Abstract
This study characterizes the distribution of salp and doliolid species in the northeastern East China Sea during spring and autumn and identifies the factors that regulate their abundance. The data were collected over four years (2019–2023, except 2020). During the survey period, the Tsushima Warm Current Surface and Bottom (TWCS and TWCB, respectively) and Yellow Sea Cold Bottom Water were influential in spring, while the Changjiang Diluted Water, Coastal Water, TWCS, and TWCB were dominant in autumn. The mean sea surface temperature (SST) and sea surface salinity (SSS) in spring and the mean SST in autumn statistically decreased (p < 0.05), while the mean SSS in autumn statistically increased (p < 0.05). The spatial distribution of salp and doliolid species remained consistent in spring and autumn, although the dominant assemblage shifted seasonally. Spring was predominantly characterized by an abundance of salp species, whereas doliolid species were dominant in autumn, with their distribution and abundance influenced by different environmental factors such as temperature and salinity in spring and food availability in autumn. Our results provide valuable data on the factors affecting the presence of salp and doliolid species in the northeastern East China Sea.
1. Introduction
Pelagic thaliaceans (salps, doliolids, and pyrosomes) are planktonic, gelatinous zooplankton belonging to the phylum Chordata. These organisms are filter feeders that consume phytoplankton of various sizes in marine ecosystems [1,2,3]. Salps and doliolids exhibit a unique life cycle that alternates between asexual and sexual reproduction to maintain their population [4]. In particular, salps typically alternate between blastozooids and oozooids, while doliolids switch between phorozooids and gonozooids [5]. These traits enable them to rapidly proliferate with high growth rates, significantly impacting marine ecosystems by influencing primary productivity and enhancing vertical carbon flux through fecal pellet production [6,7].
The distribution of thaliaceans is known to be influenced by hydrodynamic conditions such as the sea temperature and salinity [3,8], the influx intensity of water masses within the study region [3,9], the availability of food [10], and various biological factors, including prey competition and predator–prey relationships [11,12,13]. Salpa fusiformis is the widest-ranging and most abundant thaliacean species worldwide, occurring in Pacific waters [12,13,14,15,16]. Mass aggregations of S. fusiformis have been reported to cause blockages in the cooling water supply screens of South Korea’s nuclear power plants and to damage the nets of coastal fish farms [17,18,19,20]. Additionally, S. fusiformis can alter ecosystem nutrient dynamics by affecting the productivity of other taxa such as Euphausiacea and Copepoda via food competition and by modifying predator–prey relationships [7]. However, despite the importance of these interspecies interactions, research on salps in the northeastern East China Sea has primarily focused on mass occurrences and the spatial distribution of S. fusiformis [17,19,21,22].
The northeastern East China Sea is bordered by the southern region of South Korea and is characterized by complex rias-style coasts. With an average depth of 70 m and a maximum depth of 139 m, this area is influenced by various water masses, including the Tsushima Warm Current (TWC), Yellow Sea Cold Bottom Water (YSCBW), Korean Coastal Water (KCW), and Changjiang Diluted Water (CDW) [23,24,25,26]. The Changjiang River discharge is regulated by meteorological conditions such as rainfall and typhoons, and reports suggest that its discharge may influence the northeastern East China Sea depending on the season [27]. Furthermore, seasonal freshwater inputs from the Nakdong, Seomjin, and Yeongsan Rivers from southern Korea and dense fish and shellfish farming along the coast contribute to frequent eutrophication and phytoplankton blooms in spring and autumn [28,29,30,31].
The present study sought to compile a species list of salps and doliolids occurring in spring and autumn in the northeastern East China Sea, analyze the occurrence patterns of dominant species, and strengthen the understanding of the environmental factors influencing salps and doliolids by comparing them with species from other seas. This study was motivated by previous research showing that the mass aggregation of salps (predominantly S. fusiformis, Doliolum denticulatum, and D. nationalis) is strongly correlated with chlorophyll a (Chl-a) concentrations in the northeastern East China Sea [17,19,21] and that the food environment in the study area is predominantly shaped by phytoplankton blooms occurring in spring and autumn [28,29,30,31]. To this end, data were collected over the course of four years from 2019 to 2023 (except 2020 due to COVID-19 restrictions) during the food-rich spring and autumn seasons in the northeastern East China Sea.
2. Materials and Methods
2.1. Environmental Factors
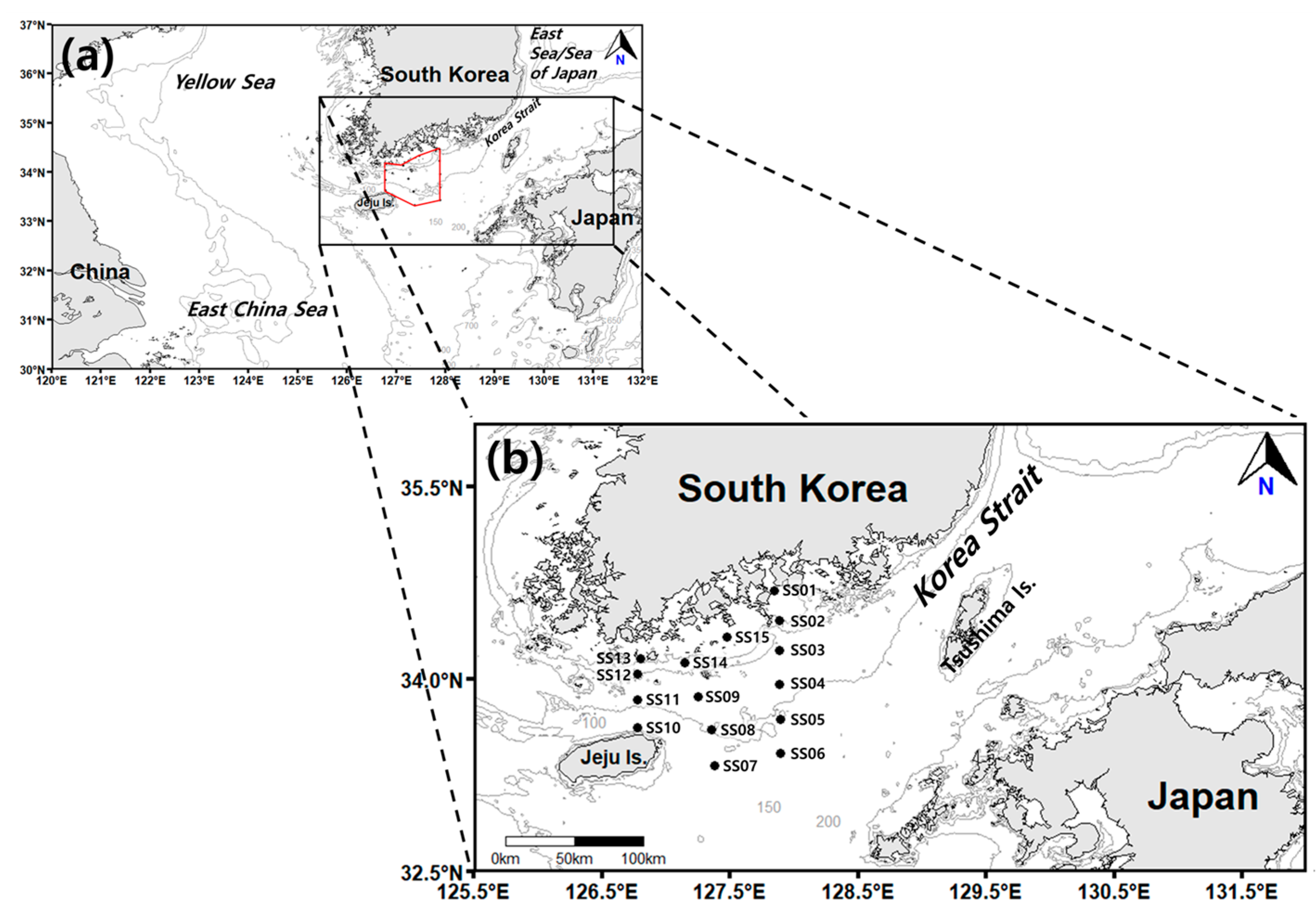
Surveys were conducted in spring and autumn to characterize the occurrence patterns of salp and doliolid species in the northeastern East China Sea during the two periods of the year when they are most abundant (Figure 1). From 2019 to 2023, a total of eight surveys (four in spring and four in autumn) were conducted at 15 stations aboard the Saedongbaek-Ho training vessel of Chonnam National University. However, surveys could not be conducted in 2020 due to global COVID-19 pandemic restrictions, including government lockdowns. During the study period, the observation stations varied due to meteorological conditions. Table 1 summarizes the survey locations and their depth during the study period. Basic marine environmental data such as the seawater temperature, salinity, and Chl-a concentration were measured using a portable submersible fluorometer (ASTD102, JFE Advantech Co. Ltd., Nishinomiya, Japan) and a conductivity, temperature, and depth (CTD) profiler (SBE19, Sea-Bird Co., Washington, DC, USA). The fluorescence of the samples, which was used to represent Chl-a levels obtained from the CTD profiler, was calibrated using measurements from filtered water samples collected from different water layers at each station (the surface, subsurface chlorophyll maximum, and bottom).
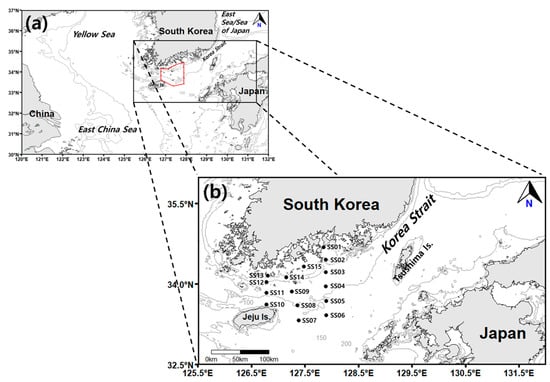
Figure 1.
(a) Map of the study area. (b) Locations of the sampling stations. The contour lines indicate the water depth (m).

Table 1.
Net information for the survey period. The white circles indicate the surveyed stations. N/S = not sampled.
2.2. Zooplankton Sample Collection
Zooplankton samples were collected vertically from the bottom (2 m above the seabed) to the surface using a bongo net (net mouth diameter: 60 cm; mesh sizes: 150 and 200 μm), with only the 200 μm samples used due to the size of thaliaceans. To determine the filtered seawater volume, a flowmeter (Model 438115, Hydro-Bios Co., Altenholz, Germany) was attached to the net entrance. Following their collection, the samples were immediately fixed on-site with a neutralized formaldehyde solution at a final concentration of 5%. In addition to the sampling gear, the Multiple Opening/Closing Net and Environmental Sampling System (MOCNESS) was employed to confirm the vertical distribution of salps and doliolids based on the water mass distribution across six sampling stations (unpublished data). Thaliaceans within the zooplankton samples were identified and counted using a UNESCO counting chamber. The zooplankton abundance was expressed as individuals per 100 cubic meters (inds. 100 m−3). Individuals that could not be easily identified were immersed in Rose Bengal dye diluted with deionized water for 1 min, after which they were identified and classified down to the species level using a stereo microscope (SMZ645, Nikon, Tokyo, Japan). Salp and doliolid species were identified as described in previous studies [32,33,34,35], with taxonomic systematics adhering to the guidelines of the WoRMS Editorial Board [36]. Additionally, although pyrosomes have been reported in the study region [37], they were not observed during the survey period and thus were not considered in this study.
2.3. Data Analysis
To assess the seasonal variability in the abundance of salps and doliolids during the survey period, the data were transformed using the log10(x + 1) index for normalization. In our study region, which is characterized by continental shelf areas with depths of less than 200 m, understanding these migration dynamics presents a challenge. The sea surface temperature (SST), sea surface salinity (SSS), sea surface fluorescence (SSF), and sea surface turbidity (SSTb) were all averaged using surface data [16]. The trends in the salp and doliolid abundance in response to these environmental factors were then determined using linear regression analysis. Temperature–salinity (T-S) bubble plots based on the abundance of salps and doliolids during the study period were generated to confirm their occurrence patterns. For canonical correspondence analysis (CCA), all of the environmental factors were standardized using z-scores to ensure comparability, and outliers were eliminated. Subsequently, CCA was used to calculate weighted scores for the environmental factors influencing the abundance of salps and doliolids, thus providing insights into the seasonal variation in environmental factors that affect their abundance. The significance level for the statistical analyses was set at p < 0.05. All visualizations and statistical analyses were performed in R (version 4.1.0) [38].
3. Results
3.1. Seasonal Variation in the Environmental Factors
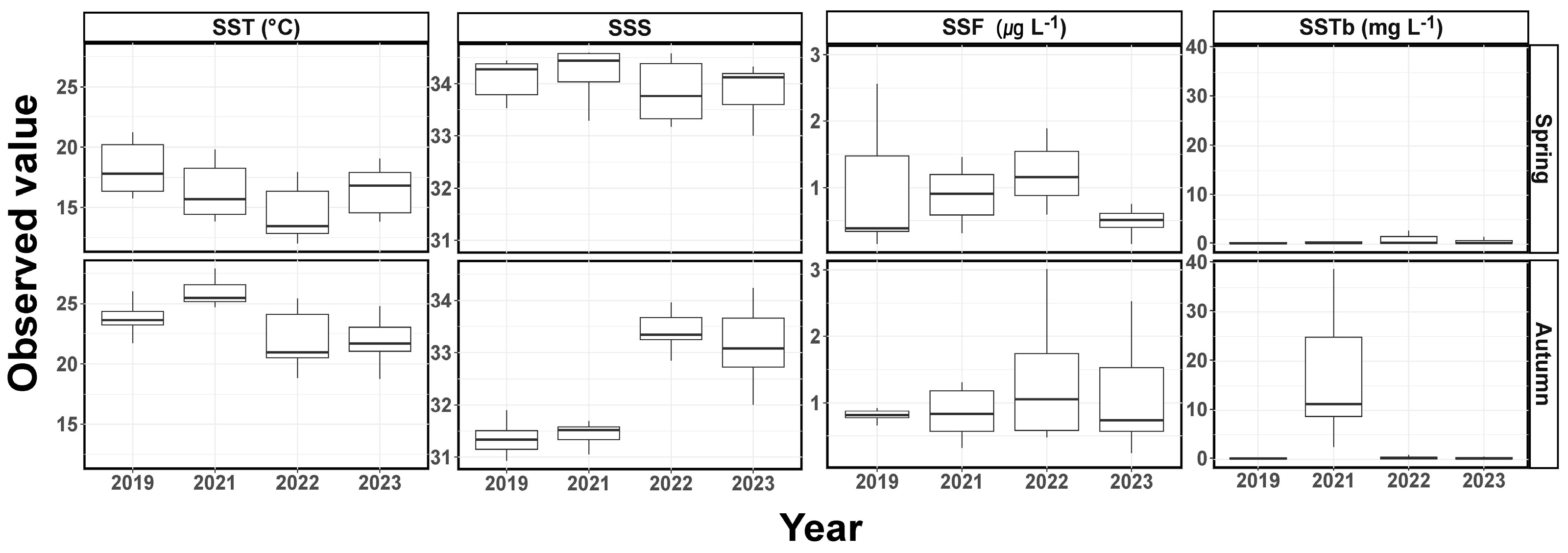
In spring, the SST in the survey area ranged from 15.8 to 21.2 °C in 2019, 13.9 to 19.8 °C in 2021, 12.0 to 17.9 °C in 2022, and 13.8 to 19.1 °C in 2023 (Figure 2). The SST exhibited statistically significant differences between survey years, with the mean SST in spring 2023 significantly lower than in spring 2019 (p < 0.05, ANOVA). SSS ranged from 33.53 to 34.44 in 2019, 33.29 to 34.59 in 2021, 33.17 to 34.58 in 2022, and 33.00 to 34.33 in 2023. SSS exhibited a statistically significant decrease in 2023 compared to 2021 (p < 0.05, ANOVA), while there was no significant difference between the SSS in 2019 and that in 2023 (p > 0.05, ANOVA). SSF ranged from 0.15 to 2.56 μg L−1 in 2019, 0.31 to 2.50 μg L−1 in 2021, 0.59 to 4.45 μg L−1 in 2022, and 0.15 to 3.64 μg L−1 in 2023. There was a statistically significant decrease in the mean SSF in 2023 compared to 2021 (p < 0.05, ANOVA), while there was no significant difference between the mean SSF in 2019 and 2023 (p > 0.05, ANOVA). SSTb ranged from 0.11 to 0.41 mg L−1 in 2019, 0.12 to 2.90 mg L−1 in 2021, 0.10 to 2.73 mg L−1 in 2022, and 0.06 to 1.50 mg L−1 in 2023, with no significant difference observed during the study period (p > 0.05, ANOVA).
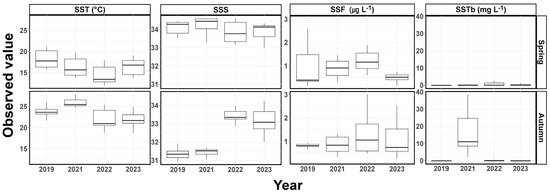
Figure 2.
Seasonal variation in the environmental factors in spring and autumn. SST, sea surface temperature; SSS, sea surface salinity; SSF, sea surface fluorescence; SSTb, sea surface turbidity.
In autumn, the SST in the surveyed area ranged from 21.7 to 26.0 °C in 2019, 24.7 to 27.9 °C in 2021, 18.9 to 25.4 °C in 2022, and 18.8 to 24.8 °C in 2023 (Figure 2). There was a statistically significant decrease in the mean SST in 2023 compared to 2019, with statistically significant differences observed between the survey years (p < 0.05, ANOVA). SSS ranged from 30.93 to 31.90 in 2019, 30.36 to 33.41 in 2021, 32.01 to 33.96 in 2022, and 32.01 to 34.24 in 2023. Lower salinity ranges were observed in 2019 and 2021, whereas 2022 and 2023 exhibited relatively higher salinity ranges. The mean SSS increased during the study period, with statistically significant differences observed between each survey period (p < 0.05, ANOVA). SSF ranged from 0.66 to 1.74 μg L−1 in 2019, 0.32 to 1.31 μg L−1 in 2021, 0.48 to 3.01 μg L−1 in 2022, and 0.25 to 2.53 μg L−1 in 2023. There were no statistically significant differences in the mean SSF between the survey periods (p > 0.05, ANOVA). SSTb ranged from 0.11 to 0.24 mg L−1 in 2019, 2.53 to 38.74 mg L−1 in 2021, 0.10 to 0.89 mg L−1 in 2022, and 0.08 to 1.13 mg L−1 in 2023. There was a statistically significant decrease in the mean SSTb in 2023 compared to 2021 (p < 0.05, ANOVA), but, except for 2021, there were almost no statistically significant differences in the mean (p > 0.05, ANOVA). During the study period, to verify whether the observed trends in the mean SST and SSS were within the normal range expected within a season, data from the Korea Oceanographic Data Center (KODC) were consulted, and these are presented in Figure S1.
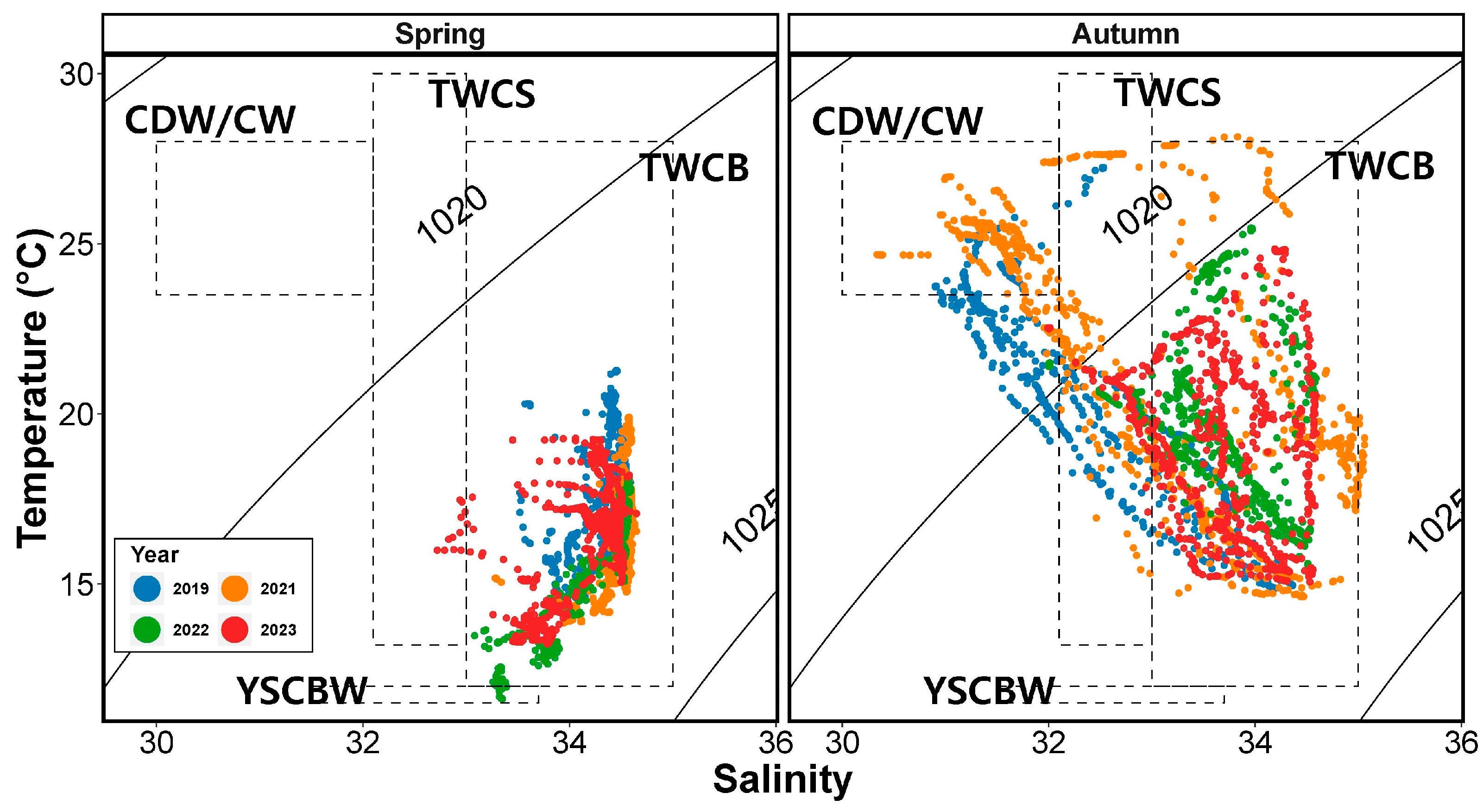
Using the temperature and salinity profiles obtained during the survey period, we constructed T-S diagrams (Figure 3). Based on previous studies, the observed water masses in the study region included the Tsushima Warm Current Surface and Bottom (TWCS and TWCB, respectively) and YSCBW in spring, while in autumn, Coastal Water (CW), CDW, TWCS, and TWCB were observed. CDW and CW are both characterized by low salinity and high temperatures and represent mixed water that cannot be separated during the inflow into the study region, and thus they are referred to together as CDW/CW in this study. In spring, TWCB was the primary influence in the study region, with some influence from YSCBW observed in 2022. In autumn, the influence of the low-salinity CDW/CW was observed in 2019 and 2021, while TWC predominantly influenced the region in 2022 and 2023. The influence of YSCBW was not observed during autumn.
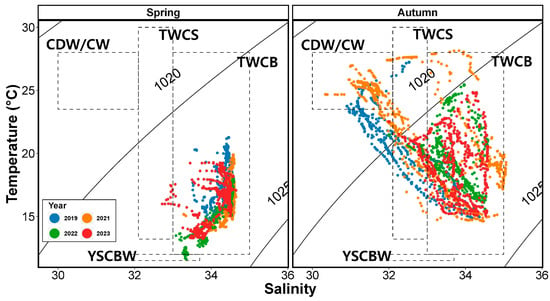
Figure 3.
Temperature–salinity (T-S) diagrams for the abundance of salp and doliolid species in the study area in spring and autumn. Abbreviations: CW, Coastal Water, as reported by Lim [8]; CDW, Changjiang Diluted Water, as reported by Lie et al. [24]; YSCBW, Yellow Sea Cold Bottom Water, as reported by Choi [26]; TWCS and TWCB, Tsushima Warm Current Surface and Bottom, respectively, as reported by Hur et al. [25].
3.2. Spatial Distribution of Salp and Doliolid Assemblages
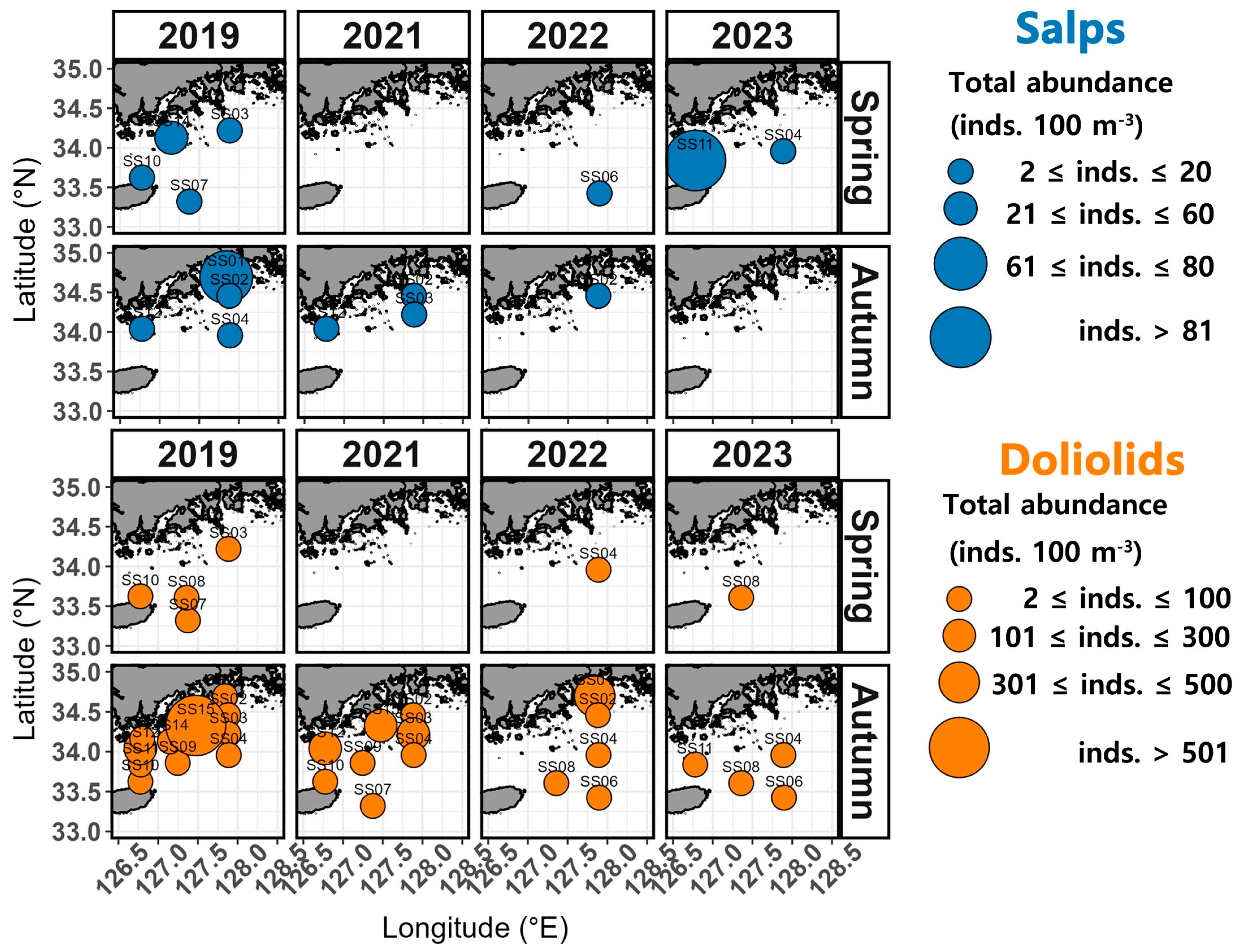
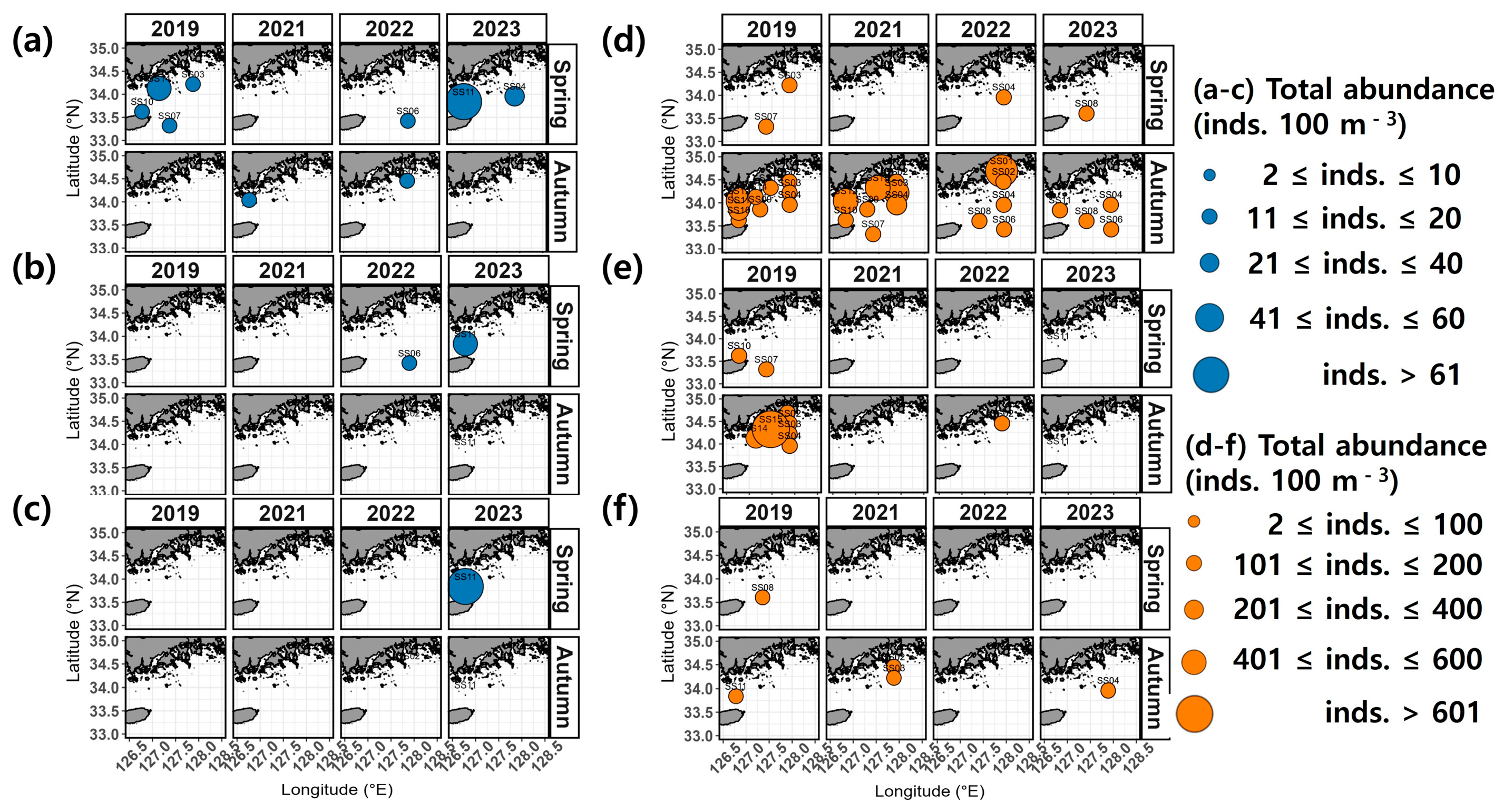
In spring, the occurrence of salp species ranged from 2 to 39 inds. 100 m−3 in 2019, spanning both coastal and offshore areas (Figure 4). In 2021, salp species were absent from all stations. In 2022, salp species were only observed at the offshore station SS06, with an abundance of 11 inds. 100 m−3. In 2023, the abundance of salp species ranged from 11 to 194 inds. 100 m−3, with the highest (194 inds. 100 m−3) recorded west of the northeastern East China Sea (between Wando and Jeju, SS11). In autumn, the abundance of salp species ranged between 2 and 71 inds. 100 m−3 in 2019, with the highest (71 inds. 100 m−3) recorded closer to the coast at station SS01. In 2021, a few occurrences were recorded at SS02, SS03, and SS12. In 2022, only 2 inds. 102 m−3 were identified in the samples from station SS02, whereas in 2023, salp species were entirely absent.
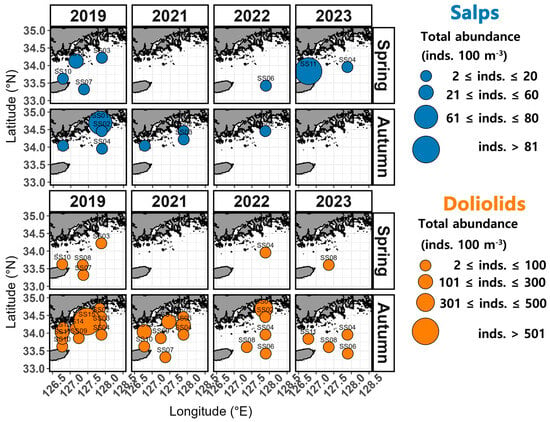
Figure 4.
Spatial distribution of the total abundance of salps and doliolids.
In terms of doliolid species, 1 to 17 inds. 100 m−3 were detected in the spring of 2019, with the highest abundance (17 inds. 100 m−3) recorded at the offshore station SS07 (Figure 4). In 2022 and 2023, a few occurrences were recorded only at offshore stations (SS04 and SS08). In autumn, the abundance of doliolid species varied widely between 7 and 776 inds. 100 m−3 in 2019 and, while they were observed in both coastal and offshore areas, they were predominantly detected at coastal station SS15, where the highest abundance was recorded (776 inds. 100 m−3) (Figure 4). In 2021, they were observed at numbers ranging between 1 and 250 inds. 100 m−3, mainly at coastal station SS15 (250 inds. 100 m−3). In 2022, they were present at levels of 1 to 415 inds. 100 m−3, with the highest abundance recorded at coastal station SS01 (415 inds. 100 m−3). In 2023, they were observed at levels of 1 to 16 inds. 100 m−3 only at stations distant from the coast (SS04, SS06, SS08, and SS11).
During the survey period, the majority of salps observed in spring were attributed to Salpa fusiformis, while only a few were observed at some coastal stations (SS02 and SS12) in autumn (Figure 4 and Figure 5a). The salp species that occurred only in specific years were Cyclosalpa affinis and C. bakeri (Figure 5b,c). Doliolids observed in spring exhibited irregular spatial patterns and occurred in lower abundances throughout the survey period (Figure 5d–f). In autumn, the dolioid species Doliolum nationalis was consistently observed, primarily at coastal stations (Figure 5d). The doliolid species that occurred only in specific years included Dolioletta gegenbauri and Doliolum denticulatum (Figure 5e,f).
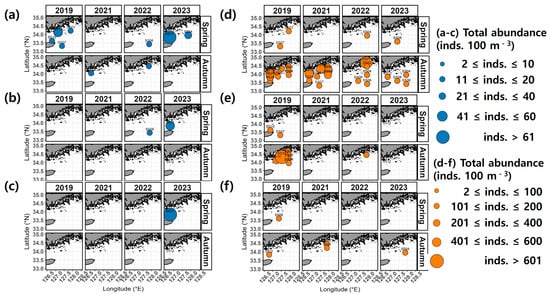
Figure 5.
Spatial distribution of dominant species. (a) Salpa fusiformis, (b) Cyclosalpa affinis, (c) Cyclosalpa bakeri, (d) Doliolum nationalis, (e) Dolioletta gegenbauri, and (f) Doliolum denticulatum.
3.3. Seasonal Variation in Salp and Doliolid Assemblages
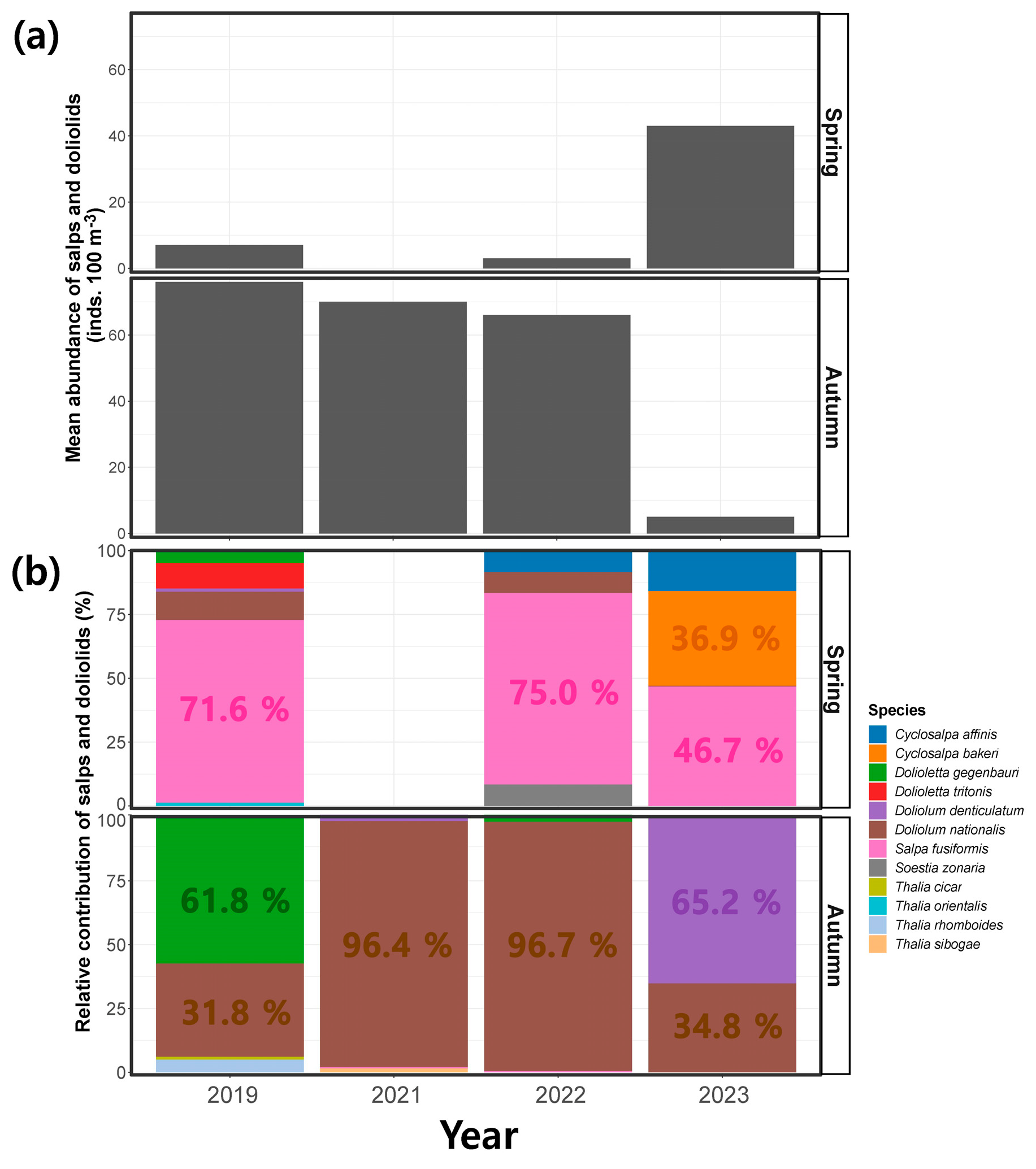
During the spring period, the mean abundance of salps and doliolids observed across the study region was 7 ± 11 inds. 100 m−3 in 2019, 0 inds. 100 m−3 in 2021, 3 ± 4 inds. 100 m−3 in 2022, and 43 ± 36 inds. 100 m−3 in 2023 (Figure 6a). In contrast, during the autumn period, the mean abundance of salps and doliolids was 76 ± 169 inds. 100 m−3 in 2019, 70 ± 95 inds. 100 m−3 in 2021, 660 ± 154 inds. 100 m−3 in 2022, and 5 ± 6 inds. 100 m−3 in 2023, thus exhibiting a decreasing tendency in the average abundance over the survey period (Figure 6a). The relative contributions of individual species were examined to investigate whether the observed variation in the seasonal abundance of salps and doliolids was regulated at the species level (Figure 6b and Table 2). In spring, Salpa fusiformis was the most dominant species, with a relative contribution of 71.6% in 2019, followed by Doliolum nationalis (11.1%) and Dolioletta tritonis (9.9%). However, no salps or doliolids were detected in 2021. In 2022, S. fusiformis was the dominant species, with a relative contribution of 75.0%, followed by Cyclosalpa affinis, D. nationalis, and Soestia zonaria with 8.3% each. In 2023, among the salps, S. fusiformis (46.7%), Cyclosalpa bakeri (36.9%), and C. affinis (15.9%) were the most dominant, whereas D. nationalis was less abundant (0.5%) during this period. In autumn, Dolioletta gegenbauri (61.8%) was the most dominant species in 2019, followed by D. nationalis (31.8%), and Thalia rhomboides (5.0%). In 2021 and 2022, D. nationalis was the dominant species, accounting for 96.4% and 96.7% of the abundance, respectively. In 2021, Doliolum denticulatum, S. fusiformis, and Thalia sibogae each had a relative abundance of less than 1%, while in 2022, D. gegenbauri and S. fusiformis occurred and made only small contributions to the assemblage, accounting for 2.8% and 0.4%, respectively. Doliolum denticulatum was the most dominant species in 2023 at 65.2%, with D. nationalis occurring at 34.8%. No Cyclosalpa species were detected in autumn from 2019 to 2023.
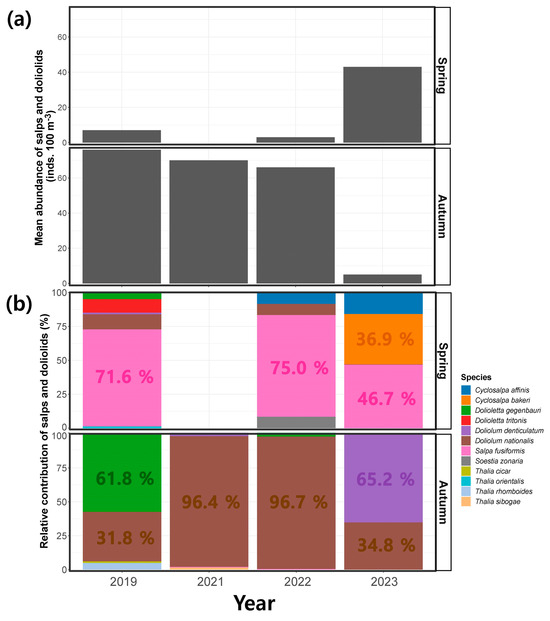
Figure 6.
(a) Seasonal variation in the abundance and (b) relative contribution of salps and doliolids during the spring and autumn periods.

Table 2.
Occurrence of salp and doliolid species during the study period. N/S = not sampled. The asterisks indicate the relative abundance of the salp and doliolid species (* 1 ≤ inds. 100 m−3 ≤ 200, *** ≥401 inds. 100 m−3).
3.4. Environmental Envelopes for Salps and Doliolids
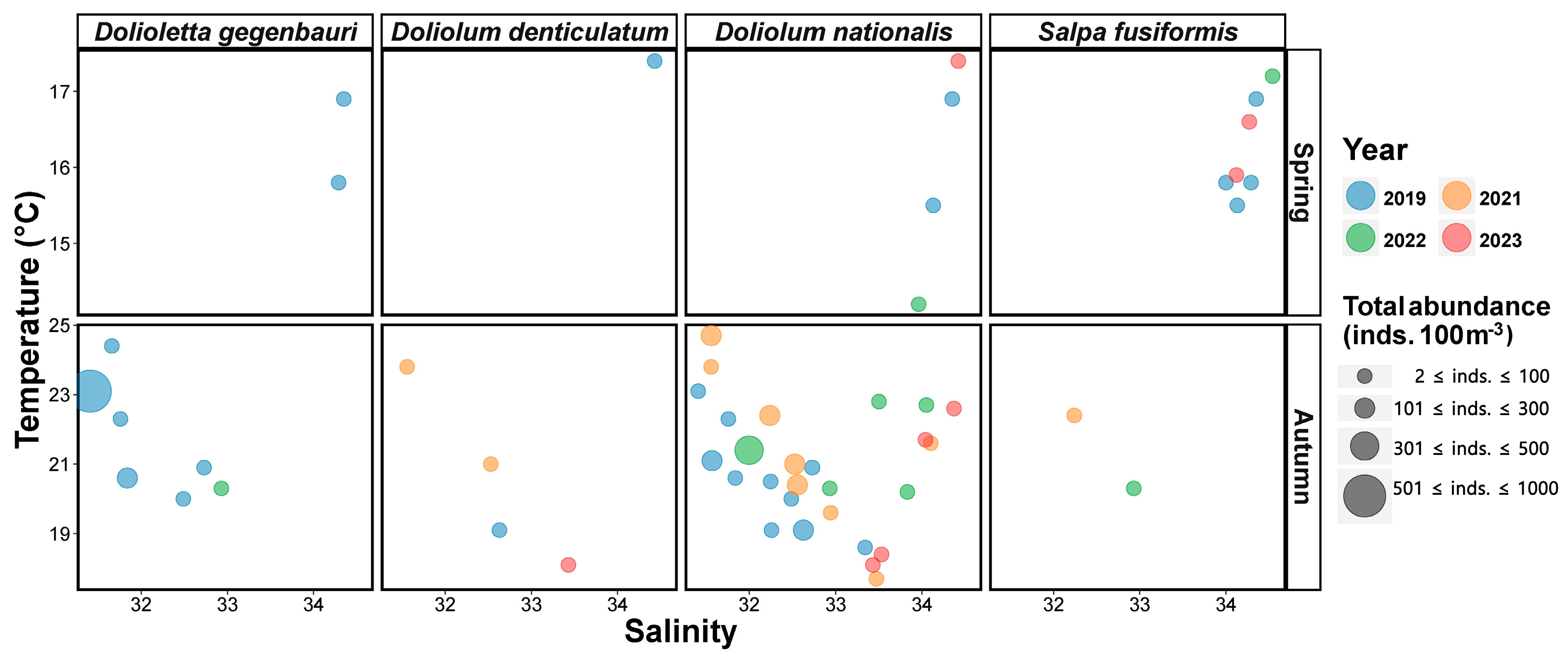
T-S bubble plots of species-specific occurrence patterns were generated to characterize the seasonal occurrence patterns of dominant salps and doliolids during the study period (Figure 7). Dolioletta gegenbauri was recorded only in 2019 and exhibited its highest abundance (3 inds. 100 m−3) at 16.9 °C and a salinity of 34.35. Doliolum denticulatum was also observed only in 2019 and reached its highest abundance (1 inds. 100 m−3) at 17.4 °C and a salinity of 34.43. Doliolum nationalis occurred every year from 2019 to 2023 (excluding 2021), with its highest abundance recorded at 16.9 °C with a salinity of 34.35 in 2019 (6 inds. 100 m−3), 14.2 °C with a salinity of 33.96 in 2022 (1 inds. 100 m−3), and 17.4 °C with a salinity of 34.42 in 2023 (1 inds. 100 m−3). Salpa fusiformis, which was present in spring from 2019 to 2023 (excluding 2021), was consistently observed, with the highest abundances recorded at 15.8 °C with a salinity of 34.00 in 2019 (39 inds. 100 m−3), 17.2 °C with a salinity of 34.54 in 2022 (9 inds. 100 m−3), and 15.9 °C with a salinity of 34.12 in 2023 (81 inds. 100 m−3).
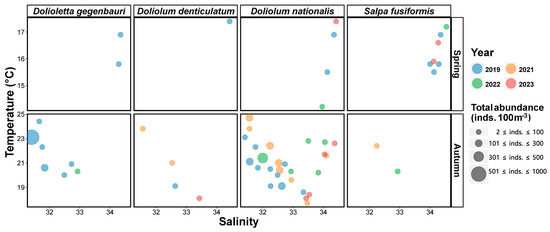
Figure 7.
Occurrence of dominant salps and doliolids in spring and autumn.
In autumn, Dolioletta gegenbauri was recorded only in 2019 and 2022, with its highest abundance recorded at 23.1 °C with a salinity of 31.41 in 2019 (774 inds. 100 m−3) and at 20.3 °C with a salinity of 32.93 in 2022 (13 inds. 100 m−3) (Figure 7). Doliolum denticulatum was observed every year from 2019 to 2023 (excluding 2022), with its highest abundance recorded at 19.1 °C with a salinity of 32.63 in 2019 (5 inds. 100 m−3), 23.8 °C with a salinity of 31.64 in 2021 (10 inds. 100 m−3), and 16.1 °C with a salinity of 33.43 in 2023 (15 inds. 100 m−3). Doliolum nationalis was detected every year from 2019 to 2023, with the highest abundance observed at 21.1 °C with a salinity of 31.57 in 2019 (207 inds. 100 m−3), 24.7 °C with a salinity of 31.56 in 2021 (250 inds. 100 m−3), 21.4 °C with a salinity of 32.00 in 2022 (415 inds. 100 m−3), and 18.4–21.7 °C with a salinity of 33.53–34.04 in 2023 (3 inds. 100 m−3). Salpa fusiformis occurred only in 2021 and 2022, with a maximum abundance at 22.4 °C with a salinity of 32.34 in 2021 (3 inds. 100 m−3) and at 20.3 °C with a salinity of 32.93 in 2022 (2 inds. 100 m−3).
Collectively, our findings revealed that only a few doliolids were present at low abundances in spring, with salps more dominant during this season (Figure 7). In contrast, doliolids were more abundant than salps in autumn. The temperature and salinity ranges for the dominant species are summarized in Table 3.

Table 3.
Dominant salps and doliolids observed during the study period and the conditions under which they were observed. Delta (Δ) values represent the difference between the maximum and minimum temperature and salinity, which indicates the adaptability of dominant species to environmental changes. Refer to Figure 5 for the raw data.
3.5. Relationship between Chlorophyll a Concentrations and the Abundance of Salps and Doliolids
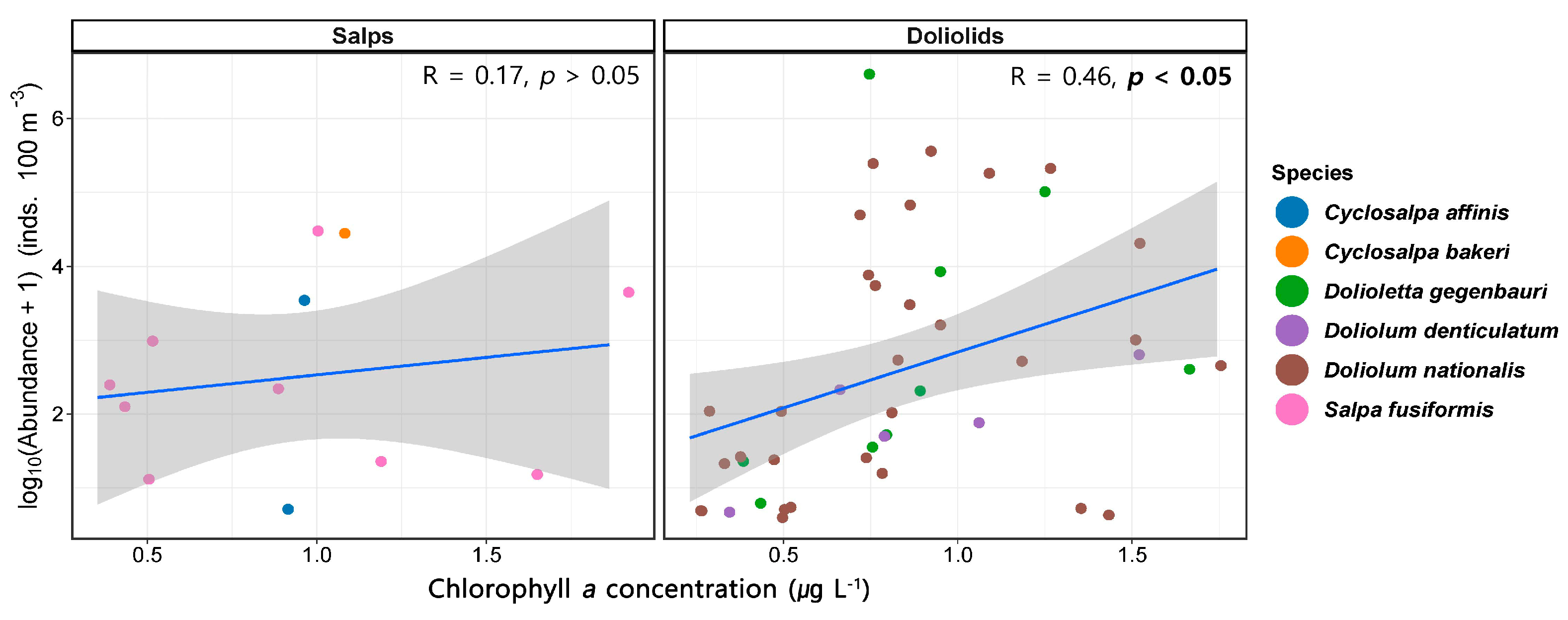
Linear regression analysis was conducted to investigate the relationship between the abundance of salps and doliolids and Chl-a concentrations in the northeastern East China Sea. Our analysis sought to determine whether the quantitative variation in the salp and doliolid abundance was influenced by prey availability, as represented by Chl-a levels. In this study, the linear relationship between salps and doliolids and Chl-a concentrations at the species level was not considered due to the low correlations and high p-values. The linear relationship between the abundance of salps and doliolids and the Chl-a concentrations is presented in Figure 8. Salps had no correlation with Chl-a (R = 0.17, p > 0.05), whereas doliolids exhibited a positive correlation (R = 0.46, p < 0.05).
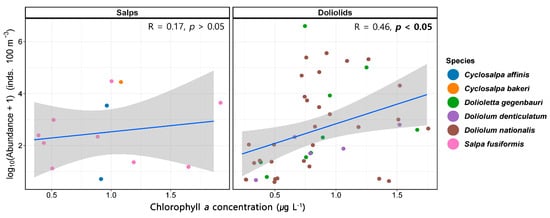
Figure 8.
Relationship between the abundance of salps and doliolids and Chlorophyll a (Chl-a) concentrations.
3.6. Factors Determining the Year-to-Year Variation in Dominant Species in Spring and Autumn
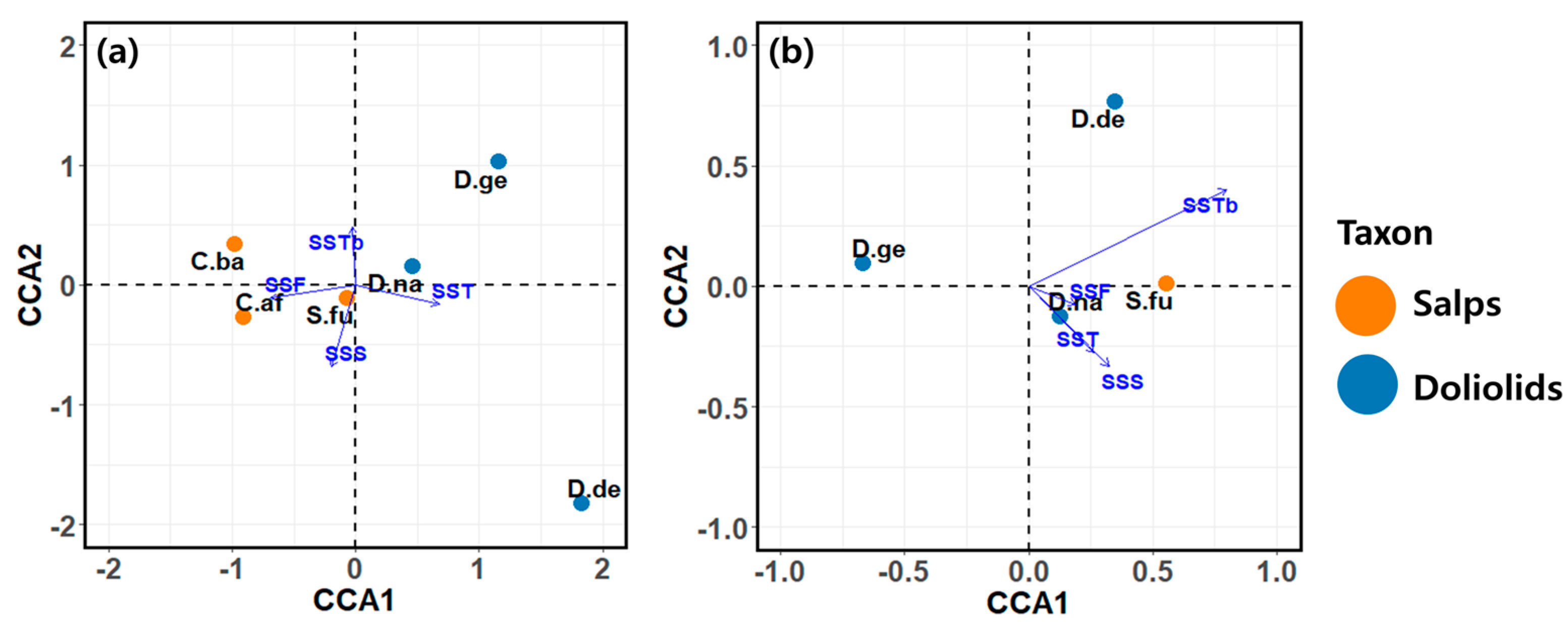
CCA was conducted to explore the environmental factors influencing the variability of dominant species populations in spring and autumn (Figure 9 and Table 4). In spring, the CCA results explained 60.4% and 30.5% of the contributions to the total data on the first and second axes, respectively, and accounted for 90.9% of the variation in the abundance of the recorded species (Figure 9a and Table 4). A notable feature of this result is the distinction between salps and doliolids based on the first axis. The dominant species in the first quadrant included Dolioletta gegenbauri and D. nationalis. The environmental factors positively affecting these species were SSTb and SST, while SSF and SSS had a negative impact. C. bakeri was the dominant species in the second quadrant. The factors that positively affected the abundance of this species were SSTb and SSF, whereas SST had a negative effect. C. affinis and S. fusiformis were the dominant species in the third quadrant and were positively affected by SSF and SSS. However, for C. affinis, the SST was found to have a negative effect. The dominant species located in the fourth quadrant was D. denticulatum, with the positive factor for this species being the SST.
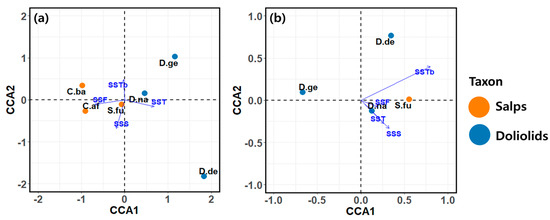
Figure 9.
Ordination plot of canonical correspondence analysis (CCA) showing the variations in the dominant species in response to physicochemical and biological factors in (a) spring and (b) autumn. C.af, Cyclosalpa affinis; C.ba, C. bakeri; D.ge, Dolioletta gegenbauri; D.de, D. denticulatum; D.na, Doliolum nationalis; S.fu, Salpa fusiformis; SST, sea surface temperature; SSS, sea surface salinity; SSF, sea surface fluorescence; SSTb, sea surface turbidity.

Table 4.
Summary of canonical correspondence analysis (CCA) results for salp and doliolid abundance vs. an environmental factor matrix. CCA1 and CCA2 represent that the eigenvalue and cumulative proportion of the variance of the relationships between the species and the environmental factors. These axes provide insights into how much of the total variance in the species abundance can be explained by environmental factors.
In autumn, the CCA results explained 59.1% and 31.6% of the contributions to the total data on the first and second axes, respectively, and accounted for 90.7% of the variation in the abundances of the dominant species (Figure 9b and Table 4). Unlike spring, the CCA results for autumn did not exhibit distinct differences at the species or family level between salps and doliolids based on the first and second axes. The dominant species in the first quadrant included D. denticulatum and S. fusiformis; D. denticulatum had a strong positive correlation with SSTb, while S. fusiformis had positive correlations with SSTb and SSF. The dominant species Dolioletta gegenbauri in the second quadrant exhibited negative correlations with SSF, SST, and SSS, while it had no correlation with SSTb. The dominant species D. nationalis in the fourth quadrant was positively correlated with all of the environmental factors (i.e., SSTb, SST, SSS, and SSF).
4. Discussion
During the survey period, a T-S diagram was generated to identify the water masses influencing the northeastern East China Sea during spring and autumn (Figure 3). Previous studies on the water masses affecting the study area have indicated that CW, CDW, TWCS, TWCB, and YSCBW mainly influence the region in spring, whereas CW, TWCS, and TWCB are the primary influencers in autumn [23,24,25,26]. However, in the present study, the effects of CW and CDW were not observed during the spring period, though the influence of TWCB was dominant throughout the survey period. Additionally, some effects of YSCBW were only observed in 2022. In contrast, during autumn, the effects of CW/CDW (characterized by high temperature and low-salinity waters) were observed only in 2019 and 2021, whereas the influence of YSCBW was not observed at all during the survey period.
During the autumn period, the observed SST and SSS were higher than in spring (Figure 2; both p < 0.05). Our spring observations revealed a general decrease in the mean SST and mean SSS over the study period, whereas our autumn observations revealed a decrease in the mean SST and an increase in the mean SSS (Figure 2). These differences can be attributed to the timing of the data collection. The surveys in autumn were conducted in September in 2019 and 2021, whereas those in 2020 and 2022 were conducted in October. These discrepancies in the seasonal trends were investigated further by reviewing data from the KODC (Figure S1). This analysis confirmed a consistent trend of a decreasing mean temperature in the western northeastern East China Sea from 2019 to 2023, aligning with our observations. Despite potential variation in the types and intensity of the water masses impacting the northeastern East China Sea, the species records for each season remained relatively consistent, facilitating their grouping based on the survey time.
Interestingly, the influence of TWC (including surface and bottom waters) was identified in all years during the autumn period. Studies conducted in this region, including the study area, have reported that currents such as CDW, TWC, and YSCBW influence the region, with the effects of CDW and TWC increasing from spring to autumn (Figure 3; [23,24,25,26]). The impact of CW or CDW on the northeastern East China Sea can vary depending on environmental factors such as typhoons, wind, and the discharge volume of the Changjiang River [39]. After the summer monsoon period (in September), this region can be significantly affected by high temperatures and low salinities [40,41]. The influence of CDW may weaken due to the mixing and movement of water masses, while the dominance of TWC may strengthen over time [23,42].
During the survey period, the species identified in the northeastern East China Sea were similar to those found in the neighboring waters of Northeast Asia (Table 5). The list of salps and doliolids from countries neighboring South Korea includes a total of 30 species across 13 genera, including 13 species identified in our study [3,15,16,43,44,45,46]. Particularly, we confirmed that doliolid species such as Dolioletta gegenbauri, Doliolum denticulatum, and Doliolum nationalis and salp species such as Cyclosalpa affinis, Salpa fusiformis, Thalia cicar, T. orientalis, and T. sibogae are commonly distributed around South Korea, China, Japan, and Taiwan (Table 5).

Table 5.
List of salp and doliolid species observed in Northeast Asia. Black circles and empty squares indicate species occurrences in the previous studies and not occurred species, respectively.
According to our findings, salp species (S. fusiformis, C. affinis, and C. bakeri) were dominant in spring, whereas doliolid species (D. denticulatum, D. nationalis, and Dolioletta gegenbauri) were dominant in autumn (Figure 4, Figure 5 and Figure 6). Previous research by Ishak et al. [16] raised the question of whether the spatial distribution of salps and doliolids is influenced by marine environmental conditions. Xu et al. [44] also noted that the dominance of salps and doliolids tends to vary within the East China Sea.
Previous studies have reported spatial differentiation between salps and doliolids, with salps generally observed in offshore regions and doliolids primarily found near coastal regions [3,10]. Additionally, the shift between a salp- and doliolid-dominant community structure appears to be influenced by marine conditions such as the temperature and/or salinity, which can also lead to differences in spatial distribution patterns [15]. However, our study revealed that, although the dominant species in spring differed from those in autumn, salps and doliolids as taxonomic groups were spatially indistinguishable from each other over the same period (Figure 4). This suggests that seasonal trends may be stronger than spatial trends. The seasonal variation in salp and doliolid abundance in Taiwan waters [3,9], the North Yellow Sea [47], and the southern South China Sea [48] was similar to our study. Figure 3 and the results of previous studies suggest that our study region is affected by summer monsoons and CDW/CW. During the low-salinity period in autumn (2019 and 2021), doliolid abundance was higher than salp abundance (Figure 4), indicating that the low salinity was a limiting factor in salp reproduction, allowing doliolids to become more widely distributed at a higher abundance [3,11,49,50,51].
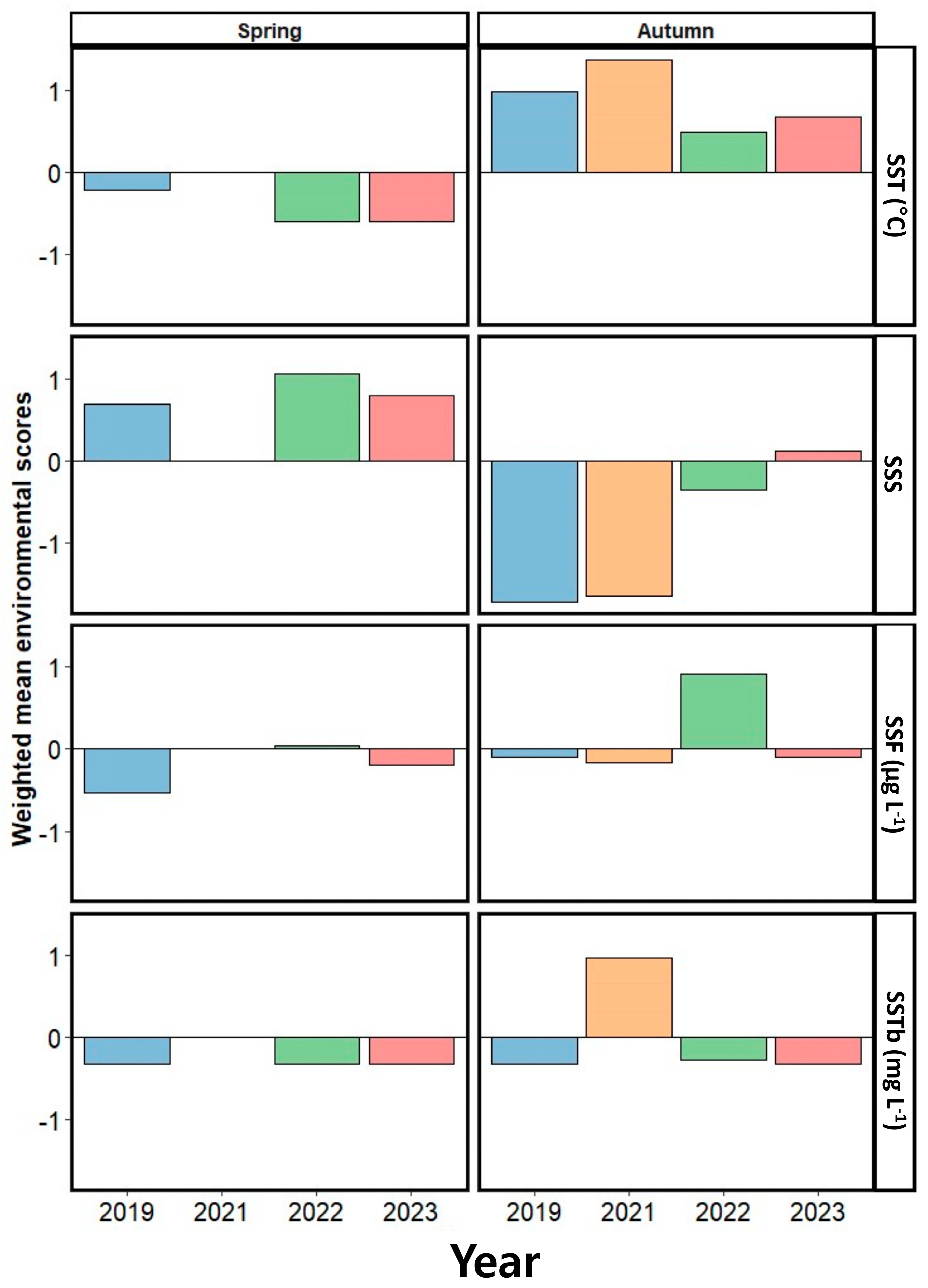
In examining the influence of seasonal factors on the abundance of dominant populations, we observed contrasting trends in the weighted average scores of the environmental factors in spring and autumn, with physical factors such as the SST and SSS having a particularly notable effect (Figure 10). In spring, the primary environmental factor influencing the annual variation in the dominant species abundance was SSS. Initially, SSS and SSS negatively affected species abundance in 2019, while SST had a negative impact in 2022 and 2023. Moreover, SSTb consistently had a negative influence on the variation in the dominant species abundance across the entire study period. In autumn, the SST was the primary positive factor affecting the seasonal variation in dominant species abundance over all of the examined years, with SSS having a negative influence (excluding 2023). SSF had a negative impact on the variation in the abundance of the dominant species in 2019, 2021, and 2023, whereas it had a strong positive impact in 2022. SSTb exhibited a negative impact on dominant species abundance in 2019, 2022, and 2023, while it had a strong positive impact in 2021.
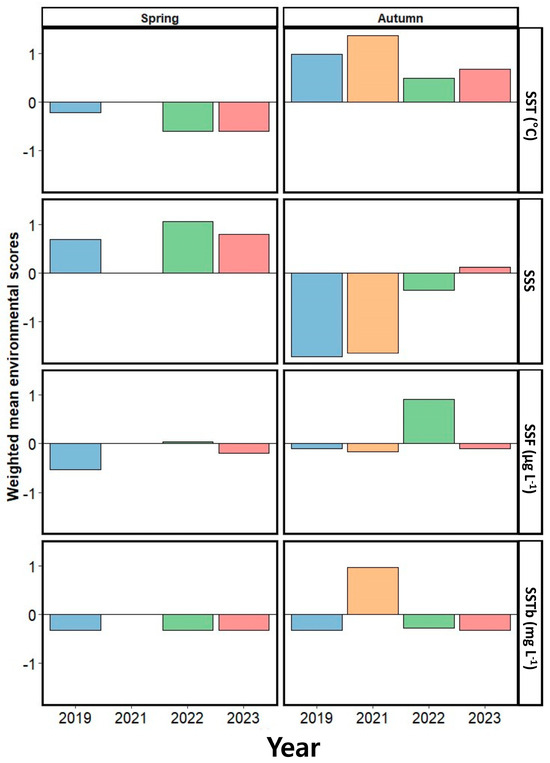
Figure 10.
Weighted mean scores of the examined environmental factors, representing the degree to which they affect the year-to-year variation in the abundance of dominant species in spring and autumn. SST, sea surface temperature; SSS, sea surface salinity; SSF, sea surface fluorescence; SSTb, sea surface turbidity.
The six dominant salp and doliolid species identified in this study were found at temperatures ranging from 10.0 to 30.0 °C and salinities from 31.00 to 34.50 (Figure 7). However, the optimal temperature and salinity for asexual reproduction vary between species. Among salps, the highest abundance of S. fusiformis was recorded at temperatures of 15.8 to 15.9 °C and salinities of 34.00 to 34.12, indicating a weak positive correlation with salinity (Figure 7 and Figure 9). In Taiwan, S. fusiformis did not occur in summer, but was abundant in winter (28.96 ± 0.17 °C), with an abundance of 480 ± 250 inds. 100 m−3 [3]. In Japan, S. fusiformis was reported in low-temperature low-salinity environments (10.0 to 15.0 °C, 32.50 to 33.50) with an abundance of 4 to 145 inds. 100 m−3, suggesting that it can inhabit a broad range of temperatures and salinities, although it tends to avoid high temperatures [16]. The asexual reproduction of salps has been linked to Chl-a concentrations [22,52,53]. In particular, the negative correlation between Chl-a concentrations and the occurrence of salp blooms suggests that the proliferation of salps relies on phytoplankton as a source of food. However, our findings did not identify a positive correlation between S. fusiformis and Chl-a (Figure 8 and Figure 9). Rather, C. affinis and C. bakeri had a negative correlation with the SST and a positive correlation with SSF (Figure 9). The absence of a correlation between the environmental factors and S. fusiformis abundance could be attributed to its widespread distribution within the study area.
Doliolids thrive at lower temperatures and salinities than salps, and are known to be more abundant along the coast than offshore. For example, D. nationalis exhibited a peak abundance at temperatures of 21.0 to 25.0 °C and salinities of 31.00 to 32.00 (Figure 7). In Taiwan, D. nationalis was only detected in summer, with a high abundance at 22.74 °C [3]. In the present study, the distribution of D. nationalis exhibited no distinct pattern in spring, but significantly expanded towards the coast in autumn, particularly at shallow stations (i.e., with depths less than 50 m) such as SS02, SS11, SS12, and SS15 (Figure 5). During spring, D. nationalis had a positive correlation with the SST and a negative correlation with SSS, whereas in autumn it showed a strong positive correlation with both the SST and SSS (Figure 9). Dolioletta gegenbauri was particularly abundant at temperatures of 20–23 °C and salinities of 31.00–32.00. Similarly, in Jiaozhou Bay, China, it was the most dominant species, occurring in temperatures ranging from 11 to 23 °C and salinities from 29.00 to 32.00 [54]. Dolioletta gegenbauri exhibited peak abundance at temperatures of 18.0 to 23.0 °C and salinities of 31.00 to 33.00, although its abundance was lower compared to other doliolid species in the same season (Figure 7). In contrast, the abundance of D. denticulatum was highest at temperatures of 18.0 to 23.0 °C and salinities of 31.00 to 33.00, although its abundance was lower compared to other doliolid species in the same season (Figure 5 and Figure 7). During spring, D. denticulatum exhibited a positive correlation with both the SST and SSS, while in autumn, it was more strongly influenced by SSTb (Figure 9).
When assessing and predicting the dynamics of marine systems, including physical, chemical, and biological factors, the role of predators in controlling primary productivity is crucial [55]. Salp and doliolid species, which are primary and secondary consumers within marine ecosystems, have unique physiological characteristics that enable them to rapidly reproduce in large numbers, significantly impacting phytoplankton populations. Moreover, the shifts in dominance between crustaceans and gelatinous filter feeders are important indicators that should be considered for long-term marine monitoring [56,57].
5. Conclusions
Based on the findings of our study, we identified distinct seasonal patterns in the abundance and distribution of salps and doliolids in the northeastern East China Sea. During spring, salp species were dominant, while doliolids were more abundant in autumn. Our observations revealed that, although the dominant species differed between seasons, the spatial distribution of salps and doliolids remained consistent within each season. These results suggest that individuals with strong resistance to salinity may be able to reproduce under favorable conditions.
Furthermore, our analysis demonstrates consistent shifts in dominant salp and doliolid communities between spring and autumn, emphasizing the influence of environmental factors. Exploring long-term data in connection with climate change indices (e.g., the Pacific Decadal Oscillation, El Niño–Southern Oscillation, and North Pacific Gyre Oscillation) will be crucial for understanding the broader effects of climate change on the dynamics of these species. Thus, there is a need for further research to investigate the long-term effects of climate change on marine ecosystems in this region.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jmse12060862/s1, Figure S1. Seawater temperature and salinity trends monitored over a five-year period at 10-meter intervals in a neigh-boring survey area. (a) These observations were conducted along neighboring survey lines (203, 204, 205) as part of the NIFS Serial Oceanographic Observation program, during both the (b) spring and (c) autumn seasons. The data was obtained from the KODC database (Korea Oceanographic Data Center; https://www.nifs.go.kr/kodc/eng/index.kodc, accessed on 18 May 2024).
Author Contributions
Conceptualization, H.-U.C., Y.S.J., S.C. and H.Y.S.; Data curation, H.-U.C., Y.S.J. and S.C.; Funding acquisition, H.Y.S.; Investigation, H.-U.C., Y.S.J. and S.C.; Methodology, Y.S.J. and H.Y.S.; Project administration, H.Y.S.; Resources, H.Y.S.; Supervision, H.Y.S.; Validation, Y.S.J. and S.C.; Visualization, Y.S.J. and S.C.; Writing—original draft, H.-U.C., Y.S.J. and H.Y.S.; Writing—review and editing, H.-U.C., Y.S.J., S.C. and H.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Korea (RS-2018-KS181192).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the crew of the T/V Saedongbaek-Ho and the members of the Zooplankton Species Diversity Laboratory at Chonnam National University for their support in the field. We also extend our sincere gratitude to the anonymous reviewers and the editor for their constructive and invaluable suggestions and comments.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Bone, Q.; Braconnot, J.C.; Carré, C.; Ryan, K.P. On the Filter-Feeding of Doliolum (Tunicata: Thaliacea). J. Exp. Mar. Bio. Ecol. 1997, 214, 179–193. [Google Scholar] [CrossRef]
- Hereu, C.M.; Lavaniegos, B.E.; Goericke, R. Grazing Impact of Salp (Tunicata, Thaliacea) Assemblages in the Eastern Tropical North Pacific. J. Plankton Res. 2010, 32, 785–804. [Google Scholar] [CrossRef]
- Liao, Z.H.; Hsieh, H.Y.; Lo, W.T. Influence of Monsoon-Driven Hydrographic Features on Thaliacean Distribution in Waters around Taiwan, Western North Pacific Ocean. Zool. Stud. 2013, 52, 49. [Google Scholar] [CrossRef]
- Loeb, V.J.; Santora, J.A. Population Dynamics of Salpa thompsoni near the Antarctic Peninsula: Growth Rates and Interannual Variations in Reproductive Activity (1993–2009). Prog. Oceanogr. 2012, 96, 93–107. [Google Scholar] [CrossRef]
- Henschke, N.; Everett, J.D.; Richardson, A.J.; Suthers, I.M. Rethinking the Role of Salps in the Ocean. Trends Ecol. Evol. 2016, 31, 720–733. [Google Scholar] [CrossRef]
- Pauli, N.C.; Flintrop, C.M.; Konrad, C.; Pakhomov, E.A.; Swoboda, S.; Koch, F.; Wang, X.L.; Zhang, J.C.; Brierley, A.S.; Bernasconi, M.; et al. Krill and Salp Faecal Pellets Contribute Equally to the Carbon Flux at the Antarctic Peninsula. Nat. Commun. 2021, 12, 7168. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Stamieszkin, K.; Maas, A.E.; Durkin, C.A.; Passow, U.; Estapa, M.L.; Omand, M.M.; McDonnell, A.M.P.; Karp-Boss, L.; Galbraith, M.; et al. The Outsized Role of Salps in Carbon Export in the Subarctic Northeast Pacific Ocean. Glob. Biogeochem. Cycles 2023, 37, e2022GB007523. [Google Scholar] [CrossRef]
- Pinchuk, A.I.; Batten, S.D.; Strasburger, W.W. Doliolid (Tunicata, Thaliacea) Blooms in the Southeastern Gulf of Alaska as a Result of the Recent Marine Heat Wave of 2014–2016. Front. Mar. Sci. 2021, 8, 625486. [Google Scholar] [CrossRef]
- Tew, K.S.; Lo, W.T. Distribution of Thaliacea in SW Taiwan Coastal Water in 1997, with Special Reference to Doliolum denticulatum, Thalia democratica and T. orientalis. Mar. Ecol. Prog. Ser. 2005, 292, 181–193. [Google Scholar] [CrossRef]
- Mianzan, H.; Pájaro, M.; Alvarez Colombo, G.; Madirolas, A. Feeding on Survival-Food: Gelatinous Plankton as a Source of Food for Anchovies. Hydrobiologia 2001, 451, 45–53. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Siegel, V.; Litvinov, F.; Loeb, V.; Watkins, J. Salp Distribution and Size Composition in the Atlantic Sector of the Southern Ocean. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2004, 51, 1369–1381. [Google Scholar] [CrossRef]
- Lüskow, F.; Galbraith, M.D.; Kwong, L.E.; Pakhomov, E.A. Biology and Distribution of Salps in the Subarctic Northeast Pacific. Mar. Biol. 2022, 169, 84. [Google Scholar] [CrossRef]
- Fender, C.K.; Décima, M.; Gutiérrez-Rodríguez, A.; Selph, K.E.; Yingling, N.; Stukel, M.R. Prey Size Spectra and Predator to Prey Size Ratios of Southern Ocean Salps. Mar. Biol. 2023, 170, 40. [Google Scholar] [CrossRef]
- Kim, S.W.; Won, J.H.; Lee, J.H.; Kim, C.B. A New Record of Ihlea punctata and Redescription of Salpa fusiformis (Salpida: Salpidae) in Korean Waters. Anim. Syst. Evol. Divers. 2010, 26, 353–356. [Google Scholar] [CrossRef]
- Ishak, N.H.A.; Tadokoro, K.; Okazaki, Y.; Kakehi, S.; Suyama, S.; Takahashi, K. Distribution, Biomass, and Species Composition of Salps and Doliolids in the Oyashio–Kuroshio Transitional Region: Potential Impact of Massive Bloom on the Pelagic Food Web. J. Oceanogr. 2020, 76, 351–363. [Google Scholar] [CrossRef]
- Ishak, N.H.A.; Motoki, K.; Miyamoto, H.; Fuji, T.; Taniuchi, Y.; Kakehi, S.; Kuroda, H.; Setou, T.; Takahashi, K. Basin-Scale Distribution of Salps and Doliolids in the Transition Region of the North Pacific Ocean in Summer: Drivers of Bloom Occurrence and Effect on the Pelagic Ecosystem. Prog. Oceanogr. 2022, 204, 102793. [Google Scholar] [CrossRef]
- Chae, J.; Choi, H.W.; Lee, W.J.; Kim, D.; Lee, J.H. Distribution of a Pelagic Tunicate, Salpa fusiformis in Warm Surface Current of the Eastern Korean Waters and Its Impingement on Cooling Water Intakes of Uljin Nuclear Power Plant. J. Environ. Biol. 2008, 29, 585–590. [Google Scholar]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K.; Leone, A.; Piraino, S. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2021, 29, 242–259. [Google Scholar] [CrossRef]
- Yoon, W.; Choi, B.-J.; Yoo, H.; Kim, B.; Bok, Y.; Chae, J. Unusual Mass Appearance of Salpa fusiformis (Thaliacea: Salpida) in Early Spring at a Nuclear Power Plant at Mid-Western Boundary of the East Sea. Ocean Sci. J. 2022, 57, 269–278. [Google Scholar] [CrossRef]
- Pitt, K.A.; Haberlin, D.; Stantic, B.; Doyle, T.K. Mitigating and Managing the Impacts of Gelatinous Zooplankton on Finfish Aquaculture. Aquaculture 2024, 581, 740403. [Google Scholar] [CrossRef]
- Kang, Y.S.; Jo, Y.J.; Go, W.; Kim, S.S.; Jeon, K.A.; Oh, H.J. Swarm of Salps (Tunicata: Thaliaca) and Its Impact on Marine Ecosystem in the South Sea of Korea. J. Korean Soc. Oceanogr. 2000, 5, 47–58. [Google Scholar]
- Kang, H.K.; Kim, G.; Kang, J.H.; Kim, M.; Noh, J.H. Mass Occurrence of the Salp Salpa fusiformis during Spring 2017 in the Southern Waters of Korea and the Northern East China Sea. Ocean Polar Res. 2019, 41, 135–145. [Google Scholar]
- Lim, D.B. The Movements of the Waters off the South Coast of Korea. J. Korean Soc. Oceanogr. 1976, 11, 77–88. [Google Scholar]
- Lie, H.J. A Note on Water Masses and General Circulation in the Yellow Sea (Hwanghae). J. Korean Soc. Oceanogr. 1984, 19, 187–194. [Google Scholar]
- Hur, H.B.; Jacobs, G.A.; Teague, W.J. Monthly Variations of Water Masses in the Yellow and East China Seas, November 6, 1998. J. Oceanogr. 1999, 55, 171–184. [Google Scholar] [CrossRef]
- Choi, Y. The Characteristics of Yellow Sea Bottom Cold Water in September, 2006. J. Korean Soc. Fish. Mar. Edu. 2011, 23, 425–432. [Google Scholar]
- Baek, S.H.; Lee, M.; Park, B.S.; Lim, Y.K. Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters. Sustain. Sci. Pract. Policy 2020, 12, 2781. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.J.; Kang, H.Y.; Kwon, K.Y.; Lee, W.C.; Kwak, J.H. Seasonal Variations in Primary Productivity and Biomass of Phytoplankton in Geoje-Hansan Bay on the Southern Coast of Korea. Ocean Sci. J. 2019, 54, 213–227. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.; Lim, Y.K.; Kim, Y.J.; Baek, S.H. Occurrence Characteristics of Harmful and Non-Harmful Algal Species Related to Coastal Environments in the Southern Sea of Korea. Mar. Freshw. Res. 2019, 70, 794. [Google Scholar] [CrossRef]
- Lim, Y.K.; Baek, S.H.; Lee, M.; Kim, Y.O.; Choi, K.-H.; Kim, J.H. Phytoplankton Composition Associated with Physical and Chemical Variables during Summer in the Southern Sea of Korea: Implication of the Succession of the Two Toxic Dinoflagellates Cochlodinium (a.k.a. Margalefidinium) polykrikoides and Alexandrium affine. J. Exp. Mar. Bio. Ecol. 2019, 516, 51–66. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, D.H. Coastal Warming Heightens Direct Impacts of Seawater Temperature on Nutrients near Aquaculture Farms in Korea. Sci. Total Environ. 2023, 892, 164643. [Google Scholar] [CrossRef] [PubMed]
- Chihara, M.; Murano, M. An Illustrated Guide to Marine Plankton in Japan; Tokai University Press: Tokyo, Japan, 1997; p. 1574. (In Japanese) [Google Scholar]
- Apablaza, P.; Palma, S. First Record of Dolioletta gegenbauri (Uljanin, 1884) and Doliolum nationalis Borgert, 1893 for Chilean Waters (Tunicata, Doliolida). Investig. Mar. 2005, 33, 127–130. [Google Scholar] [CrossRef]
- Kim, S.W.; Won, J.-H.; Kim, C.-B. Report for Eight Species of Salpinae (Thaliacea: Salpida: Salpidae) from Korean Waters. J. Asia Pac. Biodivers. 2017, 10, 453–459. [Google Scholar] [CrossRef]
- Ishak, N.H.B.A.; Adam, N.A.B.; Kassim, Z. A Taxonomic Revision of the Genus Thalia Blumenbach, 1798; Weelia Yount, 1954; Brooksia Metcalf, 1918 (Salpida: Salpidae) from East Coast of Peninsular Malaysia. Zootaxa 2018, 4422, 451–477. [Google Scholar] [CrossRef] [PubMed]
- WoRMS Editorial Board WoRMS Editorial Board. Available online: https://www.marinespecies.org (accessed on 2 April 2024).
- Seo, S.Y.; Kim, S.W.; Won, J.H. Two New Records of Thaliacea (Chordata: Tunicata) in Korea. Anim. Syst. Evol. Diversity 2023, 39, 10–15. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Lim, D.; Cho, Y.G.; Jeong, D.; Kim, J.; Xu, Z.; Chang, T. A Comprehensive Investigation of Coastal and Shelf Sediment Sources in the South Sea of Korea: A Marginal Sea of the Northwestern Pacific. Ocean Sci. J. 2023, 58, 28. [Google Scholar] [CrossRef]
- Jung, S.W.; Park, J.G.; Jeong, D.H.; Lim, D. Seasonal Changes in Water Masses and Phytoplankton Communities in the Western Part of South Coastal Waters, Korea. Korean J. Environ. Biol. 2012, 30, 328–338. [Google Scholar]
- Yoon, Y.H. Characteristics on Spatial Distributions of Phytoplankton Communities in Relation to Water Masses in the Western South Sea, Korea in Early Autumn 2021. Korean J. Environ. Biol. 2021, 39, 559–572. [Google Scholar] [CrossRef]
- Moon, J.H.; Pang, I.C.; Yoon, J.H. Response of the Changjiang Diluted Water around Jeju Island to External Forcings: A Modeling Study of 2002 and 2006. Cont. Shelf Res. 2009, 29, 1549–1564. [Google Scholar] [CrossRef]
- Franco, P.; Dahms, H.U.; Lo, W.T.; Hwang, J.-S. Pelagic Tunicates in the China Seas. J. Nat. Hist. 2017, 51, 917–936. [Google Scholar] [CrossRef]
- Xu, Z.L.; Lin, M.; Zhang, J. Changes in Dominant Species of Thaliacea in the East China Sea. Acta Zool. Sin. 2006, 52, 53–62. [Google Scholar]
- Kim, S.W.; Won, J.H.; Kim, C.B. Taxonomic Study of the Genus Thalia (Thaliacea: Salpida: Salpidae) from Korea. Anim. Syst. Evol. Divers. 2011, 27, 142–150. [Google Scholar] [CrossRef][Green Version]
- Li, K.; Yin, J.; Huang, L.; Zhang, J.; Lian, S.; Liu, C. Distribution and Abundance of Thaliaceans in the Northwest Continental Shelf of South China Sea, with Response to Environmental Factors Driven by Monsoon. Cont. Shelf Res. 2011, 31, 979–989. [Google Scholar] [CrossRef]
- Franco, P.; Chen, H.; Liu, G. Distribution and Abundance of Pelagic Tunicates in the North Yellow Sea. J. Ocean Univ. China 2014, 13, 782–790. [Google Scholar] [CrossRef]
- Kamaruddin, N.A.; Ishak, N.H.A. Distribution and Diversity of Gelatinous Zooplankton in the Southern South China Sea. IOP Conf. Ser. Earth Environ. Sci. 2021, 944, 012019. [Google Scholar] [CrossRef]
- Daponte, M.C.; Capitanio, F.L.; Esnal, G.B. A Mechanism for Swarming in the Tunicate Salpa thompsoni (Foxton, 1961). Antarct. Sci. 2001, 13, 240–245. [Google Scholar] [CrossRef]
- Gibson, D.M.; Paffenhöfer, G.A. Asexual Reproduction of the Doliolid, Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). J. Plankton Res. 2002, 24, 703–712. [Google Scholar] [CrossRef]
- Deibel, D.; Paffenhöfer, G.A. Predictability of Patches of Neritic Salps and Doliolids (Tunicata, Thaliacea). J. Plankton Res. 2009, 31, 1571–1579. [Google Scholar] [CrossRef]
- Zeldis, J.R.; Davis, C.S.; James, M.R.; Ballara, S.L.; Booth, W.E.; Chang, F.H. Salp Grazing: Effects on Phytoplankton Abundance, Vertical Distribution and Taxonomic Composition in a Coastal Habitat. Mar. Ecol. Prog. Ser. 1995, 126, 267–283. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, S.; Zhang, G. Seasonal Variation in Abundance, Diel Vertical Migration and Body Size of Pelagic Tunicate Salpa fusiformis in the Southern Yellow Sea. Chin. J. Oceanol. Limnol. 2012, 30, 92–104. [Google Scholar] [CrossRef]
- Wang, S.; Wan, A.; Zhang, G.; Sun, S. Northward Expansion of a Warm-Water Doliolid Dolioletta gegenbauri (Uljanin, 1884) into a Temperate Bay, China. Water 2022, 14, 1685. [Google Scholar] [CrossRef]
- Richardson, A.J.; Schoeman, D.S. Climate Impact on Plankton Ecosystems in the Northeast Atlantic. Science 2004, 305, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Choo, S.; Soh, H.Y. Influence of Rainfall Events on Zooplankton Community Characteristics and Feeding Habits in Estuarine–Coastal Environments. Front. Mar. Sci. 2022, 9, 950695. [Google Scholar] [CrossRef]
- Choo, S.; Kwak, M.T.; Cho, Y.K.; Yoon, Y.H.; Soh, H.Y. Effects of Water Masses on the Zooplankton Community Structure in the Northern East China Sea during the East Asian Summer Monsoon in 2020. Ecol. Indic. 2023, 154, 110847. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).