3.4. Description of Male
Five males examined (one broken at pleosome-urosome level), 6.0–7.1 mm BL (n = 4).
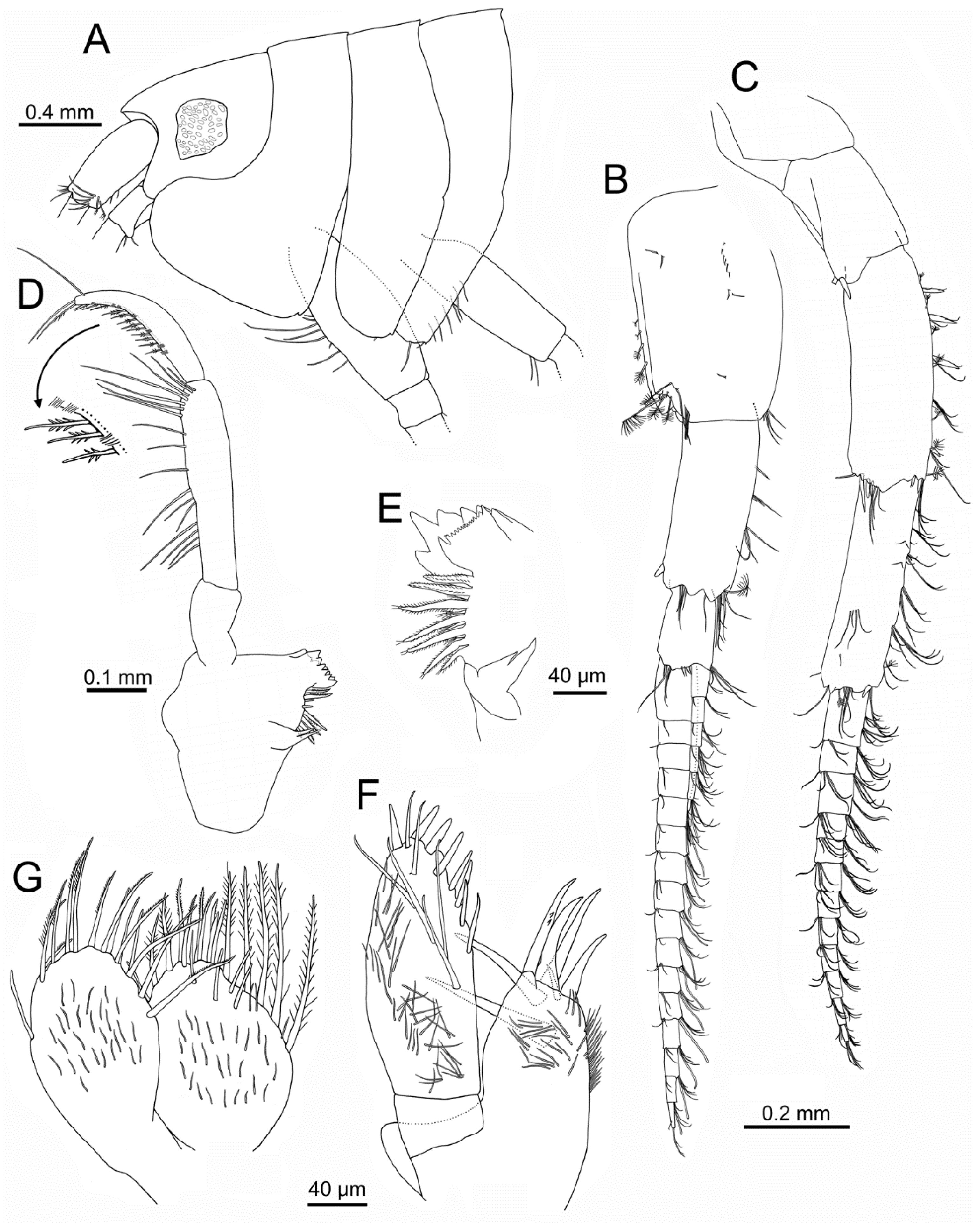
Head (
Figure 2 and
Figure 3A): 1.8 times as long as pereonite 1 and 8% of body length. Eyes well developed, ommatidia covering most of eye, strong dark pigmentation; rostrum short; ventral margin produced anteriorly and rounded.
Antenna 1 (
Figure 3B): peduncle article 1 1.6 times as long as wide, with several simple short setae on external surface, plumose setae on distal half of posterior margin, cluster of setae on anterodistal margin; article 2 0.8 times as long as article 1, with one spine on posterodistal margin and clusters of setae on anterior and distal margins, one plumose seta on anterodistal corner; article 3 0.46 times as long as article 2, with setae on distal margin. Flagellum with twelve to seventeen articles, each provided with two clusters of setae and one aesthetasc on anterodistal margin; accessory flagellum with five to six articles, with several setae mostly on distal margin or minute distal article; ratio accessory flagellum/flagellum: 0.3; ratio flagellum/peduncle article 1: 2.0.
Antenna 2 (
Figure 3C): subequal in length to antenna 1. Peduncle article 3 with one to two posterodistal spines; article 4 about three times as long as wide, with a row of five spines on anterior margin each associated with simple and plumose setae; two spines on anterodistal margin, and three spines on posterodistal margin, plus several associated setae; article 5 3.5 times as long as wide, with several clusters of setae on anterior margin and external face on distal third, one spine on anterodistal corner, and several terminal plumose and simple setae. Flagellum about 0.6 times as long as peduncle, with ten to twelve articles, each with two clusters of setae on distal margin.
Mandible (
Figure 3D,E): distolateral corner not forming a definite tooth; left lacinia mobilis with six triangular teeth; right lacinia mobilis with anterior margin with sharp small teeth (>10) and two larger acute teeth; incisor process with five triangular teeth, one much longer than others, six to seven raker robust setae with many setules, with several shorter setae in between; molar process distinct, with two distally acute setae. Left and right mandibular palp similar; palp article 1 about 1.6 times as long as wide and 0.4 times as long as article 2, lacking setae; article 2 4.3 times as long as wide, with a row of medial and subdistal setae; article 3 curved, widest at mid-length, 0.7 times as long as article 2, with posterior row of D3-setae not increasing in length and with a parallel row of short, very thin B3-setae grouped in clusters of four or five, one long subdistal B3-seta, and two to three E3-setae.
Maxilla 1 (
Figure 3F): palp article 1 about 0.25 times as long as article 2; article 2 with eight stout setae of increasing thickness on mesial and apical margins, facial setae including six long setae and numerous thinner and shorter setae. Outer plate with seven distal simple and serrate stout setae, one much shorter than others, and numerous shorter setae on surface of distal half.
Maxilla 2 (
Figure 3G): inner plate slightly wider than outer plate; both plates with long setae on distal part, most plumose; inner plate with several facial setae near anterior margin, and numerous shorter setae along surfaces of both plates.
Maxilliped (
Figure 4A–C): palp length ratio of article 1–4 about 1:3:1.8:1.7; article 1 with one seta; article 2 with long medial setae, and cluster of setae on distolateral corner; article 3 with medial setae on distal half and cluster of setae on distolateral corner; article four slender, slightly curved, unguis about 0.25 times as long as dactylus, with two basal setae. Outer plate reaching proximal third of palp article 2, with five to seven lateral setae and five distal spines of growing thickness. Inner plate small, with four simple setae, one spine, and two plumose setae on margin of distal half, posterodistal margin with one to two longer setae.
Gnathopod 1 (
Figure 3A and
Figure 4D,E): larger than gnathopod 2. Coxa broad, 1.2 times as long as wide, with a posterodistal tooth, anterior margin rounded. Length ratio of articles from basis to dactylus about 5.4:1:1.5:1.5:6.6:7.2. Basis with row of setae on anterior (second third) and posterior margin (proximal third). Ischium with one small posterodistal seta. Merus with a posterodistal cluster of setae, lacking distal tooth. Carpus with a posterodistal cluster of setae. Propodus roughly triangular in shape, 1.7 times as long as wide, length ratio of anterior, posterior and palmar margins about 2.25:1:1.6; with four clusters of setae along posterior margin; palm defined by two spines of different length associated with several setae, palm margin almost straight with two processes on distal third near to dactylus, one lateral row of simple, shorter setae and medial row of longer setae with several spinules, posterodistal palmar margin with one simple seta and one pappose seta, inner face of palm proximal half with several rows of simple setae; two anterodistal small setae near dactylus. Dactylus strongly curved, dactylar teeth as small indentations on second quarter; anterior margin with one proximal seta.
Gnathopod 2 (
Figure 3A and
Figure 4F): coxa 1.6 times as long as wide, anterior margin slightly convex and posterior margin nearly straight, with a posterodistal small tooth and one seta. Length ratio of articles from basis to dactylus about 3.6:1:1.2:1.5:3.6:2.5. Basis with two setae on posterior margin, anterior margin with one acute process on distal third. Ischium with one posterior subdistal seta. Merus posterodistal corner with conspicuous triangular tooth and several setae of different length. Carpus with three clusters of setae along posterior margin. Propodus with anterior and posterior border nearly straight, 1.4 times as long as wide, length ratio of anterior, posterior and palmar margins about 1.4:1:0.9; posterior margin border crested, with seven clusters of setae on posterior margin, each cluster arising from one crest; palm defined by two spines of different length, palm border smooth, with four lateral setae widely spaced, two medial rows of about twenty-five (outer row) and ten to twelve (inner) hooked stout setae regularly spaced and medial row of setae with spinules, inner face with several setae; six anterodistal setae near dactylus. Dactylus lacking teeth; anterior margin with one proximal seta.
Pereopod 3 (
Figure 5A): coxa nearly rectangular, 2.3 times as long as wide, with several setae on posterior margin and small tooth with one seta on posterodistal corner. Length ratio of articles from basis to dactylus about 4.7:1:2.4:1.7:2.7:1.4. Basis with several setae on distal third of anterior margin. Merus and carpus with several anterodistal and posterodistal setae, carpus also with two setae on posterior margin. Propodus with one anterodistal seta and six setae on posterior margin. Dactylus slender, slightly curved along distal half, without setae.
Pereopod 4 (
Figure 5B): coxa broad, 1.25 times as long as wide, anterior and posterior margins parallel, with notch on anterodistal corner. Length ratio of articles from basis to dactylus about 4.7:1:2.3:2.1:3.1:1.7. Basis with two setae on distal third of anterior margin. Merus with one anterodistal seta. Carpus with one anterodistal seta and two posterodistal setae. Propodus with one anterodistal seta and several setae along posterior margin. Dactylus slender, slightly curved along distal half, without setae.
Pereopod 5 (
Figure 5C,D): coxa 0.7 times as long as wide; anterior and posterior margins convex to slightly convex, lacking tooth. Length ratio of articles from basis to dactylus about 3.6:1:2.9:3:3.3:1.4. Basis broad, 1.35 times as long as wide, anterior margin strongly convex, posterior margin regularly convex, anterior margin with seven conical stout spines along distal half and three distal setae, posterior margin with seven to eight serrations along ¾ of distal half, each serration usually with one seta, distal corner rounded and produced into a lobe. Ischium with three anterodistal setae and one small spine. Merus 3.2 times as long as broad, with two to three pairs of setae on anterior margin, two spines on posterior margin on second third, three anterodistal setae, posterodistal corner with two setae and one spine about 0.25 times as long as merus. Carpus anterior margin with two setae on second third, anterodistal and posterodistal setae similar to merus. Propodus with 6 setae on anterior margin, propodal apical tuft with five anterodistal setae (two clearly longer than dactylus). Dactylus slender, slightly curved along distal half, with subterminal short seta.
Pereopod 6 (
Figure 5E): broken in all specimens at carpus-merus level. Coxa 0.65 times as long as wide; anterior margin almost straight, posterior border convex, lacking tooth. Length ratio of articles from basis to merus about 4.9:1:4.5. Basis broad, 1.35 times as long as wide, anterior and posterior margins slightly and regularly convex, anterior margin with five spines along distal half and two distal setae, ten serrations all along posterior margin, each serration usually with one seta, distal corner rounded and produced into a small lobe. Ischium with three anterodistal setae, without spine. Merus 3.4 times as long as broad, with two pairs of setae on anterior margin, three spines on posterior margin on proximal ⅔, four anterodistal, and four posterodistal setae.
Pereopod 7 (
Figure 5F): broken in all specimens at carpus-merus level. Coxa 0.6 times as long as wide; anterior margin almost straight, posterior margin slightly convex, lacking tooth. Length ratio of articles from basis to merus about 4.3:1:4.7. Basis broad, 1.5 times as long as wide, anterior and posterior margins almost parallel, anterior margin with five spines and one distal longer seta, posterior margin with 11 serrations, some accompanied by one small seta. Ischium with two anterodistal setae, without spine. Merus 3.4 times as long as broad, with two pairs of setae on anterior margin, four spines on posterior margin on proximal ⅔, three anterodistal, and three posterodistal setae.
Pleonites (
Figure 6A): all pleonites lacking posterodistal teeth. Pleonite 1 posterior margin weakly convex, posteroventral corner slightly acute. Pleonite 2 posterior margin slightly convex, posteroventral corner with small tooth. Pleonite 3 posterior margin clearly convex on inferior half, posteroventral corner produced into a well-developed tooth.
Urosomites (
Figure 6B): all lacking posterodorsal tooth; urosomite 1 with posteroventral acute tooth.
Uropod 1 (
Figure 6C): peduncle 1.2 times as long as rami, five dorsolateral spines and a pair of distal spines consisting of a small one and a long spine pointing backwards, dorsomedial border with a row of sixteen to twenty-three spines and a pair distal of spines similar to dorsolateral ones; outer ramus with four outer and three medial spines, two spines on tip (one much longer); inner ramus with two outer spines and four medial longer and thicker spines, tip with large spine.
Uropod 2 (
Figure 6D): peduncle subequal to rami, one distal dorsolateral spine and one distal dorsomedial spine; outer ramus with three outer and two medial spines, two spines on tip (one much longer); inner ramus with one outer and three medial spines, tip with three spines (central much longer).
Uropod 3 (
Figure 6E): peduncle 0.7 times as long as rami, one dorsolateral and two dorsomedial spines, one anterodistal spine; outer and inner ramus subequal, outer ramus 2-articulated, article 2 0.4 times as long as article 1, article 1 with three pairs of spines on outer side; inner ramus with two outer and four medial spines on medial border, and one subdistal seta.
Telson (
Figure 6B,F): medial cleft 0.9 times as long as telson total length; medial tooth of each lobe about twice as long as outer tooth; each lobe with two spines, medial the longest and about 2–2.2 times as long as outer spine, each pair accompanied by plumose seta; pair of short seta with two to threeapical setules on each lobe dorsal face at distal third.
3.5. Description of Ovigerous Female
Six ovigerous females examined, 6.75–8.75 mm BL (n = 6).
Head: 1.85 times as long as pereonite 1, and 8.5% of body length. Eyes well developed, similar to male.
Antenna 1 (
Figure 7A): peduncle article 1 1.7 times as long as wide; article 2 0.65 times as long as article 1; article 3 0.5 times as long as article 2. Flagellum with 13–18 articles; accessory flagellum with 5–7 articles, minute distal article; ratio accessory flagellum/flagellum: 0.3; ratio flagellum/peduncle article 1: 2.0.
Antenna 2 (
Figure 7B): subequal in length to antenna 1. Peduncle article 3 with one long spine; article 4 2.8 times as long as wide, with two spines on inner face near anterior margin proximal third, two spines on the anterodistal margin, and two spines on the posterodistal margin; article 5 3.6 times as long as wide, one spine on anterodistal corner. Flagellum about 0.6 times as long as peduncle, with 9–12 articles.
Mandible (
Figure 7C,D): distolateral corner not forming a definite tooth; mandible (laciniae, processes) similar to male. Similar left and right mandibular palp; palp article 1 about 1.6 times as long as wide and 0.35 times as long as article 2, lacking setae; article 2 4.35 times as long as wide; article 3 strongly curved at distal half, widest at mid-length, 0.7 times as long as article 2.
Maxilla 1 (
Figure 7E): palp article 1 about 0.23 times as long as article 2. Outer plate similar to male.
Maxilliped (
Figure 8A,B): palp length ratio of article 1–4 about 1:2.5:1.1:1.2; article 1 with two setae; article 3 setae similar to male, also including three facial setae; article 4 slender, slightly curved, unguis 0.25 times as long as dactylus, with one basal seta. Outer plate reaching proximal third of palp article 2, five distal spines of growing thickness. Inner plate small, including three plumose setae, posterodistal margin with four to five setae.
Gnathopod 1 (
Figure 8C): different to male gnathopod 1. Coxa broad, about as long as wide, with a posterodistal tooth (sometimes indistinct) and one seta, anterior margin rounded. Length ratio of articles from basis to dactylus about 4.3:1:1.2:1.2:3.6:3.4. Basis with row of setae on second third anterior margin, and two setae on proximal third posterior margin. Ischium with one small posterodistal seta. Merus with a posterodistal cluster of setae, lacking distal tooth. Carpus with a posterodistal cluster of setae. Propodus oval in shape, 1.6 times as long as wide; length ratio of anterior, posterior, and palmar margins about 2:1:1.15, with five clusters of setae along posterior margin; palm defined by two spines of different length associated with several setae, palm margin with two medial rows of hooked stout setae (>30), and medial row of setae with several spinules; three anterodistal simple setae near dactylus; inner face with several setae. Dactylus gently curved along its length, lacking teeth; anterior margin with one proximal seta.
Gnathopod 2 (
Figure 8D,E): similar to gnathopod 1. Coxa oval, wider at base, 1.5 times as long as wide, margins nearly straight, with a posterodistal small tooth and anteroventral notch each with one seta, several setae on inner face near posterodistolateral margin; oostegite well developed 1.5 times as long as coxa, with long setae on anterior and posterior margins. Length ratio of articles from basis to dactylus about 2.9:1:1.1:1.25:2.75:2.3. Merus posterodistal corner with conspicuous triangular tooth. Propodus posterior margin border with small crests, with eight clusters of setae. Palm defined by two spines of different length, palm border smooth, setae and spines similar to gnathopod 1. Dactylus gently curved along length, lacking teeth.
Pereopod 3 (
Figure 9A): coxa nearly rectangular, 2.1 times as long as wide, with a posterodistal small tooth and anteroventral notch each with one seta, several setae on inner face near posterodistolateral margin; oostegite well developed, 1.4 times as long as coxa, with long setae on anterior and posterior margins. Length ratio of articles from basis to dactylus about 4.6:1:2:1.6:3:1.5. Dactylus slender, almost straight, without setae.
Pereopod 4 (
Figure 9B): coxa broad, 1.3 times as long as wide, anterior and posterior margins almost straight; oostegite well developed 1.25 times as long as coxa, with long setae on anterior and posterior margins. Length ratio of articles from basis to dactylus about 4.5:1:2.25:1.75:2.75:1.5. Basis with two setae on distal third of anterior margin and one posterodistal seta. Dactylus slender, almost straight, without setae.
Pereopod 5 (
Figure 9C): coxa 0.8 times as long as wide; anterior and posterior margins convex to slightly convex, lacking tooth; oostegite well developed, 1.8 times as long as coxa, with long setae on anterior and posterior margins. Length ratio of articles from basis to dactylus about 5:1:3.75:3.1:4.1:1.5. Basis broad, 1.5 times as long as wide, anterior margin convex, posterior margin slightly convex, anterior margin with four conical stout spines along distal half and two distal setae, posterior margin with five to six serrations all along distal half, each serration usually with one seta, distal corner rounded and produced into a small lobe. Merus 3.2 times as long as broad, with two to three pairs of setae on anterior margin, three spines on posterior margin, two anterodistal setae, posterodistal corner with two setae and one spine about 0.25 times as long as merus. Propodus with four setae on anterior margin, propodal apical tuft with five anterodistal setae (two clearly longer than dactylus). Dactylus slender, slightly curved along distal half.
Pereopod 6 (
Figure 9D): broken in all specimens at carpus-merus level. Coxa 0.95 times as long as wide; anterior margin almost straight, posterior border convex, lacking tooth. Length ratio of articles from basis to merus about 4.8:1:4.5. Basis broad, 1.35 times as long as wide, anterior and posterior margins slightly and regularly convex, anterior margin with five spines along distal half and two distal setae, ten serrations all along posterior margin, each serration usually with one seta, distal corner rounded and produced into a small lobe. Ischium with three anterodistal setae, without spine. Merus 3.4 times as long as broad, with two pairs of setae on anterior margin, three spines on posterior margin on proximal two thirds, four anterodistal and four posterodistal setae.
Pereopod 7 (
Figure 9E): broken in all specimens at carpus-merus level. Coxa 0.7 times as long as wide; anterior margin almost straight, posterior margin convex, lacking tooth. Length ratio of articles from basis to merus about 4.5:1:4.9. Basis broad, 1.35 times as long as wide, anterior and posterior margins convex, anterior margin with five spines and two distal setae, posterior margin with nine serrations, some accompanied by one small seta. Merus 3.3 times as long as broad, with two to three pairs of setae on anterior margin, five spines on posterior margin on proximal ⅔, five anterodistal and five posterodistal setae.
Pleonites (
Figure 10A): all pleonites lacking posterodistal teeth. Pleonite 1 posterior margin almost straight, posteroventral corner rounded to slightly pointed. Pleonite 2 posterior margin straight, posteroventral corner with small tooth. Pleonite 3 posterior margin clearly convex on inferior half, posteroventral corner produced into a well-developed tooth.
Urosomites: similar to male.
Uropod 1 (
Figure 10B): peduncle 1.1 times as long as rami, six dorsolateral spines and a pair of distal spines consisting of a small one and a long spine pointing backwards, dorsomedial border with a row of nineteen to twenty-five spines and one distal spine; outer ramus with five outer and four medial spines, three spines on tip (one much longer); inner ramus with two outer spines and four medial longer and thicker spines, tip with three spines similar to outer ramus.
Uropod 2 (
Figure 10C): peduncle subequal to rami, one distal dorsolateral spine and one distal dorsomedial spine; outer ramus with four outer and two medial spines, two spines on tip (one much longer); inner ramus with two outer and three medial spines, tip with three spines (central much longer).
Uropod 3 (
Figure 10D): peduncle 0.7 times as long as rami, one anterodistal and two dorsomedial spines; outer and inner ramus subequal, outer ramus 2-articulated, article 2 0.35 times as long as article 1, article 1 with one spine and three pairs of spines on outer side; inner ramus with two outer and four medial spines on medial border.
Telson (
Figure 10E): medial cleft 0.85 times as long as telson length; tooth and setae similar to male.