Mussel Culture Activities Facilitate the Export and Burial of Particulate Organic Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Methodology
2.3. Two End-Member Mixing Model
3. Results
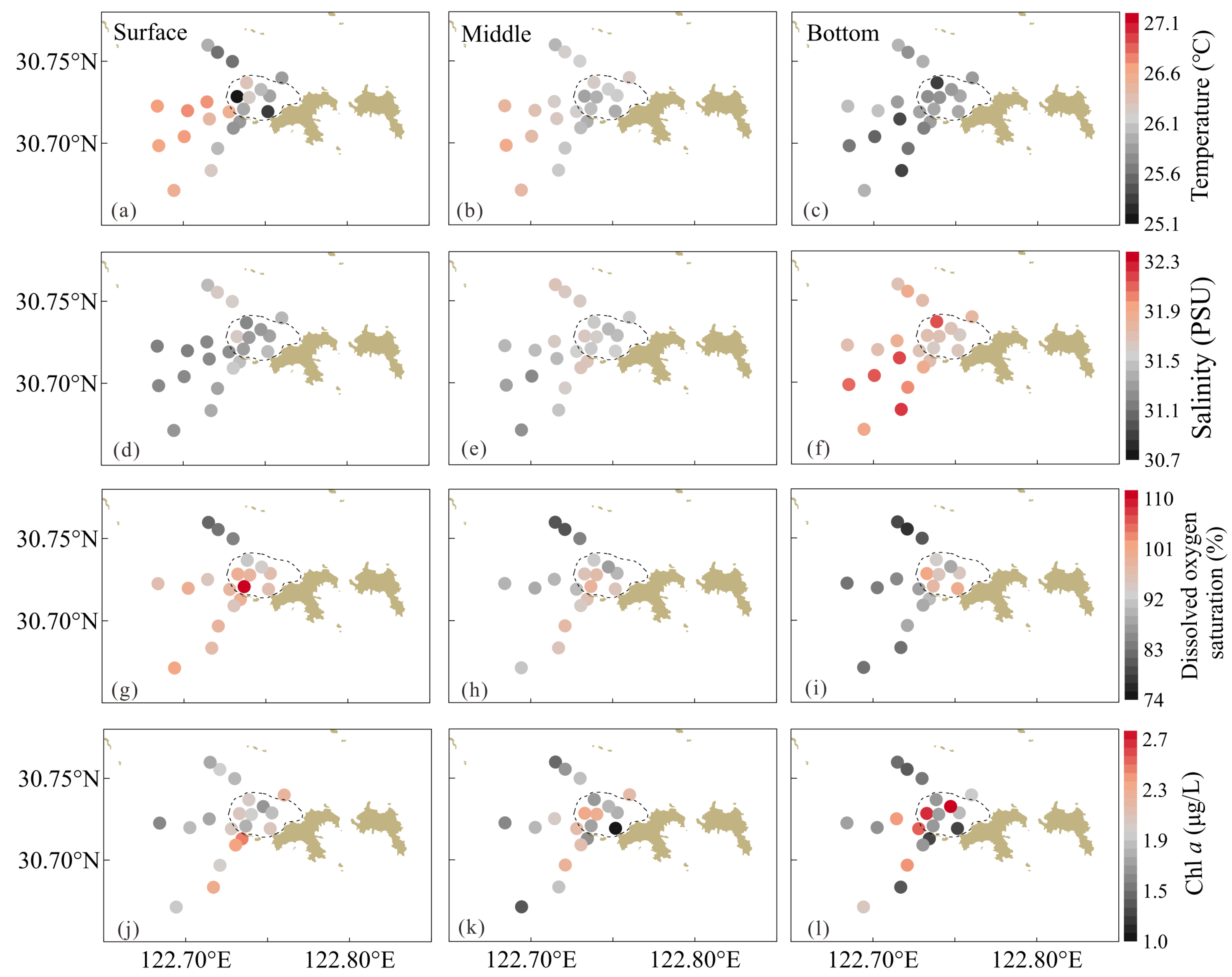
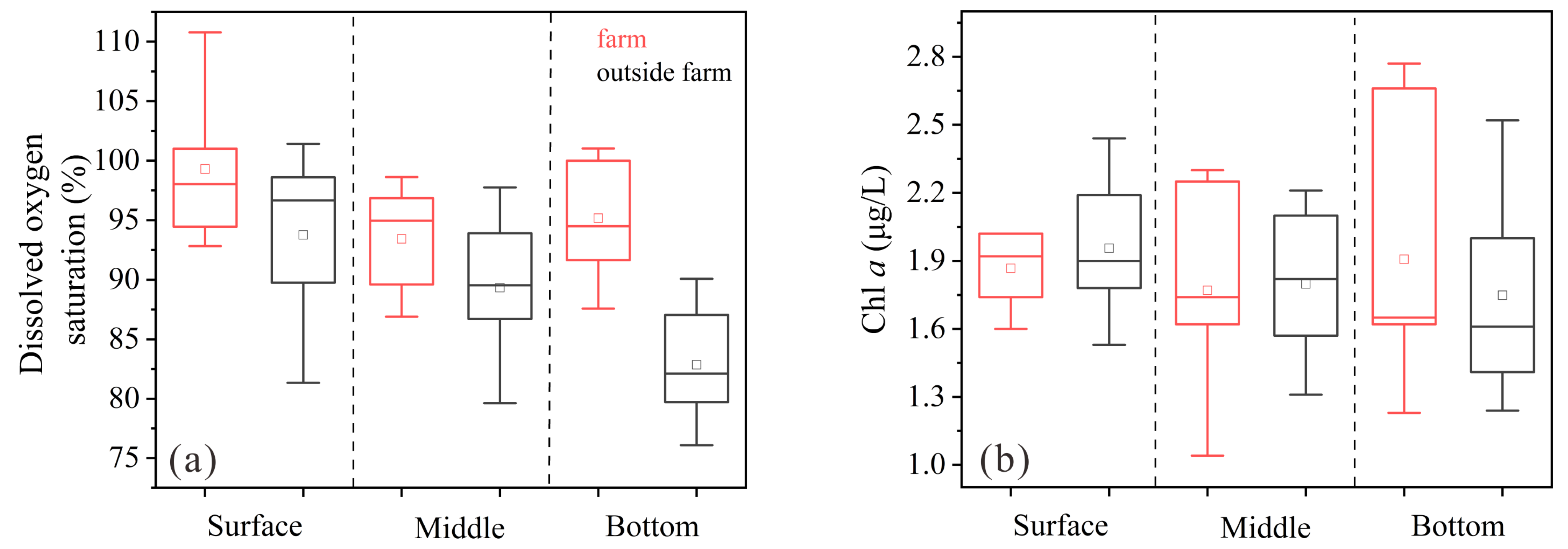
3.1. Hydrological Conditions, DO, and Chl a
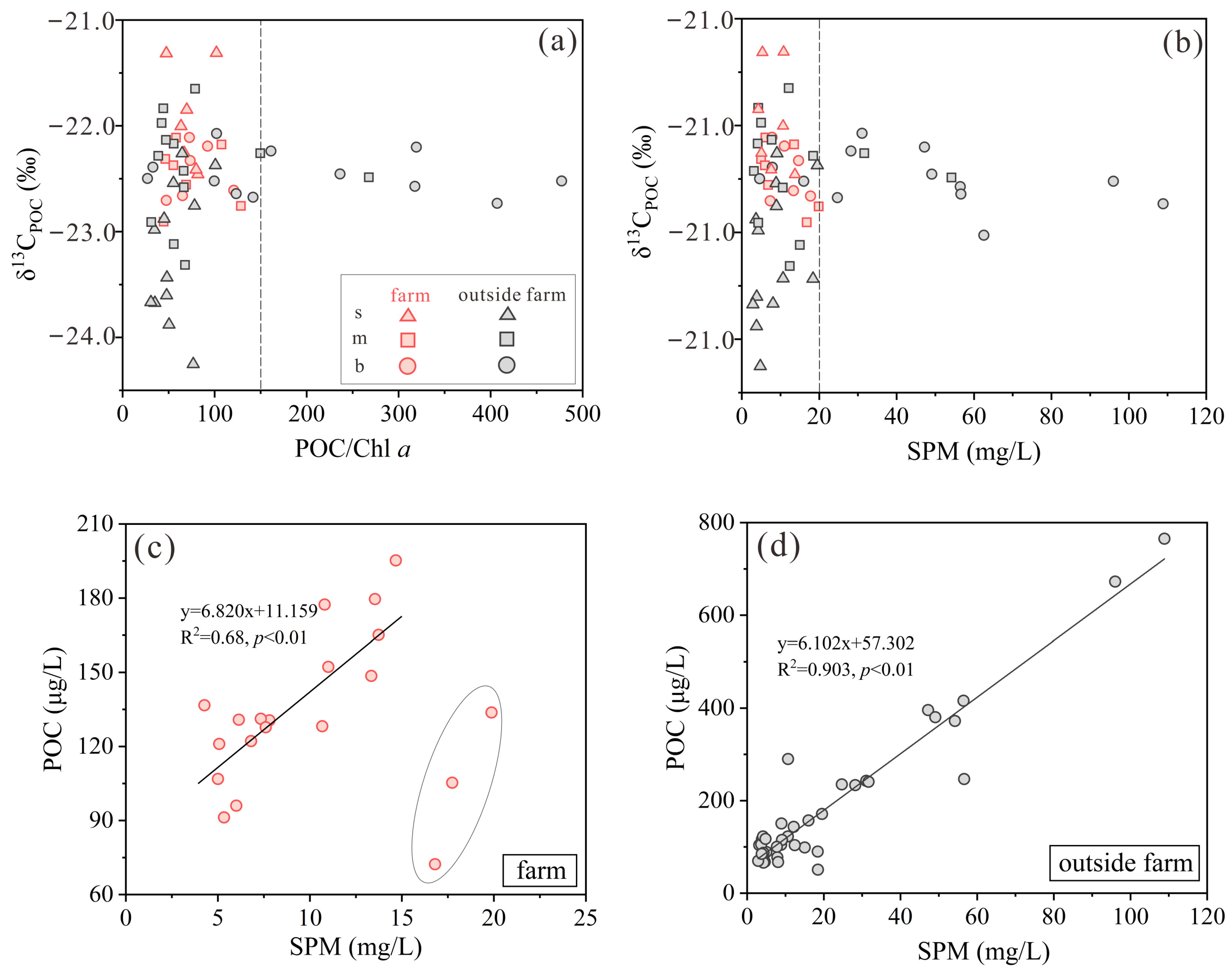
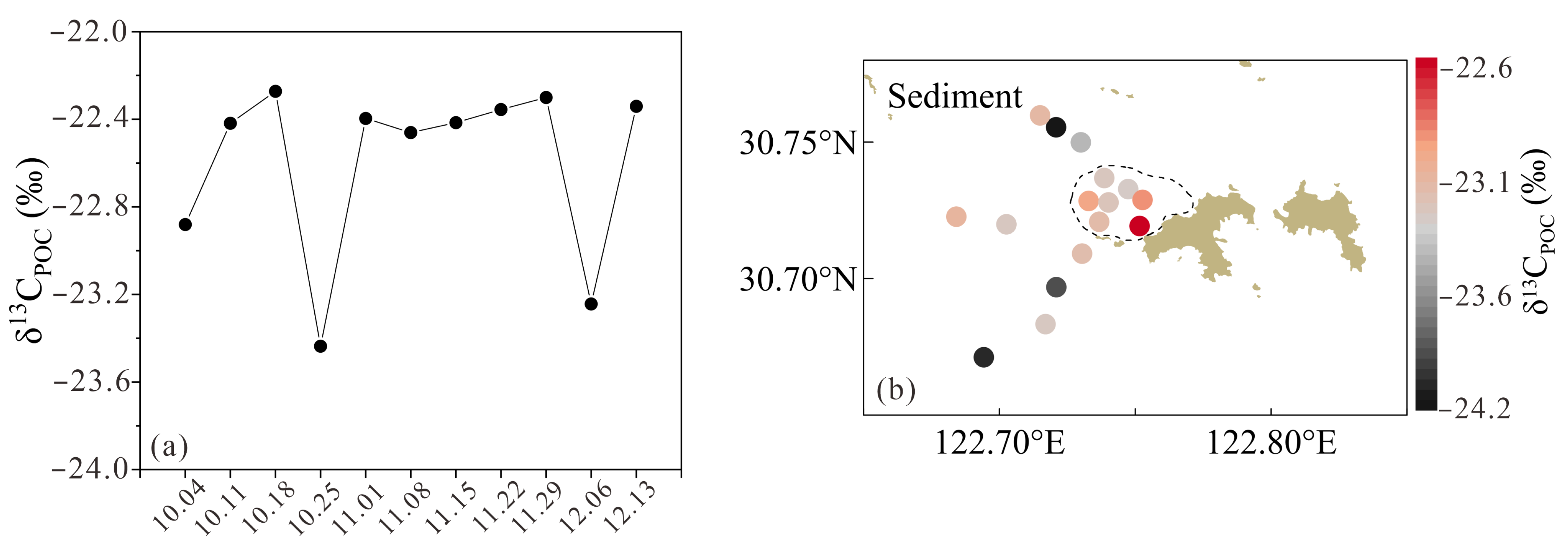
3.2. SPM, POC, δ13CPOC, and δ15NPN
3.3. δ13CPOC of Sinking Particles and Surface Sediments
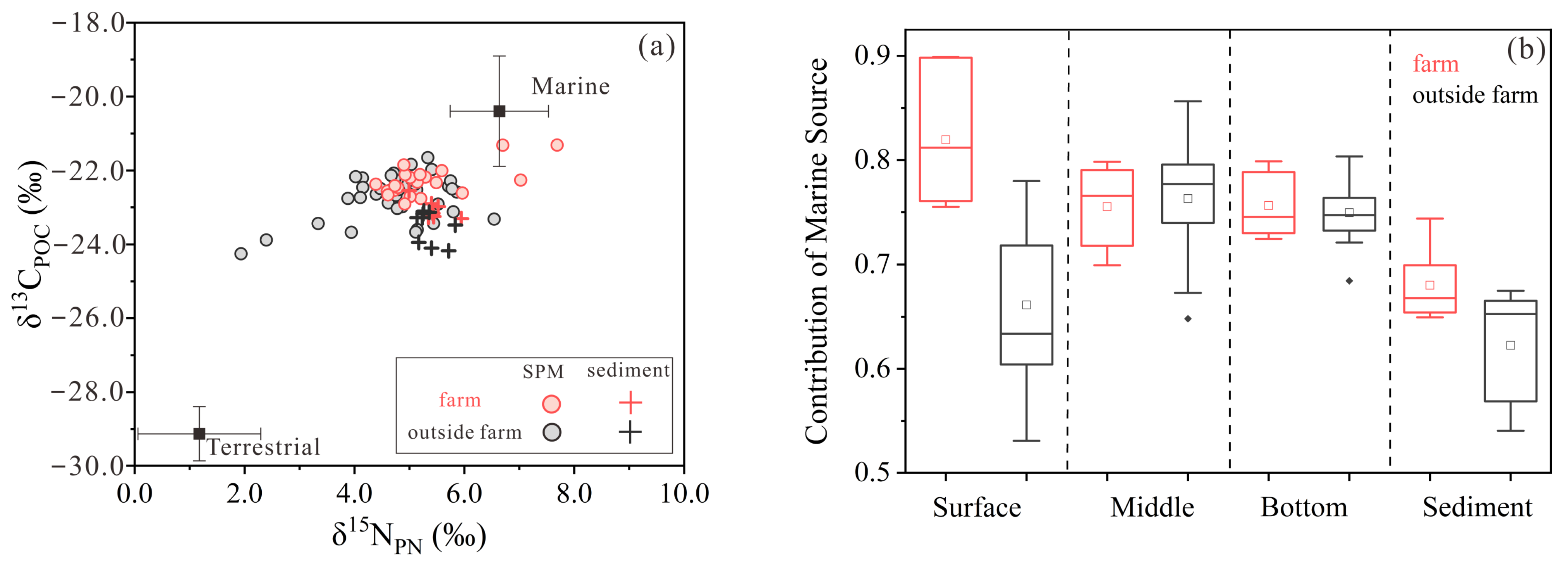
3.4. The Proportion of Marine Organic Matter in Suspended Particulate Matter and Sediment
4. Discussion
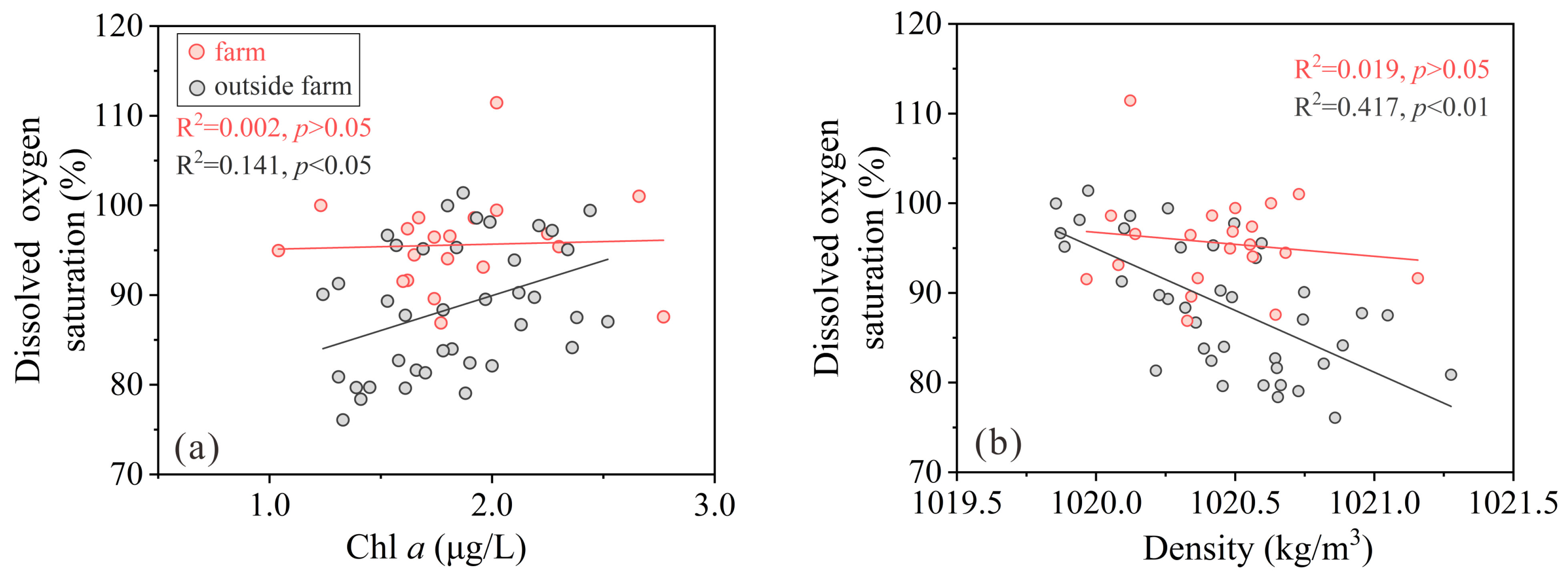
4.1. Preservation of Particulate Organic Matter and the Effects of Sediment Resuspension
4.2. Sources of Particulate Organic Matter
4.3. Effects of Mussel Feeding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubchenco, J.; Haugan, P.M.; Pangestu, M.E. Five priorities for a sustainable ocean economy. Nature 2020, 588, 30–32. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.; Mamun-Ur-Rashid, M. Quantifying greenhouse gas emissions from global aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef] [PubMed]
- van der Schatte Olivier, A.; Jones, L.; Vay, L.L. Christie, A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquacult. 2020, 12, 3–25. [Google Scholar] [CrossRef]
- Pan, Z.; Tan, Y.M.; Gao, Q.F.; Dong, S.L.; Fang, X.D.; Yan, J.L. A 120-year record of burial fluxes and source apportionment of sedimentary organic carbon in Alian Bay, China: Implication for the influence of mariculture activities, and regional environment changes. Aquaculture 2021, 535, 736421. [Google Scholar] [CrossRef]
- Dame, R.F.; Zingmark, R.G.; Haskin, E. Oyster reefs as processors of estuarine materials. J. Exp. Mar. Biol. Ecol. 1984, 83, 239–247. [Google Scholar] [CrossRef]
- Martini, A.; Calì, M.; Capoccioni, F.; Martinoli, M.; Pulcini, D.; Buttazzoni, L.; Moranduzzo, T.; Pirlo, G. Environmental performance and shell formation-related carbon flows for mussel farming systems. Sci. Total Environ. 2022, 831, 154891. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Turolla, E.; Lanzoni, M.; Moore, D.; Castaldelli, G. Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci. Total Environ. 2022, 848, 157508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liao, Y.; Zhu, J.; Shi, X.; Shou, L.; Zeng, J.; Chen, Q.; Chen, J. Influence of biodeposition by suspended cultured oyster on the distributions of trace elements in multiple media in a semi-enclosed bay of China. J. Hazard. Mater. 2023, 443, 130347. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Riegman, R.; van der Meer, J. Impact of the blue mussel Mytilus edulis on the microbial food web in the western Wadden Sea, The Netherlands. Mar. Ecol. Prog. Ser. 2015, 527, 119–131. [Google Scholar] [CrossRef]
- Morris, J.P.; Humphreys, M.P. Modelling seawater carbonate chemistry in shellfish aquaculture regions: Insights into CO2 release associated with shell formation and growth. Aquaculture 2019, 501, 338–344. [Google Scholar] [CrossRef]
- Jaramillo, E.; Bertran, C.; Bravo, A. Mussel biodeposition in an estuary in southern Chile. Mar. Ecol. Prog. Ser. 1992, 82, 85–94. [Google Scholar] [CrossRef]
- Zhong, W.; Lin, J.; Zou, Q.; Yamazaki, H.; Yang, W.; Yang, G. Impacts of large-scale suspended mussel farm on seston depletion. Estuar. Coast. Shelf Sci. 2024, 300, 108710. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Kawahata, T.; Fujibayashi, M.; Nishimura, O.; Sakamaki, T. Sources and oxygen consumption of particulate organic matter settling in oyster aquaculture farms: Insights from analysis of fatty acid composition. Estuar. Coast. Shelf Sci. 2021, 254, 107328. [Google Scholar] [CrossRef]
- Froján, M.; Figueiras, F.G.; Zúñiga, D.; Alonso-Pérez, F.; Arbones, B.; Castro, C.G. Influence of Mussel Culture on the Vertical Export of Phytoplankton Carbon in a Coastal Upwelling Embayment (Ría de Vigo, NW Iberia). Estuaries Coasts 2016, 39, 1449–1462. [Google Scholar] [CrossRef]
- Xia, B.; Cui, Y.; Chen, B.; Cui, Z.; Qu, K.; Ma, F. Carbon and nitrogen isotopes analysis and sources of organic matter in surface sediments from the Sanggou Bay and its adjacent areas, China. Acta Oceanlol. Sin. 2014, 33, 48–57. [Google Scholar] [CrossRef]
- Sui, J.; Zhang, J.H.; Ren, S.J.; Lin, F. Organic Carbon in the Surface Sediments from the Intensive Mariculture Zone of Sanggou Bay: Distribution, Seasonal Variations and Sources. J. Ocean Univ. China 2019, 18, 985–996. [Google Scholar] [CrossRef]
- Jiao, M.; Zhou, W.; Long, C.; Zhang, L.; Xu, P.; Li, H.Y.; Suo, A.; Yue, W. Dietary reconstruction and influencing factors of oysters cultured in a typical estuarine bay of South China. J. Clean. Prod. 2024, 449, 141773. [Google Scholar] [CrossRef]
- Kotta, J.; Futter, M.; Kaasik, A.; Liversage, K.; Ratsep, M.; Barboza, F.R.; Bergstrom, L.; Bergstrom, P.; Bobsien, I.; Diaz, E.; et al. Cleaning up seas using blue growth initiatives: Mussel farming for eutrophication control in the Baltic Sea. Sci. Total Environ. 2020, 709, 136144. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, P.; Liao, Y.; Liu, Q.; Chen, Q.; Shou, L.; Zeng, J.; Chen, J. Oyster farming control on phytoplankton bloom promoted by thermal discharge from a power plant in a eutrophic, semi-enclosed bay. Water Res. 2019, 159, 1–9. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Herman, P.M. Organic matter processing in tidal estuaries. Mar. Chem. 2007, 106, 127–147. [Google Scholar] [CrossRef]
- Wang, X.C.; Chen, R.F.; Gardner, G.B. Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico. Mar. Chem. 2004, 89, 241–256. [Google Scholar] [CrossRef]
- Ge, J.; Torres, R.; Chen, C.; Liu, J.; Xu, Y.; Bellerby, R.; Shen, F.; Bruggeman, J.; Ding, P. Influence of suspended sediment front on nutrients and phytoplankton dynamics off the Changjiang Estuary: A FVCOM-ERSEM coupled model experiment. J. Mar. Syst. 2020, 204, 103292. [Google Scholar] [CrossRef]
- Barth, J.A.; Veizer, J.; Mayer, B. Origin of particulate organic carbon in the upper St. Lawrence: Isotopic constraints. Earth Planet. Sci. Lett. 1998, 162, 111–121. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, D.J.; Wei, H.; Lu, R.X. Isotope variability of particulate organic matter at the PN section in the East China Sea. Biogeochemistry 2003, 65, 31–49. [Google Scholar] [CrossRef]
- Zhong, W.; Lin, J.; Zou, Q.; Wen, Y.; Yang, W.; Yang, G. Hydrodynamic effects of large-scale suspended mussel farms: Field observations and numerical simulations. Front. Mar. Sci. 2022, 9, 973155. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, J.; Zheng, B.; Cai, W.; Lin, K.; Tang, J. Temporal and spatial distribution of red tide outbreaks in the Yangtze River Estuary and adjacent waters, China. Mar. Pollut. Bull. 2013, 72, 213–221. [Google Scholar] [CrossRef]
- Qian, W.; Dai, M.; Xu, M.; Kao, S.J.; Du, C.; Liu, J.; Wang, H.; Guo, L.; Wang, L. Non-local drivers of the summer hypoxia in the East China Sea off the Changjiang Estuary. Estuar. Coast. Shelf Sci. 2017, 198, 393–399. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.; Wang, K.; Ni, X.; Li, D.; Zeng, D.; Fan, W.; Xu, D. The Changjiang River plume shifts from carbon source to sink when net community production exceeds a threshold in early autumn. Sci. Total Environ. 2023, 888, 164126. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Shen, Z.; Laws, E.A. Nutrient Dynamics in the Upwelling Area of Changjiang (Yangtze River) Estuary. J. Coast. Res. 2009, 25, 569–580. [Google Scholar] [CrossRef]
- Yang, D.; Yin, B.; Sun, J.; Zhang, Y. Numerical study on the origins and the forcing mechanism of the phosphate in upwelling areas off the coast of Zhejiang province, China in summer. J. Mar. Syst. 2013, 123–124, 1–18. [Google Scholar] [CrossRef]
- Lü, X.; Qiao, F.; Xia, C.; Zhu, J.; Yuan, Y. Upwelling off Yangtze River estuary in summer. J. Geophys. Res. Ocean. 2006, 111, C11. [Google Scholar] [CrossRef]
- Qiao, L.; Huang, L.; Wang, Z.; Yao, Z.; Liu, S. Flux and its seasonal variation of suspended particulate matter in the Bohai Sea, Yellow Sea and East China Sea. Geol. J. 2016, 51, 22–34. [Google Scholar] [CrossRef]
- Yin, W.; Ma, Y.; Wang, D.; He, S.; Huang, D. Surface Upwelling off the Zhoushan Islands, East China Sea, from Himawari-8 AHI Data. Remote Sens. 2022, 14, 3261. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Feng, Z.; Wu, H. Rapid variations of phytoplankton blooms and their dynamics off the Changjiang River Estuary. Front. Mar. Sci. 2024, 11, 1345940. [Google Scholar] [CrossRef]
- Chen, Y.L.L.; Chen, H.Y.; Gong, G.C.; Lin, Y.H.; Jan, S.; Takahashi, M. Phytoplankton production during a summer coastal upwelling in the East China Sea. Cont. Shelf Res. 2004, 24, 1321–1338. [Google Scholar] [CrossRef]
- Gong, G.C.; Chen, Y.L.L.; Liu, K.K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Tseng, Y.F.; Lin, J.; Dai, M.; Kao, S.J. Joint effect of freshwater plume and coastal upwelling on phytoplankton growth off the Changjiang River. Biogeosciences 2014, 11, 409–423. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Li, R.; Song, Z.; Wu, J. Seasonal fluxes and source variation of organic carbon transported by two major Chinese Rivers: The Yellow River and Changjiang (Yangtze) River. Glob. Biogeochem. Cycles 2012, 26, GB2025. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Saito, Y.; Xu, G.; Chen, B.; Chen, L. Sedimentary signals of the upwelling along the Zhejiang coast, China. Estuar. Coast. Shelf Sci. 2020, 232, 106396. [Google Scholar] [CrossRef]
- Song, S.; Li, Z.; Li, C.; Yu, Z. The response of spring phytoplankton assemblage to diluted water and upwelling in the eutrophic Changjiang (Yangtze River) Estuary. Acta Oceanlol. Sin. 2017, 36, 101–110. [Google Scholar] [CrossRef]
- Maar, M.; Larsen, J.; Schourup-Kristensen, V. Intensified Mussel Farming; Impacts on Nutrient Budgets and Ecology in a Eutrophic Semi-Enclosed Fjord System. J. Geophys. Res. Biogeosciences 2023, 128, e2022JG007312. [Google Scholar] [CrossRef]
- Wiesner, M.G.; Zheng, L.; Wong, H.K.; Wang, Y.; Chen, W. Fluxes of particulate matter in the South China Sea. Part. Flux Ocea 1996, 57, 293–309. [Google Scholar]
- Li, H.; Zhang, J.; Xuan, J.; Wu, Z.; Ran, L.; Wiesner, M.G.; Chen, J. Asymmetric response of the biological carbon pump to the ENSO in the South China Sea. Geophys. Res. Lett. 2022, 49, e2021GL095254. [Google Scholar] [CrossRef]
- Carpenter, J.H. The accuracy of the winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Murrell, M.C.; Hollibaugh, J.T. Distribution and Composition of Dissolved and Particulate Organic Carbon in Northern San Francisco Bay During Low Flow Conditions. Estuar. Coast. Shelf Sci. 2000, 51, 75–90. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Liu, S.M.; Zhang, Z.F.; Yao, Q.Z.; Hong, G.H.; Cooper, L. Sources and distribution of carbon within the Yangtze River system. Estuar. Coast. Shelf Sci. 2007, 71, 13–25. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.Y.T.; Xu, M.N.; Wang, H.; Dai, M.; Kao, S.J. Nitrogen isotope constraint on the zonation of multiple transformations between dissolved and particulate organic nitrogen in the Changjiang plume. Sci. Total Environ. 2022, 818, 151678. [Google Scholar] [CrossRef]
- Liang, S.; Li, S.; Guo, J.; Yang, Y.; Xu, Z.; Zhang, M.; Li, H.; Yu, X.; Wang, X. Source, composition, and reactivity of particulate organic matter along the Changjiang Estuary salinity gradient and adjacent sea. Mar. Chem. 2023, 252, 104245. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, B.; Li, D.; Sun, X.; Xu, Z.; Sun, Q.; Jiang, Z.; Ma, X.; Jin, H.; Chen, J. Observational studies of the effects of wind mixing and biological process on the vertical distribution of dissolved oxygen off the Changjiang Estuary. Front. Mar. Sci. 2023, 10, 1081688. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, G.; Li, J.; Song, Z.; Zhang, S.; Chen, C.; Zhu, Q.; Meng, Y. Effects of Typhoon Mujigae on the Biogeochemistry and Ecology of a Semi-Enclosed Bay in the Northern South China Sea. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006031. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Sharp, J.H.; Fogel, M.L. Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol. Oceanogr. 1988, 33, 1102–1115. [Google Scholar] [CrossRef]
- Guo, W.; Ye, F.; Xu, S.; Jia, G. Seasonal variation in sources and processing of particulate organic carbon in the Pearl River estuary, South China. Estuar. Coast. Shelf Sci. 2015, 167, 540–548. [Google Scholar] [CrossRef]
- Lin, J.; Li, C.; Zhang, S. Hydrodynamic effect of a large offshore mussel suspended aquaculture farm. Aquaculture 2016, 451, 147–155. [Google Scholar] [CrossRef]
- Kao, S.J.; Lin, F.J.; Liu, K.K. Organic carbon and nitrogen contents and their isotopic compositions in surficial sediments from the East China Sea shelf and the southern Okinawa Trough. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 1203–1217. [Google Scholar] [CrossRef]
- Tan, F.C.; Cai, D.L.; Edmond, J.M. Carbon isotope geochemistry of the Changjiang estuary. Estuar. Coast. Shelf Sci. 1991, 32, 395–403. [Google Scholar] [CrossRef]
- Huang, C.; Chen, F.; Zhang, S.; Chen, C.; Meng, Y.; Zhu, Q.; Song, Z. Carbon and nitrogen isotopic composition of particulate organic matter in the Pearl River Estuary and the adjacent shelf. Estuar. Coast. Shelf Sci. 2020, 246, 107003. [Google Scholar] [CrossRef]
- Gao, L.; Li, D.; Ishizaka, J. Stable isotope ratios of carbon and nitrogen in suspended organic matter: Seasonal and spatial dynamics along the Changjiang (Yangtze River) transport pathway. J. Geophys. Res. Biogeosci. 2014, 119, 1717–1737. [Google Scholar] [CrossRef]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Ye, F.; Guo, W.; Shi, Z.; Jia, G.; Wei, G. Seasonal dynamics of particulate organic matter and its response to flooding in the Pearl River Estuary, China, revealed by stable isotope (δ13C and δ15N) analyses. J. Geophys. Res. Ocean. 2017, 122, 6835–6856. [Google Scholar] [CrossRef]
- Hu, L.; Shi, X.; Yu, Z.; Lin, T.; Wang, H.; Ma, D.; Guo, Z.; Yang, Z. Distribution of sedimentary organic matter in estuarine–inner shelf regions of the East China Sea: Implications for hydrodynamic forces and anthropogenic impact. Mar. Chem. 2012, 142–144, 29–40. [Google Scholar] [CrossRef]
- Sun, X.; Fan, D.; Cheng, P.; Hu, L.; Sun, X.; Guo, Z.; Yang, Z. Source, transport and fate of terrestrial organic carbon from Yangtze River during a large flood event: Insights from multiple-isotopes (δ13C, δ15N, Δ14C) and geochemical tracers. Geochim. Cosmochim. Acta 2021, 308, 217–236. [Google Scholar] [CrossRef]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Malet, N.; Sauriau, P.G.; Ryckaert, M.; Malestroit, P.; Guillou, G. Dynamics and sources of suspended particulate organic matter in the Marennes-Oléron oyster farming bay: Insights from stable isotopes and microalgae ecology. Estuar. Coast. Shelf Sci. 2008, 78, 576–586. [Google Scholar] [CrossRef]
- Newell, R.I.E. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2004, 23, 51–61. [Google Scholar]
- Nizzoli, D.; Welsh, D.T.; Bartoli, M.; Viaroli, P. Impacts of mussel (Mytilus galloprovincialis) farming on oxygen consumption and nutrient recycling in a eutrophic coastal lagoon. Hydrobiologia 2005, 550, 183–198. [Google Scholar] [CrossRef]
- Souchu, P.; Vaquer, A.; Collos, Y.; Landrein, S.; Deslous-Paoli, J.M.; Bibent, B. Influence of shellfish farming activities on the biogeochemical composition of the water column in Thau Lagoon. Mar. Ecol. Prog. Ser. 2001, 218, 141–152. [Google Scholar] [CrossRef]
- Hartstein, N.D.; Stevens, C.L. Deposition beneath long-line mussel farms. Aquac. Eng. 2005, 33, 192–213. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Zhang, J.; Lang, C.; Li, W.; Wu, Z.; He, X.; Wang, X.; Yu, J.; Li, Q.; Li, Y.; et al. Mussel Culture Activities Facilitate the Export and Burial of Particulate Organic Carbon. J. Mar. Sci. Eng. 2024, 12, 910. https://doi.org/10.3390/jmse12060910
Han L, Zhang J, Lang C, Li W, Wu Z, He X, Wang X, Yu J, Li Q, Li Y, et al. Mussel Culture Activities Facilitate the Export and Burial of Particulate Organic Carbon. Journal of Marine Science and Engineering. 2024; 12(6):910. https://doi.org/10.3390/jmse12060910
Chicago/Turabian StyleHan, Lisong, Jingjing Zhang, Cheng Lang, Weiying Li, Zezhou Wu, Xingju He, Xinyang Wang, Jinping Yu, Qian Li, Yijie Li, and et al. 2024. "Mussel Culture Activities Facilitate the Export and Burial of Particulate Organic Carbon" Journal of Marine Science and Engineering 12, no. 6: 910. https://doi.org/10.3390/jmse12060910



