A Pilot Study on the Diel Vertical Migration Pattern of Mesopelagic Fishes in the Southern and Central South China Sea
Abstract
1. Introduction
2. Materials and Methods
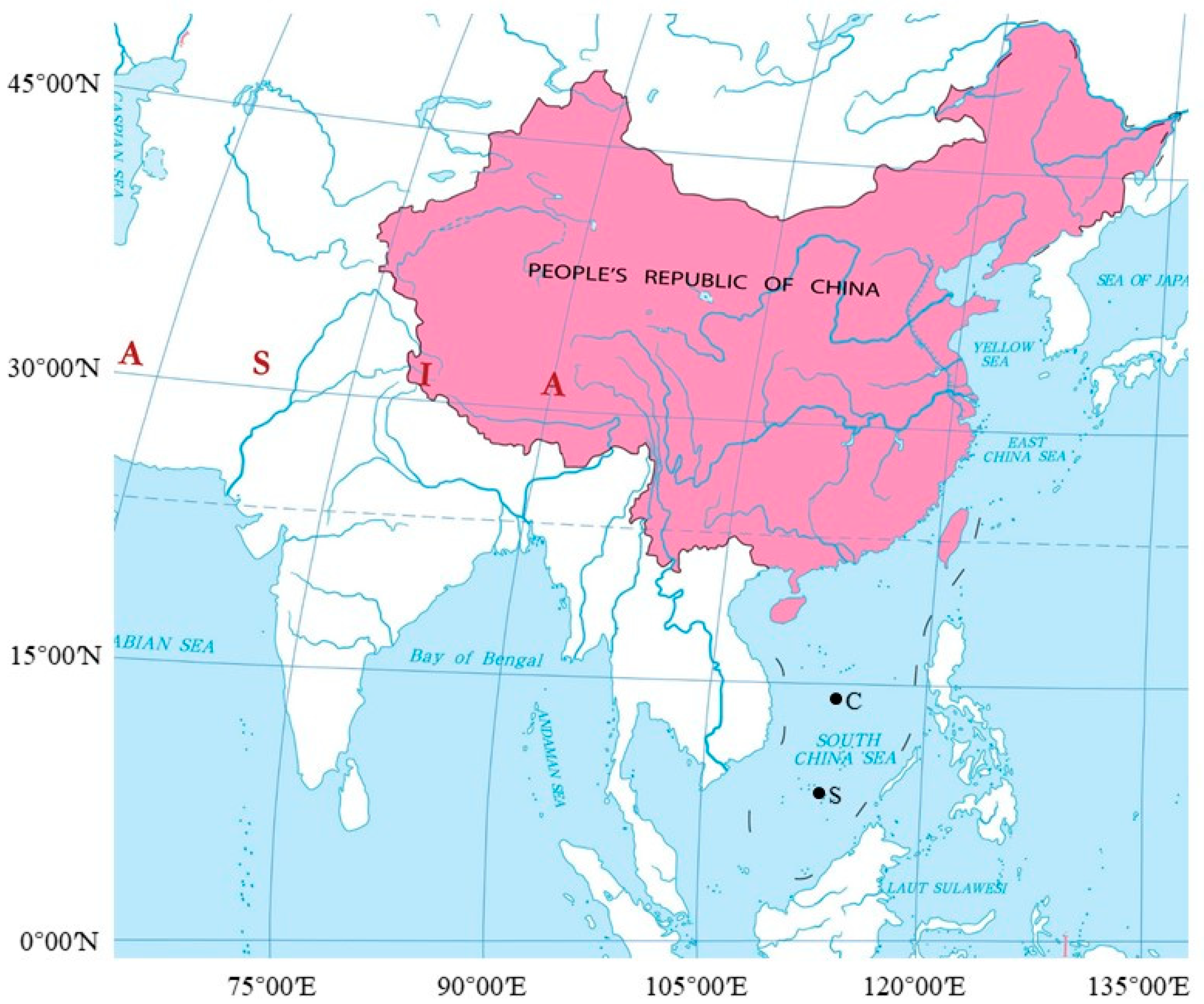
2.1. Survey Design
2.2. Data Collection
2.3. Acoustic Data Analysis
2.4. Vertical Distribution and Diel Vertical Migration
2.5. Diel Vertical Migration Patterns
3. Results
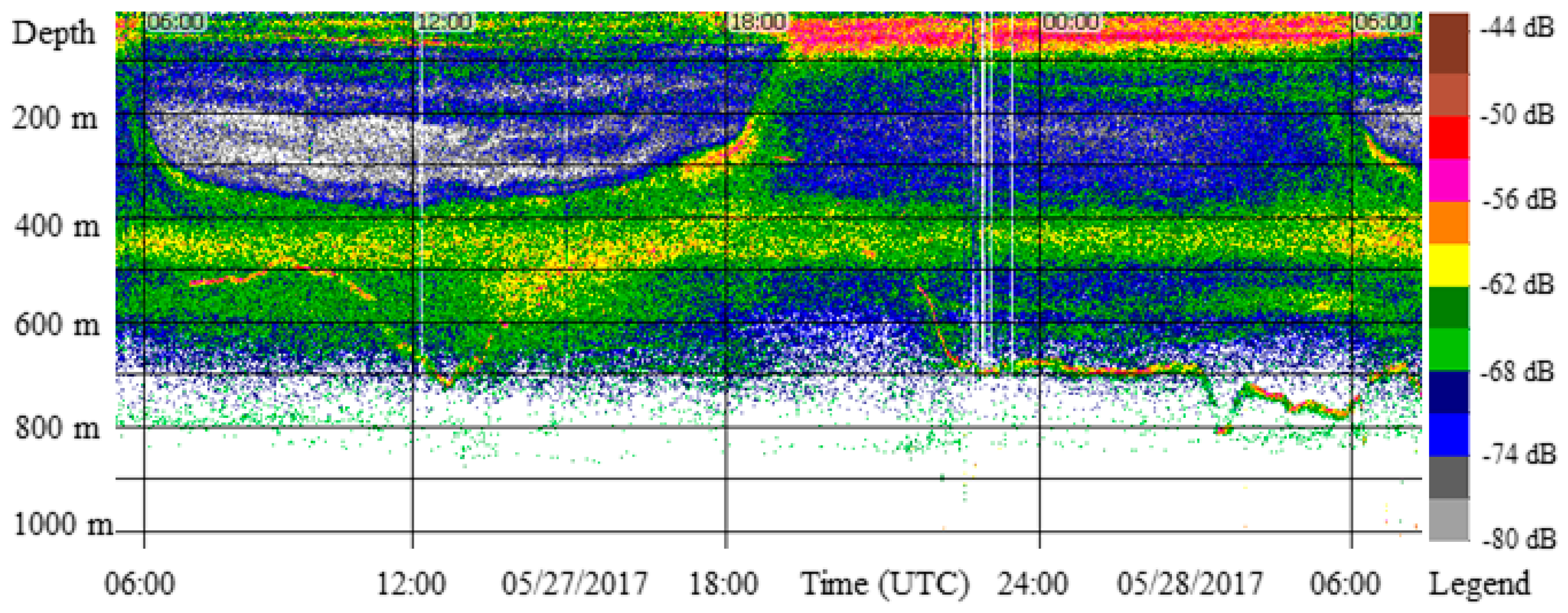
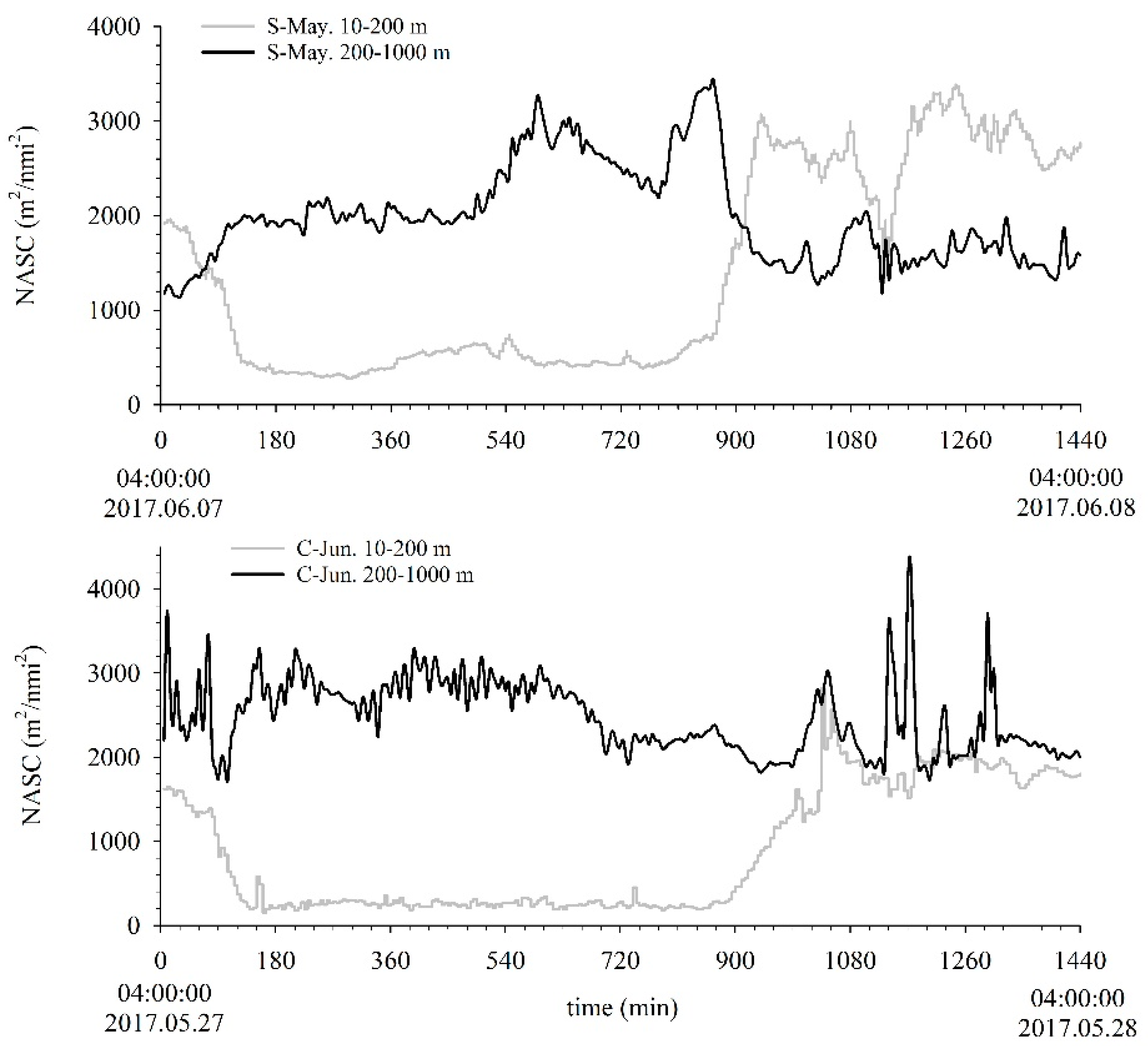
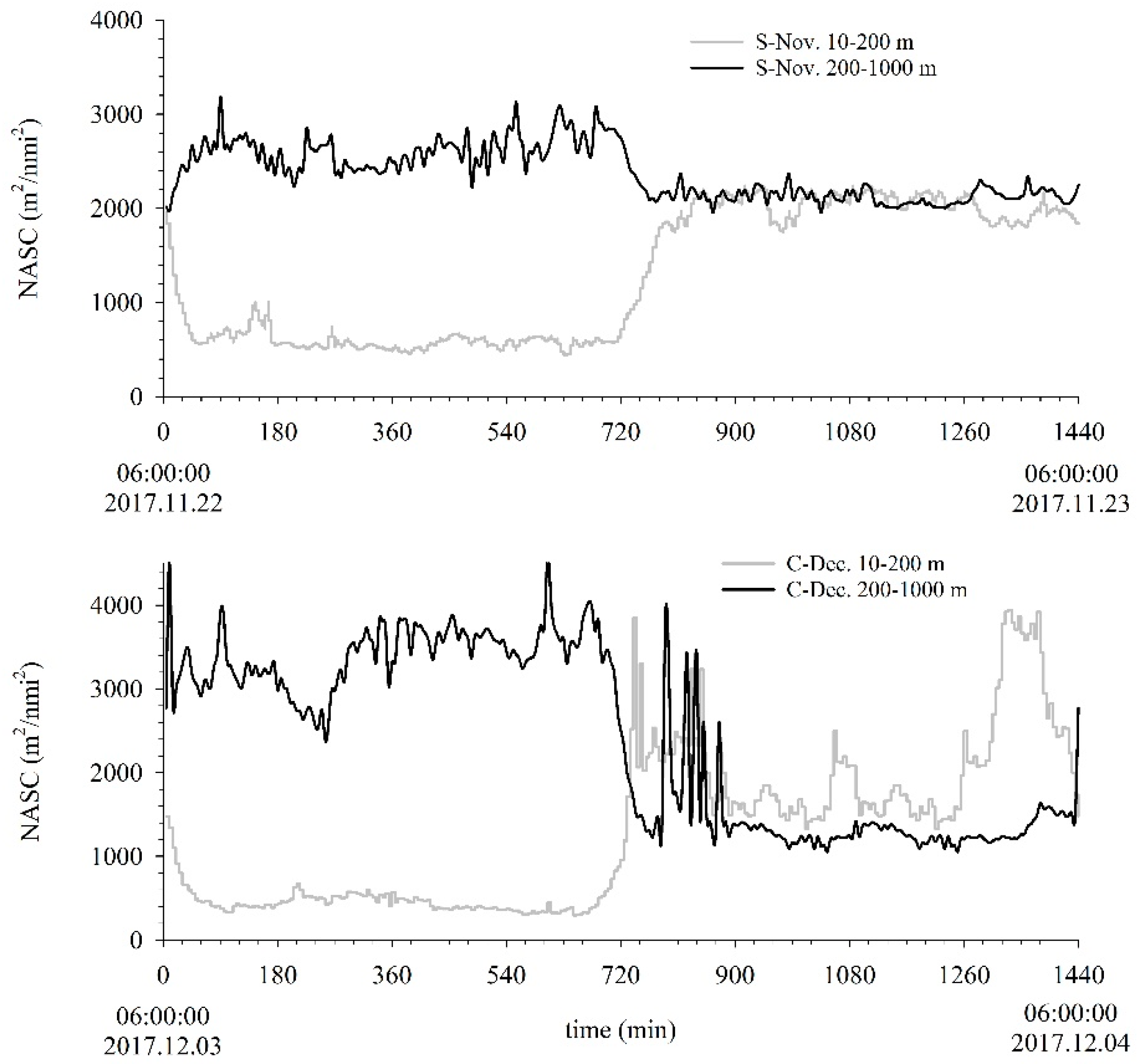
3.1. Migration Process
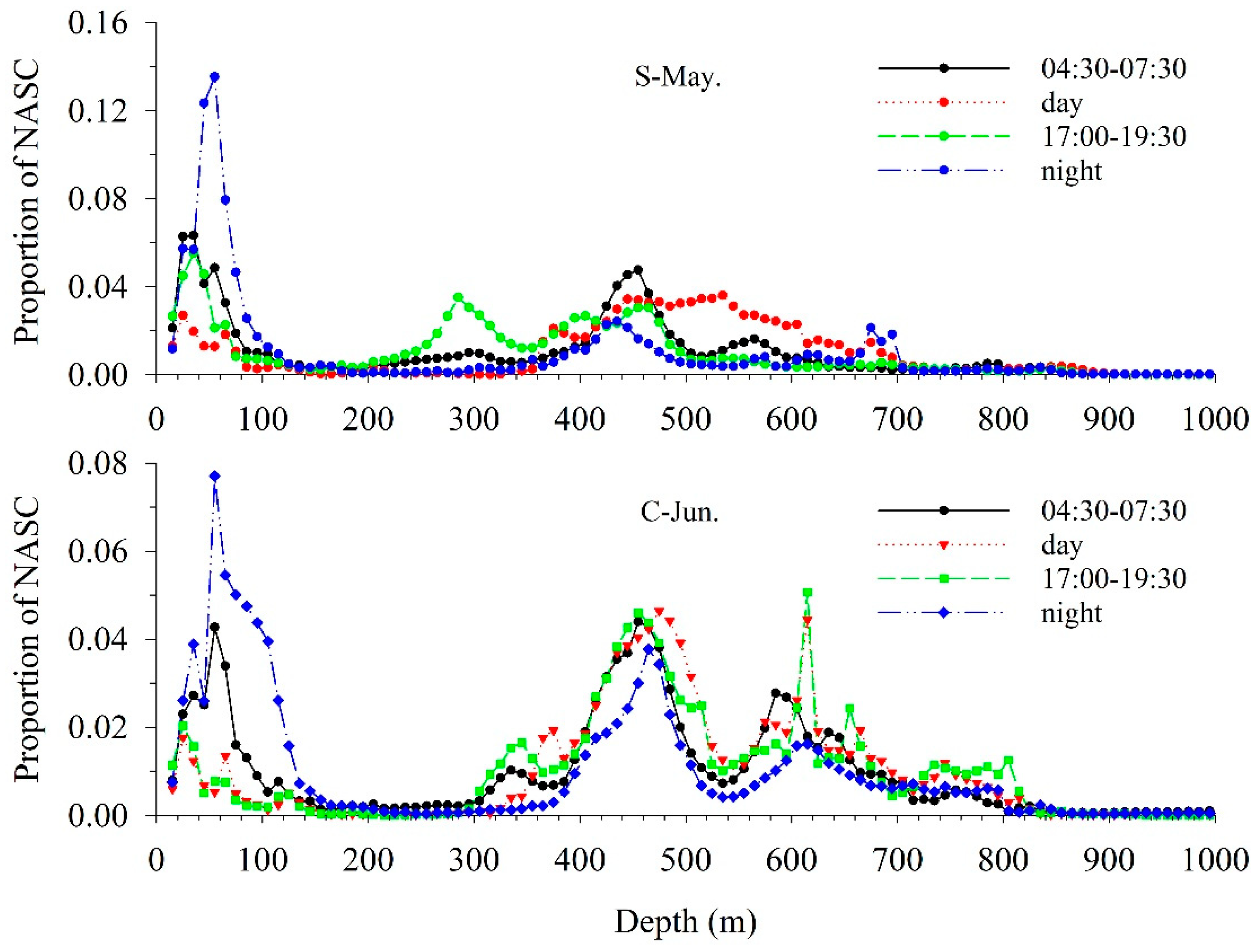
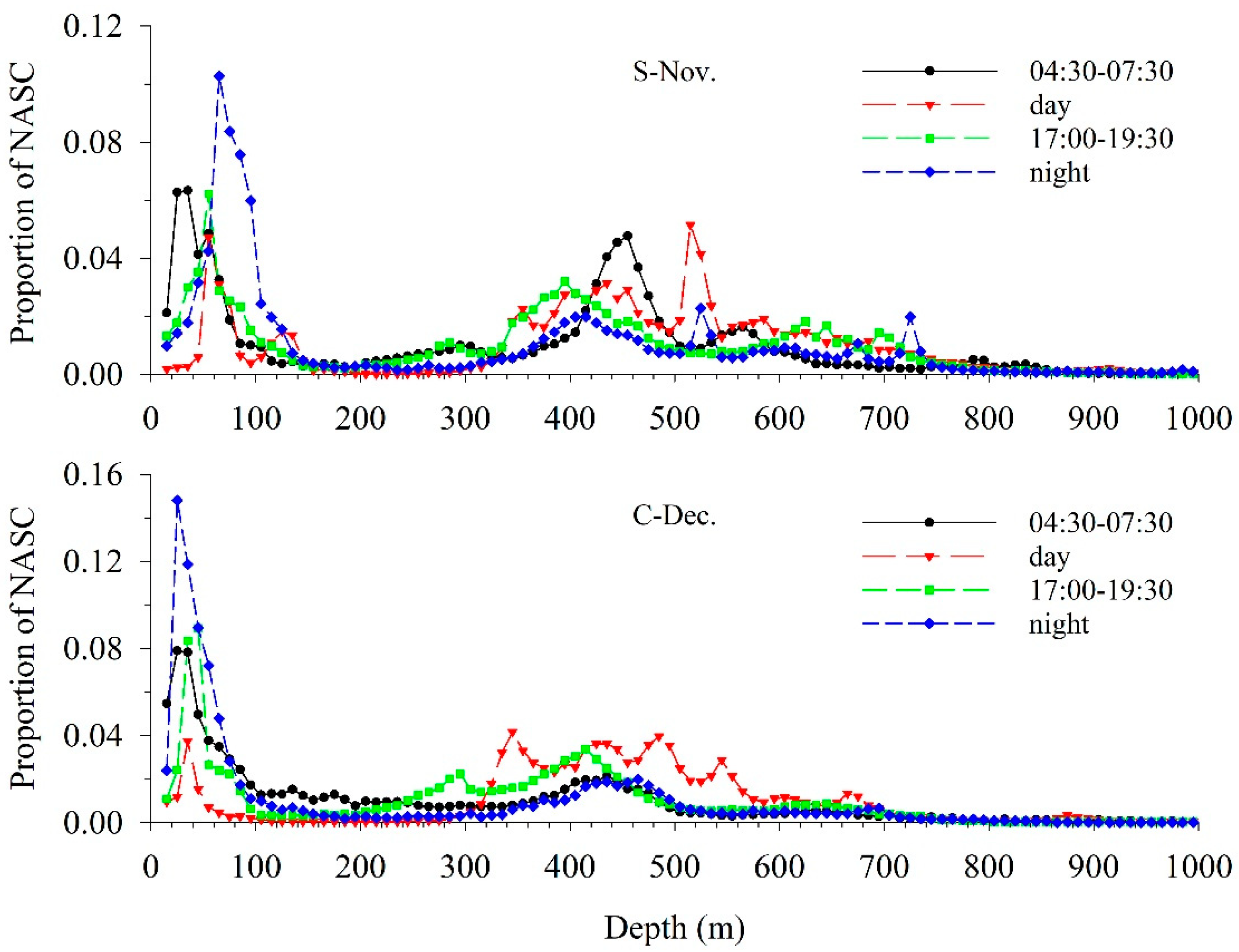
3.2. Vertical Distribution
3.3. Acoustic Migration Proportion
3.4. Diel Vertical Migration Species
4. Discussion
4.1. Diel Vertical Distribution
4.2. Diel Vertical Migration Pattern
4.3. Influencing Factors of DVM
4.3.1. Biological Factors
4.3.2. Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barham, E.G. Deep scattering layer migration and composition: Observations from a diving saucer. Science 1966, 151, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Snoeijs-Leijonmalm, P.; Flores, H.; Sakinan, S.; Hildebrandt, N.; Svenson, A.; Castellani, G.; Vane, K.; Mark, F.C.; Heuzé, C.; Tippenhauer, S.; et al. Unexpected fish and squid in the central Arctic deep scattering layer. Sci. Adv. 2022, 8, eabj7536. [Google Scholar] [CrossRef] [PubMed]
- Jakob, G. Mesopelagic fish, a large potential resource in the Arabian Sea. Deep Sea Res. Part A 1984, 31, 1019–1035. [Google Scholar]
- Salvanes, A.G.V.; Kristoffersen, J.B. Mesopelagic Fishes. In Encyclopedia of Ocean Sciences; Academic Press: Cambridge, MA, USA, 2001; Volume 3, pp. 1711–1717. [Google Scholar] [CrossRef]
- Olivar, M.P.; Bernal, A.; Molí, B.; Pena, M.; Balbín, R.; Castellón, A.; Miquel, J.; Massutí, E. Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep Sea Res. Part A 2012, 62, 53–69. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.L.; Jiang, Y.N.; Chen, Z.Z.; Zhao, X.Y.; Gong, Y.Y.; Ying, Y.P.; Li, Z.Y.; Kong, X.L.; Chen, G.B.; et al. Species composition and biomass density of mesopelagic nekton of the South China Sea continental slope. Deep Sea Res. Part II 2019, 167, 105–120. [Google Scholar] [CrossRef]
- Netburn, A.N.; Koslow, J.A. Mesopelagic fish assemblages across oceanic fronts: A comparison of three frontal systems in the southern california current ecosystem. Deep Sea Res. Part I 2018, 134, 80–91. [Google Scholar] [CrossRef]
- Gjøsaeter, J.; Kawaguchi, K. A Review of the World Resources of Mesopelagic Fish; Food & Agriculture Org.: Rome, Italy, 1980. [Google Scholar]
- Irigoien, X.; Klevjer, T.A.; Røstad, A.; Martinez, U.; Boyra, G.; Acuña, J.L.; Bode, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Hernandez-Leon, S.; et al. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 2014, 5, 3271. [Google Scholar] [CrossRef]
- Kaartvedt, S.; Staby, A.; Aksnes, D.L. Efficient trawl avoidance by mesopelagic fishes causes large underestimation of their biomass. Mar. Ecol. Prog. Ser. 2012, 456, 1–6. [Google Scholar] [CrossRef]
- Davison, P.; Lara-Lopez, A.; Koslow, J.A. Mesopelagic fish biomass in the southern California current ecosystem. Deep Sea Res. Part II 2015, 112, 129–142. [Google Scholar] [CrossRef]
- Pauly, D.; Piroddi, C.; Hood, L.; Bailly, N.; Palomares, M.L.D. The Biology of Mesopelagic Fishes and Their Catches (1950–2018) by Commercial and Experimental Fisheries. J. Mar. Sci. Eng. 2021, 9, 1057. [Google Scholar] [CrossRef]
- Bernal, A.; Olivar, M.P.; Maynou, F. Diet and feeding strategies of mesopelagic fishes in the western Mediterranean. Prog. Oceanogr. 2015, 135, 1–17. [Google Scholar] [CrossRef]
- Eduardo, L.N.; Lucena-Frédou, F.; Mincarone, M.M.; Soares, A.; Loc’H, F.L.; Frédou, T.; Ménard, F.; Bertrand, A. Trophic ecology, habitat, and migratory behaviour of the viperfish Chauliodus sloani reveal a key mesopelagic player. Sci. Rep. 2020, 11, 20996. [Google Scholar] [CrossRef] [PubMed]
- Nacari, L.A.; Escribano, R.; Harrod, C.; Oliva, M.E. Combined use of carbon, nitrogen and sulfur stable isotopes reveal trophic structure and connections in deep-sea mesopelagic and demersal fish communities from the Southeastern Pacific Ocean. Deep Sea Res. Part I 2023, 197, 104069. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, J.; Yang, Y.; Jiang, Y.; Zhang, S.; Tian, H.; Chen, Z. Feeding ecology of the short-headed lanternfish Diaphus brachycephalus and Warming’s lanternfish Ceratoscopelus warmingii (Myctophidae) in the South China Sea. J. Fish Biol. 2024, 104, 1308–1325. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, P.; Lavery, A.; Lawson, G. Biogeographic variations in diel vertical migration determined from acoustic backscattering in the northwest Atlantic Ocean. Deep Sea Res. Part I 2022, 193, 103887. [Google Scholar] [CrossRef]
- Bianchi, D.; Mislan, K. Global patterns of diel vertical migration times and velocities from acoustic data. Limnol. Oceanogr. 2016, 61, 353–364. [Google Scholar] [CrossRef]
- Macali, A.; Semenov, A.; Paladini de Mendoza, F.; Dinoi, A.; Bergami, E.; Tiralongo, F. Relative Influence of Environmental Factors on Biodiversity and Behavioural Traits of a Rare Mesopelagic Fish, Trachipterus trachypterus (Gmelin, 1789), in a Continental Shelf Front of the Mediterranean Sea. J. Mar. Sci. Eng. 2020, 8, 581. [Google Scholar] [CrossRef]
- Luo, J.; Ortner, P.B.; Forcucci, D.; Cummings, S.R. Diel vertical migration of zooplankton and mesopelagic fish in the Arabian Sea. Deep Sea Res. Part II 2000, 47, 1451–1473. [Google Scholar] [CrossRef]
- Collins, M.A.; Stowasser, G.; Fielding, S.; Shreeve, R.; Xavier, J.C.; Venables, H.J.; Enderlein, P.; Cherel, Y.; Van de Putte, A. Latitudinal and bathymetric patterns in the distribution and abundance of mesopelagic fish in the Scotia Sea. Deep Sea. Res. Part II 2012, 59, 189–198. [Google Scholar] [CrossRef]
- Saunders, R.A.; Hill, S.L.; Tarling, G.A.; Murphy, E.J. Myctophid Fish (Family Myctophidae) Are Central Consumers in the Food Web of the Scotia Sea (Southern Ocean). Front. Mar. Sci. 2019, 6, 00530. [Google Scholar] [CrossRef]
- Klevjer, T.A.; Torres, D.J.; Kaartvedt, S. Distribution and diel vertical movements of mesopelagic scattering layers in the Red Sea. Mar. Boil. 2012, 159, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Zwolinski, J.; Morais, A.; Marques, V.; Stratoudakis, Y.; Fernandes, P.G. Diel variation in the vertical distribution and schooling behaviour of sardine (Sardina pilchardus) off Portugal. ICES J. Mar. Sci. 2007, 64, 963–972. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.e.; Chen, Z.; Gong, Y.; Chen, G. Preliminary study on the nautical area scattering coefficient and distribution of mesopelagic fish species in the central-southern part of the South China Sea. J. Fish. Sci. China 2017, 24, 120–135. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J. The deep-sea fishes from the ajacent waters of Dongsha Islands in the South China Sea II: Myctophiformes. Trop. Ocean. 1983, 1, 234–255. [Google Scholar]
- Liu, S.; Tang, Y.; Chen, G.; Zhang, J.; Yao, Z.; Guo, Y.; Liu, H. Vertical distribution and diurnal movement of the deep scattering layer in the South China Sea. Adv. Mar. Sci. 2015, 33, 10–20. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, J.; Zhao, X.Y.; Chen, Z.Z.; Ying, Y.P.; Li, Z.Y.; Xu, D.F.; Liu, Z.A.; Zhou, M. Vertical distribution and diel migration of mesopelagic fishes on the northern slope of the South China sea. Deep Sea Res. Part II 2019, 167, 128–141. [Google Scholar] [CrossRef]
- Simrad. Simrad ER60 Scientific Echo Sounder Software Reference Manual; Simrad Maritime AS Kongsberg: Horten, Norway, 2008; pp. 19–31. [Google Scholar]
- Masuda, H.; Amaoka, K.; Araga, C. The Fishes of the Japanese Archipelago; Tokyo University Press: Tokyo, Japan, 1984; pp. 1–374. [Google Scholar]
- Shen, S. Fishes of Taiwan; National Taiwan University: Taibei, China, 1993; pp. 1–960. [Google Scholar]
- Chen, S. Fauna Sinica: Ostichthyes (Myctophiformmes, Cetomimiformes and Osteoglossiformes); Science Press: Beijing, China, 2002; p. 304. [Google Scholar]
- Chen, X.; Liu, B.; Wang, Y. Cephalopods of the World; Ocean Press: Beijing, China, 2009; pp. 1–714. [Google Scholar]
- Sun, D.; Chen, Z. Review of Fishes of South China Sea: Volume One; Ocean Press: Beijing, China, 2013; pp. 1–606. [Google Scholar]
- Chen, D.; Zhang, M. Marine Fishes of China; China Ocean University Press: Qingdao, China, 2015; pp. 1–2152. [Google Scholar]
- De Robertis, A.; Higginbottom, I. A post-processing technique to estimate the signal-to-noise ratio and remove echosounder background noise. ICES. J. Mar. Sci. 2007, 64, 1282–1291. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.B.; Chen, P.M.; Yu, J.; Fan, J.T. Impact of subtracting ‘Time Varied Gain Background Noise’(TVGBN) on estimates of fisheries resources derived from post-processing acoustic data. J. Appl. Ichthyol. 2013, 29, 1468–1472. [Google Scholar] [CrossRef]
- Watkins, J.L.; Brierley, A.S. A post-processing technique to remove background noise from echo integration data. ICES J. Mar. Sci. 1996, 53, 339–344. [Google Scholar] [CrossRef]
- Klevjer, T.A.; Irigoien, X.; Røstad, A.; Fraile-Nuez, E.; Benítez-Barrios, V.M.; Kaartvedt, S. Large scale patterns in vertical distribution and behaviour of mesopelagic scattering layers. Sci. Rep. 2016, 6, 19873. [Google Scholar] [CrossRef]
- Catul, V.; Karuppasamy, M.G.K. A review on mesopelagic fishes belonging to family Myctophidae. Rev. Fish Biol. Fish. 2011, 21, 339–354. [Google Scholar] [CrossRef]
- Granata, A.; Bergamasco, A.; Battaglia, P.; Milisenda, G.; Guglielmo, L. Vertical distribution and diel migration of zooplankton and micronekton in Polcevera submarine canyon of the Ligurian mesopelagic zone (NW Mediterranean Sea). Prog. Oceanogr. 2020, 183, 102298. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Aioi, K. Myctophid Fishes of the Genus Myctophum (Myctophidae) in the Pacific and Indian Oceans. J. Oceanogr. Soc. Jpn. 1972, 28, 161–175. [Google Scholar] [CrossRef]
- Peña, M.; Olivar, M.P.; Balbín, R.; López-Jurado, J.L.; Iglesias, M.; Miquel, J. Acoustic detection of mesopelagic fishes in scattering layers of the Balearic Sea (western Mediterranean). Can. J. Fish. Aquat. Sci. 2014, 71, 1186–1197. [Google Scholar] [CrossRef]
- Bernard, S.; Frédéric, M.; Emile, M. Reproductive biology of Vinciguerria nimbaria in the equatorial waters of the eastern Atlantic Ocean: Reproductive biology of Vinciguerria nimbaria. J. Fish Boil. 2003, 62, 1116–1136. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qi, L.; Xu, M.; Yang, C.; Wang, X. Impact of the migration behavior of mesopelagic fishes on the compositions of dissolved and particulate organic carbon on the northern slope of the South China Sea. Deep Sea Res. Part II 2019, 167, 46–54. [Google Scholar] [CrossRef]
- Caiger, P.E.; Lefebve, L.S.; Llopiz, J.K. Growth and reproduction in mesopelagic fishes: A literature synthesis. ICES. J. Mar. Sci. 2021, 78, 765–781. [Google Scholar] [CrossRef]
- Pearcy, W.G.; Nemoto, T.; Okiyama, M. Mesopelagic fishes of the Bering Sea and adjacent northern North Pacific Ocean. J. Oceanogr. Soc. Jpn. 1979, 35, 127–135. [Google Scholar] [CrossRef]
- Butler, M.; Bollens, S.M.; Burkhalter, B.; Madin, L.P.; Horgan, E. Mesopelagic fishes of the Arabian Sea: Distribution, abundance and diet of Chauliodus pammelas, Chauliodus sloani, Stomias affinis, and Stomias nebulosus. Deep Sea Res. Part II 2001, 48, 1369–1383. [Google Scholar] [CrossRef]
- Benoit-Bird, K.J.; Au, W.W.L. Target strength measurements of Hawaiian mesopelagic boundary community animals. J. Acoust. Soc. Am. 2001, 110, 812–819. [Google Scholar] [CrossRef]
- Cavan, E.L.; Laurenceau-Cornec, E.C.; Bressac, M.; Boyd, P.W. Exploring the ecology of the mesopelagic biological pump. Prog. Oceanogr. 2019, 176, 102125. [Google Scholar] [CrossRef]
- Gartner, J.V.; Musick, J.A. Feeding habits of the deep-sea fish, Scopelogadus beanii (Pisces: Melamphaide), in the western North Atlantic. Deep Sea Res. Part II 1989, 36, 1457–1469. [Google Scholar] [CrossRef]
| Station Code | Sampling Time | Sampling Depth (m) | Mean Bottom Depth (m) | Sun Cycle | M/D/Y |
|---|---|---|---|---|---|
| S-May-1 | 04:00–05:00 | 75 | >1000 | night | 05/27/2017 |
| S-May-2 | 20:35–21:45 | 75 | >1000 | night | 05/26/2017 |
| S-May-3 | 06:30–07:30 | 450 | >1000 | day | 05/28/2017 |
| S-May-4 | 09:35–10:35 | 450 | >1000 | day | 05/26/2017 |
| S-May-5 | 13:02–14:02 | 550 | >1000 | day | 05/27/2017 |
| S-May-6 | 21:30–22:30 | 600 | >1000 | night | 05/27/2017 |
| C-Jun-1 | 19:50–20:50 | 75 | >1000 | night | 06/07/2017 |
| C-Jun-2 | 05:47–06:47 | 75 | >1000 | night–day | 06/06/2017 |
| C-Jun-3 | 14:20–15:20 | 500 | >1000 | day | 06/07/2017 |
| C-Jun-4 | 21:07–22:07 | 500 | >1000 | night | 06/07/2017 |
| C-Jun-5 | 08:07–09:07 | 600 | >1000 | day | 06/08/2017 |
| C-Jun-6 | 20:14–21:14 | 600 | >1000 | night | 06/08/2017 |
| S-Nov-1 | 22:40–23:40 | 75 | >1000 | night | 11/21/2017 |
| S-Nov-2 | 14:14–15:14 | 300 | >1000 | day | 11/21/2017 |
| S-Nov -3 | 15:26–16:26 | 400 | >1000 | day | 11/22/2017 |
| S-Nov-4 | 22:54–23:54 | 400 | >1000 | night | 11/22/2017 |
| S-Nov-5 | 07:32–08:32 | 500 | >1000 | day | 11/23/2017 |
| S-Nov-6 | 14:30–15:30 | 600 | >1000 | day | 11/23/2017 |
| C-Dec-1 | 21:00–22:00 | 75 | >1000 | night | 12/04/2017 |
| C-Dec-2 | 15:43–16:43 | 200 | >1000 | day | 12/02/2017 |
| C-Dec-3 | 09:30–10:00 | 300 | >1000 | day | 12/04/2017 |
| C-Dec-4 | 00:40–01:40 | 400 | >1000 | night | 12/02/2017 |
| C-Dec-5 | 17:50–18:50 | 500 | >1000 | day | 12/03/2017 |
| C-Dec-6 | 08:50–09:50 | 600 | >1000 | day | 12/03/2017 |
| Order | Family | Species | Distributed Depth | Occurrence | |
|---|---|---|---|---|---|
| Min. | Max. | ||||
| 1 | Myctophidae | Diaphus fulgerns | 75 m (N) | 550 m (D) | 10/24 |
| 2 | Myctophidae | Lampadena mollis | 75 m (N) | 600 m (D) | 14/24 |
| 3 | Myctophidae | Diaphus phillipsi | 75 m (N) | 600 m (D/N) | 16/24 |
| 4 | Myctophidae | Diaphus fragilis | 75 m (N) | 600 m (D) | 19/24 |
| 5 | Myctophidae | Diaphus garmani | 75 m (N) | 600 m (D/N) | 22/24 |
| 6 | Myctophidae | Diaphus perspicillatus | 75 m (N) | 600 m (D/N) | 4/24 |
| 7 | Myctophidae | Diaphus chrysorhynchus | 75 m (N) | 550 m (D)/600 m (N) | 12/24 |
| 8 | Myctophidae | Diaphus malayanus | 75 m (N) | 600 m (D) | 16/24 |
| 9 | Myctophidae | Diaphus brachycephalus | 75 m (N) | 600 m (D/N) | 15/24 |
| 10 | Myctophidae | Diaphus watasei | 75 m (N) | 550 m (D) | 6/24 |
| 11 | Myctophidae | Diaphus jenseni | 75 m (N) | 550 m (D) | 5/24 |
| 12 | Myctophidae | Lampanyctus hubbsi | 75 m (N) | 600 m (D) | 8/24 |
| 13 | Myctophidae | Lampanyctus nobilis | 75 m (N) | 550 m (D) | 4/24 |
| 14 | Myctophidae | Lampanyctus omostigma | 75 m (N) | 600 m (D/N) | 10/24 |
| 15 | Myctophidae | Lampanyctus festivus | 75 m (N) | 600 m (D) | 7/24 |
| 16 | Myctophidae | Lampanyctus punctatissimus | 75 m (N) | 600 m (D) | 8/24 |
| 17 | Myctophidae | Bolinichthys longipes | 75 m (N) | 600 m (D/N) | 21/24 |
| 18 | Myctophidae | Myctophum asperum | 75 m (N) | 600 m (D) | 7/24 |
| 19 | Myctophidae | Lampadena luminosa | 75 m (N) | 600 m (D) | 6/24 |
| 20 | Myctophidae | Symbolophorus evermanni | 75 m (N) | 600 m (D/N) | 9/24 |
| 21 | Myctophidae | Hygophum proximum | 75 m (N) | 600 m (D/N) | 19/24 |
| 22 | Myctophidae | Centrobranchus andreae | 75 m (N) | 600 m (D) | 7/24 |
| 23 | Myctophidae | Ceratoscopelus warmingii | 75 m (N) | 600 m (D) | 12/24 |
| 24 | Notosudidae | Scopelosaurus hoedti | 75 m (N) | 550 m (D)/600 m (N) | 6/24 |
| 25 | Paralepididae | Stemonosudis rothschildi | 75 m (N) | 600 m (D) | 3/24 |
| 26 | Gonostomatidae | Vinciguerria nimbara | 75 m (N) | 600 m (D) | 10/24 |
| 27 | Gonostomatidae | Diplophos taenia | 75 m (N) | 550 m (D)/600 m (N) | 8/24 |
| 28 | Nomeidae | Cubiceps squamiceps | 75 m (N) | 600 m (D) | 4/24 |
| 29 | Nomeidae | Cubiceps pauciradiatus | 75 m (N) | 600 m (N) | 3/24 |
| 30 | Stomiidae | Astronesthes splendidus | 75 m (N) | ― | 2/24 |
| 31 | Chauliodontidae | Chauliodus sloani | 75 m (N) | 600 m (D) | 16/24 |
| 32 | Grammicolepidae | Xenolepidichthys dalgleishi | 75 m (N) | 600 m (D/N) | 8/24 |
| 33 | Idiacanthidae | Idiacanthus fasciola | 75 m (N) | 600 m (D) | 6/24 |
| 34 | Nomeidae | Psenes arafurensis | 75 m (N) | 450 m (D) | 4/24 |
| 35 | Bramidae | Brama myersi | 75 m (N) | 600 m (D) | 4/24 |
| 36 | Enoploteuthidae | Abralia similis | 75 m (N) | 600 m (D) | 3/24 |
| 37 | Enoploteuthidae | Abratia multihamata | 75 m (N) | 500 m (D) | 2/24 |
| 38 | Enoploteuthidae | Abraliopsis atlantica | 75 m (DW) | ― | 1/24 |
| 39 | Enoploteuthidae | Abratia andamanica | 75 m (N) | 600 m (D) | 13/24 |
| 40 | Pyroteuthidae | Pterygioteuthis giardi | 75 m (N) | 600 m (D/N) | 2/24 |
| 41 | Mastigoteuthidae | Mastigoteuthis cordiformis | 75 m (N) | 600 m (N) | 6/24 |
| 42 | Ommastrephidae | Ornithoteuthis volatilis | 75 m (N) | 600 m (D/N) | 7/24 |
| 43 | Cranchiidae | Leachia pacifica | 75 m (N) | 500 m (D)/600 m (N) | 4/24 |
| Order | Family | Species | Distributed Depth | Occurrence | |
|---|---|---|---|---|---|
| Min. | Max. | ||||
| 1 | Myctophidae | Diaphus lucidus | 200 m (TW) | 600 m (D) | 9/24 |
| 2 | Melamphaidae | Melamphaes microps | 400 m (N) | 600 m (D) | 3/24 |
| 3 | Sternoptychidae | Argyropelecus affinis | 200 m (N) | 600 m (D) | 13/24 |
| 4 | Gonostomatidae | Gonostoma gracile | 400 m (N) | 600 m (D) | 5/24 |
| 5 | Gonostomatidae | Margrethia obtusirostra | 450 m (DW) | 600 m (D) | 5/24 |
| 6 | Scopelarchidae | Scopelarchus analis | 400 m (N) | 600 m (D) | 4/24 |
| Order | Family | Species | Distributed Depth | Occurrence | |
|---|---|---|---|---|---|
| Night | Day | ||||
| 1 | Sternoptychidae | Sternoptyx obscura | 500–600 m | 450–600 m | 6/24 |
| 2 | Sternoptychidae | Argyropelecus sladeni | 400–600 m | 450–600 m | 8/24 |
| 3 | Sternoptychidae | Sternoptyx diaphana | 400 m | 400–600 m | 10/24 |
| 4 | Melamphaidae | Melamphaes polylepis | 400–600 m | 400–600 m | 13/24 |
| 5 | Melamphaidae | Melamphaes lugubris | 500–600 m | 400–600 m | 7/24 |
| 6 | Gonostomatidae | Vinciguerria attenuata | 500 m | 450–600 m | 7/24 |
| 7 | Gonostomatidae | Cyclothone pseudopallida | 400–600 m | 450–600 m | 11/24 |
| 8 | Evermannellidae | Evermannella indica | 600 m | 450–550 m | 5/24 |
| 9 | Phosichthyidae | Woodsia nonsuchae | 400–500 m | 400–600 m | 4/24 |
| 10 | Stomiidae | Heterophotus ophistoma | 500 m | 600 m | 4/24 |
| 11 | Serrivomeridae | Serrivomer beanii | 400–600 m | 400–600 m | 7/24 |
| 12 | Malacosteidae | Malacosteus niger | 500–600 m | 500–600 m | 7/24 |
| 13 | Scopelarchidae | Scopelarchus guentheri | 400 m | 400 m | 5/24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, Y.; Gong, Y.; Cai, Y.; Kong, X.; Tian, H.; Diao, Q.; Chen, Z. A Pilot Study on the Diel Vertical Migration Pattern of Mesopelagic Fishes in the Southern and Central South China Sea. J. Mar. Sci. Eng. 2024, 12, 879. https://doi.org/10.3390/jmse12060879
Zhang J, Jiang Y, Gong Y, Cai Y, Kong X, Tian H, Diao Q, Chen Z. A Pilot Study on the Diel Vertical Migration Pattern of Mesopelagic Fishes in the Southern and Central South China Sea. Journal of Marine Science and Engineering. 2024; 12(6):879. https://doi.org/10.3390/jmse12060879
Chicago/Turabian StyleZhang, Jun, Yan’e Jiang, Yuyan Gong, Yancong Cai, Xiaolan Kong, Han Tian, Qingqing Diao, and Zuozhi Chen. 2024. "A Pilot Study on the Diel Vertical Migration Pattern of Mesopelagic Fishes in the Southern and Central South China Sea" Journal of Marine Science and Engineering 12, no. 6: 879. https://doi.org/10.3390/jmse12060879
APA StyleZhang, J., Jiang, Y., Gong, Y., Cai, Y., Kong, X., Tian, H., Diao, Q., & Chen, Z. (2024). A Pilot Study on the Diel Vertical Migration Pattern of Mesopelagic Fishes in the Southern and Central South China Sea. Journal of Marine Science and Engineering, 12(6), 879. https://doi.org/10.3390/jmse12060879








