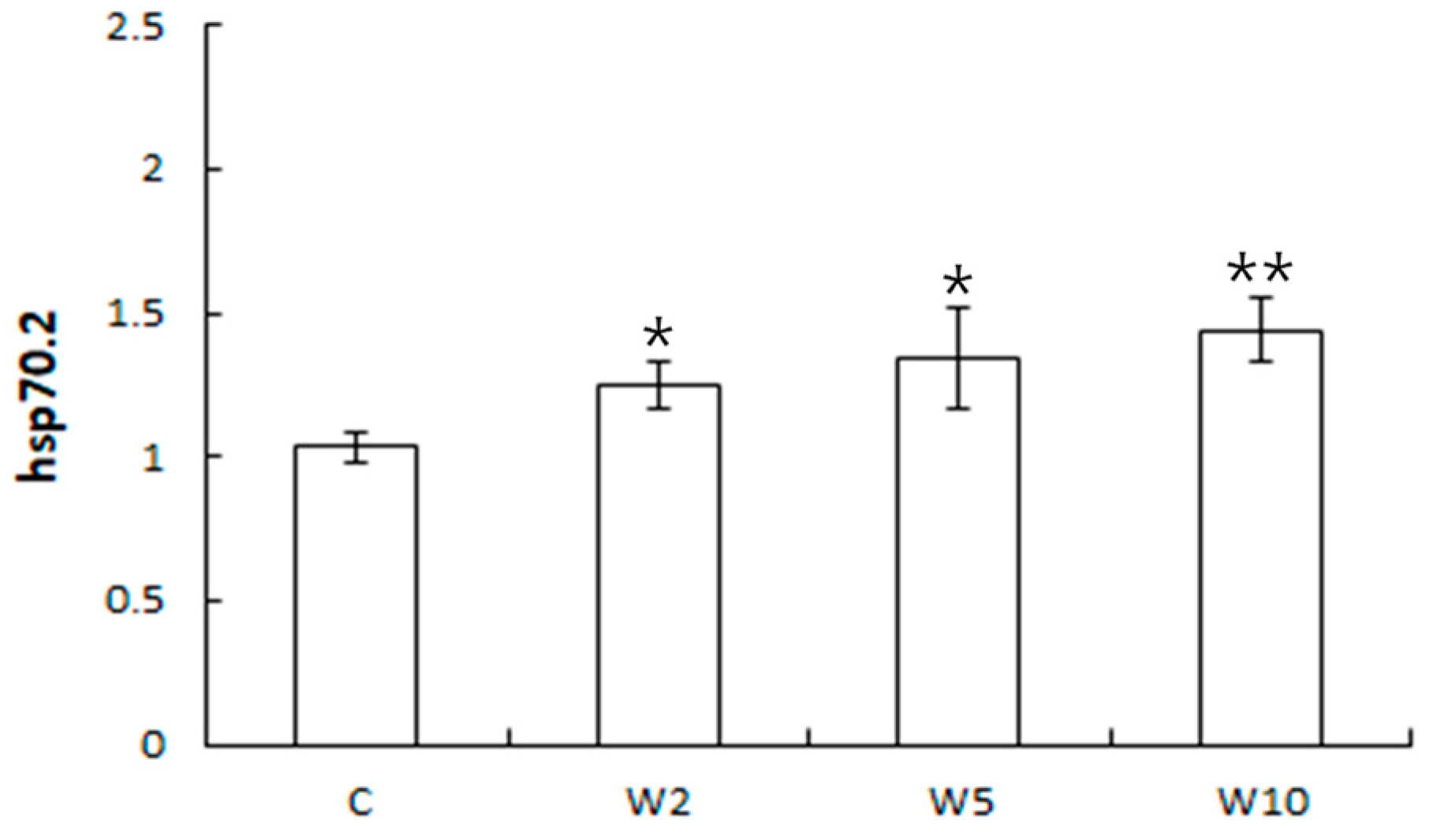
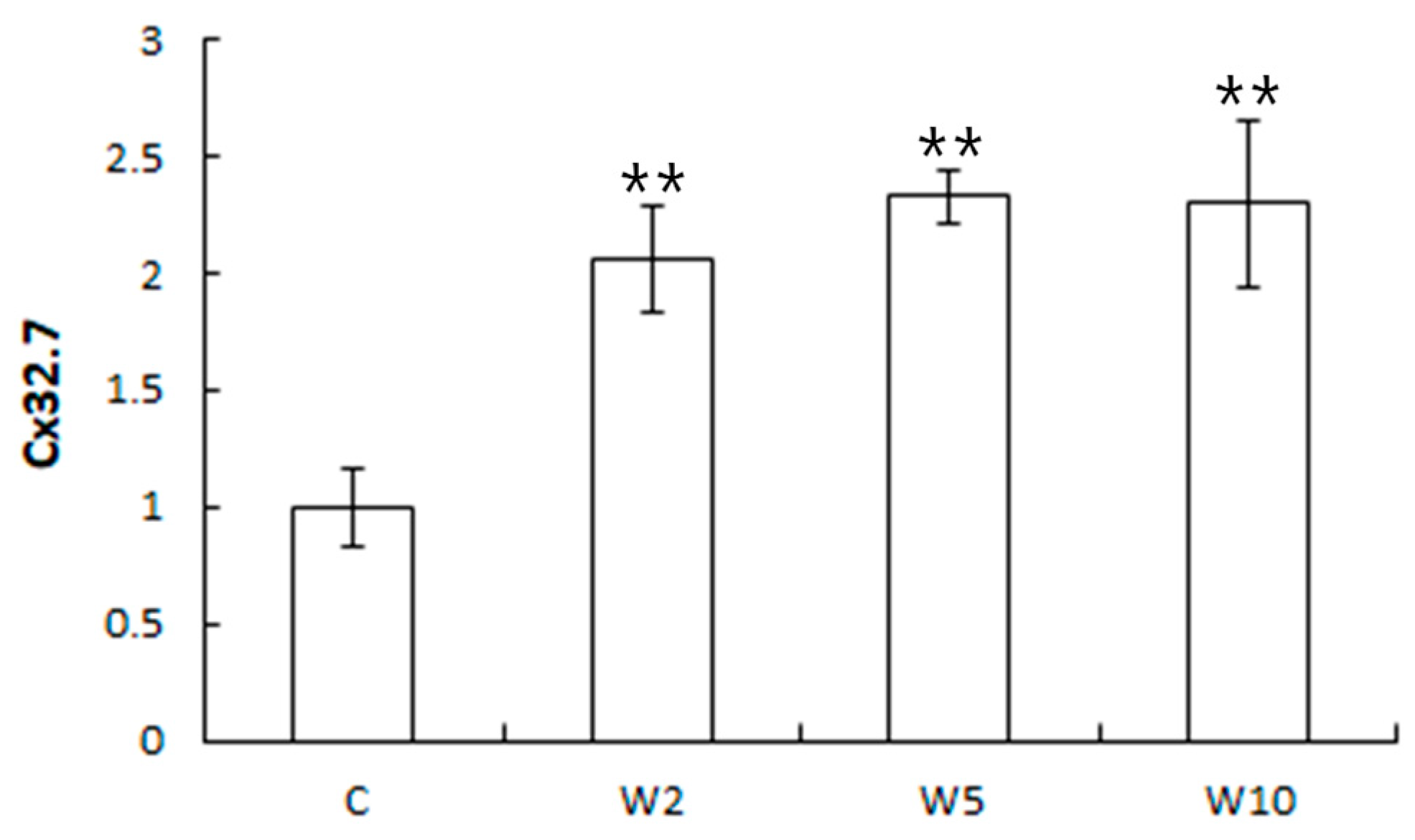
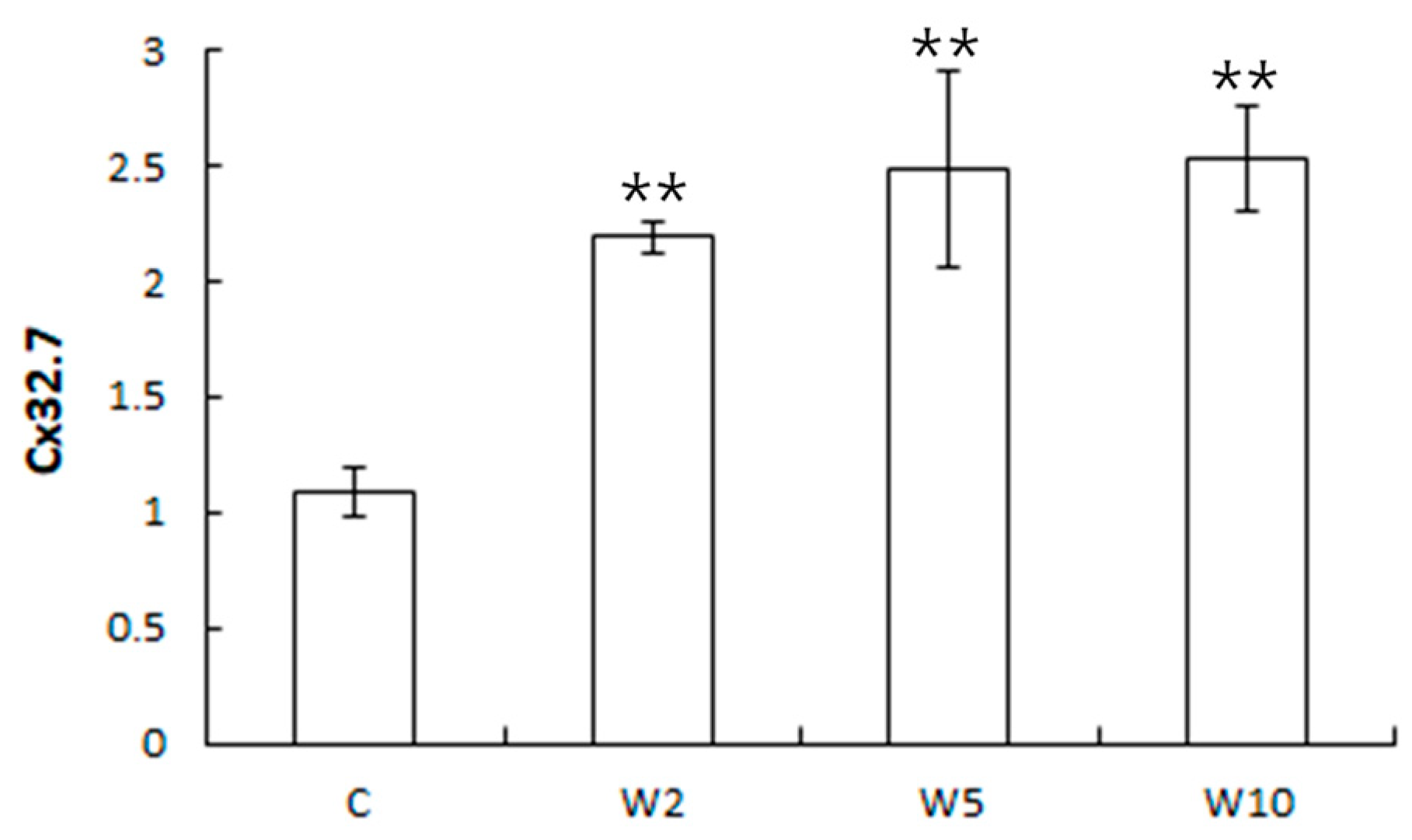
3.2. Analysis of the Expression of Health Biomarker Genes Associated with Stress in Spotted Sea Bass Equippe with Biologgers at Different Biologger/Fish Body Weight Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eddy, C.; Brill, R.; Bernal, D. Rates of at-vessel mortality and post-release survival of pelagic sharks captured with tuna purse seines around drifting fish aggregating devices (FADs) in the Equatorial eastern Pacific Ocean. Fish. Res. 2016, 174, 109–117. [Google Scholar] [CrossRef]
- Lynch, S.D.; Marcek, B.J.; Marshall, H.M.; Bushnell, P.G.; Bernal, D.; Brill, R.W. The effects of pop-up satellite archival tags (PSAT) on the metabolic rate and swimming kinematics of juvenile sandbar shark Carcharhinus plumbeus. Fish. Res. 2017, 186, 205–215. [Google Scholar] [CrossRef]
- Bridger, C.J.; Booth, R.K. The effects of biotelemetry transmitter presence and attachment procedures on fish physiology and behavior. Rev. Fish. Sci. 2003, 11, 13–34. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Rikardsen, A.H.; Alp, A.; Økland, F. The use of electronic tags in fish research—An overview of fish telemetry methods. Turk. J. Fish. Aquat. Sci. 2013, 13, 881–896. [Google Scholar]
- Macaulay, G.; Warren-Myers, F.; Barrett, L.T.; Oppedal, F.; Fore, M.; Dempster, T. Tag use to monitor fish behaviour in aquaculture: A review of benefits, problems and solutions. Rev. Aquac. 2021, 13, 1565–1582. [Google Scholar] [CrossRef]
- Wilson, S.G.; Polovina, J.J.; Stewart, B.S.; Meekan, M.G. Movements of whale sharks Rhincodon typus tagged at ningaloo reef, Western Australia. Mar. Biol. 2006, 148, 1157–1166. [Google Scholar] [CrossRef]
- Hazen, E.L.; Maxwell, S.M.; Bailey, H.; Bograd, S.J.; Hamann, M.; Gaspar, P.; Godley, B.J.; Shillinger, G.L. Ontogeny in marine tagging and tracking science: Technologies and data gaps. Mar. Ecol. Prog. Ser. 2012, 457, 221–240. [Google Scholar] [CrossRef]
- Block, B.A.; Dewar, H.; Farwell, C.; Prince, E.D. A new satellite technology for tracking the movements of Atlantic bluefin tuna. Proc. Natl. Acad. Sci. USA 1998, 95, 9384–9389. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, N.; Thorstad, E.B.; Havn, T.; Lucas, M.C. The use of external electronic tags on fish: An evaluation of tag retention and tagging effects. Anim. Biotelem. 2015, 3, 49. [Google Scholar] [CrossRef]
- Domeier, M.L.; Dewar, H.; Nasby-Lucas, N. Mortality rate of striped marlin (Tetrapturus audax) caught with recreational tackle. Mar. Freshw. Res. 2003, 54, 435–445. [Google Scholar] [CrossRef]
- Gilly, W.F.; Markaida, U.; Baxter, C.H.; Block, B.A.; Boustany, A.; Zeidberg, L.; Reisenbichler, K.; Robinson, B.; Bazzino, G.; Salinas, C. Vertical and horizontal migrations by the jumbo squid Dosidicus gigas revealed by electronic tagging. Mar. Ecol. Prog. Ser. 2006, 324, 1–17. [Google Scholar] [CrossRef]
- Counihan, T.D.; Frost, C.N. Influence of externally attached transmitters on the swimming performance of juvenile white sturgeon. Trans. Am. Fish. Soc. 1999, 128, 965–970. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Økland, F.; Finstad, B. Effects of telemetry transmitters on swimming performance of adult Atlantic salmon. J. Fish Biol. 2000, 57, 531–535. [Google Scholar] [CrossRef]
- Smircich, M.G.; Kelly, J.T. Extending the 2% rule: The effects of heavy internal tags on stress physiology, swimming performance, and growth in brook trout. Anim. Biotelem. 2014, 2, 16. [Google Scholar] [CrossRef]
- Jepsen, N.; Schreck, C.; Clements, S.; Thorstad, E.B. A brief discussion on the 2% tag/bodymass rule of thumb. In Aquatic Telemetry: Advances and Applications, Proceedings of the Fifth Conference on Fish Telemetry held in Europe, Ustica, Italy, 9–13 June 2003; Spedicato, M.T., Lembo, G., Marmulla, G., Eds.; FAO/COISPA: Rome, Italy, 2005; pp. 255–259. [Google Scholar]
- Makiguchi, Y.; Ueda, H. Effects of external and surgically implanted dummy radio transmitters on mortality, swimming performance and physiological status of juvenile masu salmon Oncorhynchus masou. J. Fish Biol. 2009, 74, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Rożyński, M.; Kapusta, A.; Demska-Zakęś, K.; Hopko, M.; Sikora, A.; Zakęś, Z. The effects of surgically implanted dummy tags on the survival, growth performance, and physiology of pikeperch Sander lucioperca. Fish Physiol. Biochem. 2017, 43, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Zakęś, Z.; Rożyński, M.; Demska-Zakęś, K. Effect of PIT tagging on hematology and plasma composition of juvenile pikeperch (Sander lucioperca (L.)). Aquacult. Int. 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Mohan, J.A.; Jones, E.R.; Hendon, J.M.; Falterman, B.; Boswell, K.M.; Hoffmayer, E.R.; Wells, R.J.D. Capture stress and post-release mortality of blacktip sharks in recreational charter fisheries of the Gulf of Mexico. Conserv. Physiol. 2020, 8, coaa041. [Google Scholar] [CrossRef] [PubMed]
- Múgica, M.; Sokolova, I.M.; Izagirre, U.; Marigómez, I. Seasondependent effects of elevated temperature on stress biomarkers, energy metabolism and gamete development in mussels. Mar. Environ. Res. 2015, 103, 1–10. [Google Scholar] [CrossRef]
- Balbi, T.; Fabbri, R.; Montagna, M.; Camisassi, G.; Canesi, L. Seasonal variability of different biomarkers in mussels (Mytilus galloprovincialis) farmed at different sites of the Gulf of La Spezia, Ligurian sea, Italy. Mar. Pollut. Bull. 2017, 116, 348–356. [Google Scholar] [CrossRef]
- Parisi, M.G.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. J. Comp. Physiol. B 2017, 187, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, X.; Wang, L.; Lu, K.; Song, K.; Ai, Q.; Mai, K.; Zhang, C. Effects of dietary arginine levels on growth, immune function of physical barriers and serum parameters of spotted seabass (Lateolabrax maculatus) reared at different water temperatures. Aquaculture 2021, 541, 736812. [Google Scholar] [CrossRef]
- Yokogawa, K.; Seki, S. Morphological and genetic differences between Japanese and Chinese sea bass of the genus Lateolabrax. Jpn. J. Ichthyol. 1995, 41, 437–445. [Google Scholar]
- Kim, Y.U.; Myoung, J.G.; Kim, Y.S.; Han, K.H.; Kang, C.B.; Kim, J.G. The Marines Fishes of Korea; Hanguel: Busan, Korea, 2001; Volume 222. (In Korean) [Google Scholar]
- Oh, S.Y.; Jeong, Y.K. Effects of external pop-up satellite archival tag (PSAT) tagging method on blood indices and PSAT attachment efficiency of yellowtail Seriola quinqueradiata. Korean J. Fish. Aquat. Sci. 2021, 54, 38–45. (In Korean) [Google Scholar]
- Yan, L.; Wang, P.; Zhao, C.; Fan, S.; Lin, H.; Guo, Y. Toxic responses of liver in Lateolabrax maculatus during hypoxia and re-oxygenation. Aquat. Toxicol. 2021, 236, 105841. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, H.; Tian, Y.; Mao, X.; Li, X.; Li, J.; Hu, Y.; Liu, Y.; Li, J.; Li, Y. HSP90 and HSP70 families in Lateolabrax mac ulatus: Genome-wide identification, molecular characterization, and expression profiles in response to various environmental stressors. Front. Physiol. 2021, 12, 784803. [Google Scholar]
- Sun, Z.; Xu, C.; Chen, Y.; Liu, D.; Wu, P.; Gao, Q. Characterization of Pannexin1, Connexin32, and Connexin43 in spotted sea bass (Lateolabrax maculatus): They are important neuro-related immune response genes involved in inflammation-induced ATP release. Front. Immunol. 2022, 13, 870679. [Google Scholar] [CrossRef]
- Wang, H.; Wen, H.; Yun, L.; Zhang, K.; Lin, Y. Evaluation of potential reference genes for quantitative RT-PCR analysis in spotted sea bass (Lateolabrax maculatus) under normal and salinity stress conditions. PeerJ 2018, 6, e5631. [Google Scholar] [CrossRef] [PubMed]
- Peres, H.; Costas, B.; Perez-Jimenez, A.; Guerreiro, I.; Oliva-Teles, A. Reference values for selected hematological and serum biochemical parameters of Senegalese sole (Solea senegalensis Kaup, 1858) juveniles under intensive aquaculture conditions. J. Appl. Ichthyol. 2015, 31, 65–71. [Google Scholar] [CrossRef]
- Cho, C.Y.; Cowey, C.B. Rainbow trout, Oncorhynchus mykiss. In Handbook of Nutritional Requirements of Finfish; Wilson, R.P., Ed.; CRS Press: Boca Raton, FL, USA, 1991; pp. 131–143. [Google Scholar]
- Hardy, R.W. Rainbow trout, Oncorhynchus mykiss. In Nutritional Requirements and Feeding of finfish for Aquaculture; Webster, C.D., Lim, C.E., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 184–202. [Google Scholar]
- Nabi, N.; Ahmed, I.; Wani, G.B. Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: Morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 2022, 29, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Denaro, M.G.; Caruso, R.; Genovese, L.; Mancari, F.; Maricchiolo, G. Short fasting and refeeding in red porgy (Pagrus pagrus, Linnaeus 1958): Response of some haematological, biochemical and non-specifc immune parameters. Mar. Environ. Res. 2012, 81, 18–25. [Google Scholar] [CrossRef]
- Davis, K.B.; Torrance, P.; Parker, N.C. Physiological stress in striped bass: Effect of acclimation temperature. Aquaculture 1990, 91, 349–358. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.Y. Physiological responses of marine fish to external attachment of pop-up satellite archival tag (PSAT). Ocean Pol. Res. 2018, 40, 169–176. [Google Scholar] [CrossRef]
- Pan, C.H.; Chien, Y.H.; Hunter, B. The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J. Exp. Mar. Biol. Ecol. 2003, 297, 107–118. [Google Scholar] [CrossRef]
- Oh, S.Y. Effect of bio-logger attachment location on blood characteristics and bio-logger attachment efficiency in spotted sea bass Lateolabrax maculatus. Korean J. Fish. Aquat. Sci. 2023, 56, 651–659. (In Korean) [Google Scholar]
- Wendel, A.; Feuerstein, S. Drug-induced lipid peroxidation in mice–I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem. Pharmacol. 1981, 30, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Woodley, C.M.; Eppard, M.B.; Brown, R.S.; Nielsen, J.L. Advancing the surgical implantation of electronic tags in fish: A gap analysis and research agenda based on a review of trends in intracoelomic tagging effects studies. Rev. Fish Biol. Fish. 2011, 21, 127–151. [Google Scholar] [CrossRef]
- Dick, M.; Eliason, E.J.; Patterson, D.A.; Robinson, K.A.; Hinch, S.G.; Cooke, S.J. Short-term physiological response profiles of tagged migrating adult sockeye salmon: A comparison of gastric insertion and external tagging methods. Trans. Am. Fish. Soc. 2018, 147, 300–315. [Google Scholar] [CrossRef]
- Runde, B.J.; Buckel, J.A.; Bacheler, N.M.; Tharp, R.M.; Rudershausen, P.J.; Harms, C.A.; Ben-Horin, T. Evaluation of six methods for external attachment of electronic tags to fish: Assessment of tag retention, growth and fish welfare. J. Fish Biol. 2022, 101, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar]
- Zhao, L.; Tang, G.; Xiong, C.; Han, S.; Yang, C.; He, K.; Liu, Q.; Luo, J.; Luo, W.; Wang, Y.; et al. Chronic chlorpyrifos exposure induces oxidative stress, apoptosis and immune dysfunction in largemouth bass (Micropterus salmoides). Environ. Pollut. 2021, 282, 117010. [Google Scholar] [CrossRef] [PubMed]
- da Rosaa, V.M.; Ariottia, K.; Bressana, C.A.; da Silvaa, E.G.; Dallaportab, M.; Júniora, G.B.; da Costab, S.T.; de Vargasc, A.C.; Baldisserottoa, B.; Finamora, I.A.; et al. Dietary addition of rutin impairs inflammatory response and protects muscle of silver catfish (Rhamdia quelen) from apoptosis and oxidative stress in Aeromonas hydrophila-induced infection. Comp. Biochem. Physiol. C 2019, 226, 108611. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cui, L.; Hou, T.; Xue, X.; Zhang, S.; Wang, Z. Stress responses of testicular development, inflammatory and apoptotic activities in male zebrafish (Danio rerio) under starvation. Dev. Comp. Immunol. 2021, 114, 103833. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.; Liu, S.; Liu, H.; Zhang, X.; Shi, J.; Ji, L.; Li, J. HSP70 alleviates spinal cord injury by activating the NF-kB pathway. J. Musculoskelet. Neuronal Interact. 2021, 21, 542–549. [Google Scholar]
- Li, S.; Wang, N.; Zhang, T.; Feng, Y.; Wang, L.; Sun, J. Characterization of three connexin32 genes and their role in inflammation-induced ATP release in the Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2020, 106, 181–189. [Google Scholar] [CrossRef]

| Biomarker Genes | Primer Sequence | Amplicon Size (bp) | Reference |
|---|---|---|---|
| bax | 5-TTCATCCGTCTGCTCTTCACAAAC-3 5-GGTGGCTGGGAGGGTATTCG-3 | 108 | Yan et al. (2021) [27] |
| hsp70-2 | 5-CCTCATCCAGGTCTACG-3 5-CTGCTCATCCTCGCTAA-3 | 388 | Sun et al. (2021) [28] |
| Cx32.7 | 5-CCCTCGTCCTCAGTCTGGTTG-3 5-TGTTCTCAGGCGATACGTTCTTG-3 | 252 | Sun et al. (2022) [29] |
| rpl7 | 5-ACCCCAACCTGAAGTCTGTG-3 5-ATGCCATATTTGCCAAGAGC-3 | 121 | Wang et al. (2018) [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-Y.; Maeng, J.-H.; Kang, H.-S. Effect of Biologger Attachment on the Stress and Health State of the Spotted Sea Bass Lateolabrax maculatus. J. Mar. Sci. Eng. 2024, 12, 793. https://doi.org/10.3390/jmse12050793
Oh S-Y, Maeng J-H, Kang H-S. Effect of Biologger Attachment on the Stress and Health State of the Spotted Sea Bass Lateolabrax maculatus. Journal of Marine Science and Engineering. 2024; 12(5):793. https://doi.org/10.3390/jmse12050793
Chicago/Turabian StyleOh, Sung-Yong, Jun-Ho Maeng, and Han-Seung Kang. 2024. "Effect of Biologger Attachment on the Stress and Health State of the Spotted Sea Bass Lateolabrax maculatus" Journal of Marine Science and Engineering 12, no. 5: 793. https://doi.org/10.3390/jmse12050793
APA StyleOh, S.-Y., Maeng, J.-H., & Kang, H.-S. (2024). Effect of Biologger Attachment on the Stress and Health State of the Spotted Sea Bass Lateolabrax maculatus. Journal of Marine Science and Engineering, 12(5), 793. https://doi.org/10.3390/jmse12050793





