Biological and Environmental Impact of Pharmaceuticals on Marine Fishes: A Review
Abstract
:1. Introduction
2. Pharmaceuticals in the Marine Environment
2.1. Concentrations and Origin of Pharmaceuticals in Seawater
| Non-Steroidal Anti-Inflammatory Drug | Environment Studied | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| Mefenamic acid | Mahdia Coast, Tunisia | Nd–0.6 | [76] |
| Eastern Mediterranean Sea, Greece | <0.2–11 | [77] | |
| Gulf of Cadiz, Southwestern Spain | Nd–4.5 | [78] | |
| Phenylbutazone | Mahdia Coast, Tunisia | Nd–2 | [76] |
| Phenazone | Gulf of Cadiz, Southwestern Spain | Nd–309.8 | [78] |
| Baltic Sea, Germany | 5.9 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 2 | [79] | |
| Indomethacin | Gulf of Cadiz, Southwestern Spain | Nd–4.5 | [78] |
| Tramadol | Eastern Mediterranean Sea, Greece | <0.1–1 | [77] |
| Nimesulide | Portuguese seawaters | Nd–7.3 | [80] |
| Codeine | Southwestern Taiwan | Nd–63.6 | [81] |
| Mediterranean coastal lagoon, Spain | 1.8 | [82] | |
| Oxycodone | Mediterranean coastal lagoon, Spain | 6.8 | [82] |
| Acetylsalicylic acid | Portuguese seawaters | Nd–534 | [80] |
| Acetaminophen | Gran Canaria Island, Spain | Nd–297 | [83] |
| Gulf of Cadiz, Southwestern Spain | Nd–41.5 | [78] | |
| Baltic Sea, Germany | 48 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 2893 | [79] | |
| Eastern Mediterranean Sea, Greece | <41 | [77] | |
| Mediterranean Sea, Israel | 12 | [79] | |
| Seawater from Victoria BC, Canada | 44.7 | [84] | |
| San Francisco Bay, USA | 85 | [79] | |
| Southern California Bight, USA | Nd–11 | [85] | |
| Portuguese seawaters | 51–584 | [80] | |
| Southwestern Taiwan | 2.6–16.7 | [81] | |
| Korean seawater | Nd–48 | [81] | |
| Red Sea, Saudi Arabian coastal waters | 2363 | [86] | |
| Santos Bay, Brazil | Nd–34.6 | [87] | |
| Ketoprofen | Gran Canaria Island, Spain | Nd–106 | [83] |
| Gulf of Cadiz, Southwestern Spain | Nd–2.6 | [78] | |
| Southern Baltic Sea, Polish coastal zone | Nd–72.7 | [88] | |
| Mahdia Coast, Tunisia | Nd–76 | [76] | |
| Portuguese seawaters | 10–90 | [80] | |
| Southwestern Taiwan | Nd–23.3 | [81] | |
| Northern Taiwan seawater | <1.7–6.59 | [89] | |
| Diclofenac | Gran Canaria Island, Spain | Nd–344 | [83] |
| Gulf of Cadiz, Southwestern Spain | Nd–319 | [78] | |
| Baltic Sea, Germany | 9.2 | [79] | |
| Mahdia Coast, Tunisia | Nd–23 | [76] | |
| Eastern Mediterranean Sea, Greece | <1.4–16 | [77] | |
| Mediterranean Sea, Israel | 6.1 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 9.7 | [79] | |
| Southern California Bight, USA | Nd–0.6 | [85] | |
| Portuguese seawaters | Nd–241 | [80] | |
| Santos Bay, Brazil | Nd–19.4 | [87] | |
| Red Sea, Saudi Arabian coastal waters | 14,020 | [86] | |
| Seawater from Singapore | <2–12 | [90] | |
| Marina Bay, Singapore | 4–38 | [91] | |
| Seawater from Northern Taiwan | <2.5–53.6 | [89] | |
| Naproxen | Eastern Mediterranean Sea, Greece | <0.01–0.8 | [77] |
| Gulf of Cadiz, South wstern Spain | Nd–95.8 | [78] | |
| Portuguese seawaters | Nd–178 | [80] | |
| Seawater from Singapore | <0.9–7 | [90] | |
| Marina Bay, Singapore | 13–30 | [91] | |
| Durban Coast, South Africa | Nd–160 | [92] | |
| Southern California Bight, USA | Nd–26 | [85] | |
| Fenoprofen | Gulf of Cadiz, Southwestern Spain | Nd–7.5 | [78] |
| Ibuprofen | Seawater from Singapore | <2–9 | [90] |
| Marina Bay, Singapore | 41–121 | [91] | |
| Red Sea, Saudi Arabian coastal waters | 508 | [86] | |
| Santos Bay, Brazil | 326–2094 | [87] | |
| Durban Coast, South Africa | Nd–166 | [92] | |
| San Francisco Bay, USA | 12 | [79] | |
| Southern California Bight, USA | Nd–30 | [85] | |
| Portuguese seawaters | Nd–222 | [80] | |
| Gulf of Cadiz, Southwestern Spain | Nd–1219.7 | [78] | |
| Aegean and Dardanelles, Greece and Turkey | 35 | [79] | |
| Baltic Sea, Germany | 109 | [79] | |
| Seawater from Tromsø, Norway | Nd–0.7 | [93] | |
| Mediterranean Sea, Israel | 7.1 | [79] | |
| Southwestern Taiwan | Nd–12.1 | [81] | |
| Seawater from Northern Taiwan | <2.5–57.1 | [89] |
| Antibiotic | Environment Studied | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| Amoxicillin | Eastern Mediterranean Sea, Greece | <128 | [77] |
| Ampicillin | Southwest Taiwan | Nd–88.7 | [81] |
| Gulf of Cadiz, Southwestern Spain | Nd–2.0 | [78] | |
| Novobiocin | Gulf of Cadiz, Southwestern Spain | Nd–0.8 | [78] |
| Clarithromycin | Eastern Mediterranean Sea, Greece | <1–1.5 | [77] |
| Aegean Sea and Dardanelles, Greece and Turkey | 16 | [79] | |
| Baltic Sea, Germany | 14 | [79] | |
| Gulf of Cadiz, Southwestern Spain | 0.2–9.4 | [78] | |
| Mediterranean coastal lagoon, Spain | 9.6 | [82] | |
| Pacific Ocean, USA | 130 | [79] | |
| Laizhou Bay, China | Nd–0.82 | [94] | |
| Bohai Sea and Yellow Sea, China | Nd–0.51 | [95] | |
| Yellow Sea, North China | 2.6 | [96] | |
| Trimethoprim | Eastern Mediterranean Sea, Greece | <0.4–3 | [77] |
| Gulf of Cadiz, Southwestern Spain | Nd–10.6 | [78] | |
| Mediterranean coastal lagoon, Spain | 1.5 | [82] | |
| Southern Baltic Sea, Polish coastal zone | Nd–2.9 | [88] | |
| Mediterranean Sea, Tunisia | Nd–3500 | [97] | |
| Southern Californi Bight, USA | Nd–2.1 | [85] | |
| Norfloxacin | Gran Canaria Island, Spain | Nd–3551 | [83] |
| Gulf of Cadiz, Southwestern Spain | Nd–207.5 | [78] | |
| Mediterranean Sea, Tunisia | Nd–20,700 | [97] | |
| Yellow Sea Coast, China | Nd–109 | [98] | |
| Laizhou Bay, China | 7.5–103 | [94] | |
| Bohai Sea, China | Nd–6800 | [99] | |
| Victoria Harbour, Hong Kong | 20.1 | [100] | |
| Hong Kong coastal waters | Nd–8 | [98] | |
| Korean seawater | Nd–0.512 | [101] | |
| Ciprofloxacin | Gran Canaria Island, Spain | Nd–303 | [83] |
| Gulf of Cadiz, Southwestern Spain | Nd–211.7 | [78] | |
| Yellow Sea Coast, China | Nd–26 | [102] | |
| Laizhou Bay, China | Nd–66 | [94] | |
| Bohai Bay, China | Nd–390 | [99] | |
| Korean seawater | Nd–1.25 | [101] | |
| Antarctica | 4–128 | [103] | |
| Clindamycin | Antarctica | <0.1 | [103] |
| Gulf of Cadiz, Southwestern Spain | Nd–4.2 | [78] | |
| Enoxacin | Laizhou Bay, China | Nd–209 | [94] |
| Ofloxacin | Bohai Bay, China | Nd–5100 | [99] |
| Laizhou Bay, China | Nd–6.5 | [94] | |
| Victoria Harbour, Hong Kong | 16.4 | [24] | |
| Korean seawater | Nd–12.4 | [101] | |
| Gulf of Cadiz, Southwestern Spain | Nd–34.4 | [78] | |
| Erythromycin | Mediterranean Sea, Southeast Spain | 0.01–0.03 | [104] |
| Gulf of Cadiz, Southwestern Spain | Nd–2.3 | [78] | |
| Mediterranean coastal lagoon, Spain | 78.4 | [82] | |
| Northern Adriatic Sea, Italy | 5.8 | [79] | |
| San Francisco Bay, USA | 217 | [79] | |
| Pacific Ocean, USA | 86 | [79] | |
| Mediterranean Sea, Tunisia | Nd–3900 | [97] | |
| Bohai Bay, China | Nd–150 | [99] | |
| Bohai Sea and Yellow Sea, China | 0.13–6.7 | [95] | |
| Yellow Sea, North China | 25.2 | [96] | |
| Laizhou Bay, China | 0.9–8.5 | [94] | |
| Hong Kong coastal waters | 16–486 | [98] | |
| Victoria Harbour, Hong Kong | 5.2 | [100] | |
| Korean seawater | Nd–0.196 | [101] | |
| Southwestern Taiwan | Nd–26.6 | [81] | |
| Spiramycin | Gulf of Cadiz, Southwestern Spain | Nd–2.1 | [78] |
| Mediterranean Sea, Tunisia | Nd–66,400 | [97] | |
| Korean seawater | Nd–7.24 | [101] | |
| Neospiramycin | Mediterranean Sea, Tunisia | Nd–4100 | [97] |
| Josamycin | Mediterranean Sea, Tunisia | Nd–1500 | [97] |
| Roxithromycin | Laizhou Bay, China | Nd–1.5 | [94] |
| Bohai Sea and Yellow Sea, China | Nd–0.26 | [95] | |
| Yellow Sea, North China | 6.9 | [96] | |
| Victoria Harbour, Hong Kong | 30.6 | [105] | |
| Gulf of Cadiz, Southwestern Spain | Nd–1.3 | [78] | |
| Baltic Sea, Germany | 16 | [79] | |
| Pacific Ocean, USA | 141 | [79] | |
| Azithromycin | Laizhou Bay, China | Nd–1.2 | [94] |
| Bohai Sea and Yellow Sea, China | Nd–0.39 | [95] | |
| Yellow Sea, North China | 2.5 | [96] | |
| Gulf of Cadiz, Southwestern Spain | Nd–17.8 | [78] | |
| Mediterranean coastal lagoon, Spain | 163.8 | [82] | |
| Lomefloxacin | Yellow Sea Coast, China | Nd–1.2 | [102] |
| Danofloxacin | Yellow Sea Coast, China | Nd–30 | [102] |
| Gulf of Cadiz, Southwestern Spain | Nd–157.5 | [78] | |
| Enrofloxacin | Yellow Sea Coast, China | 0.78–5.1 | [102] |
| Laizhou Bay, China | Nd–7.6 | [94] | |
| Gulf of Cadiz, Southwestern Spain | Nd–122 | [78] | |
| Southern Baltic Sea, Polish coastal zone | Nd | [88] | |
| Mediterranean Sea, Tunisia | Nd–40,200 | [97] | |
| Marbofloxacin | Yellow Sea Coast, China | Nd–22 | [102] |
| Fleorxacin | Yellow Sea Coast, China | Nd–1.4 | [102] |
| Orbifloxacin | Yellow Sea Coast, China | Nd–2.7 | [102] |
| Difloxacin | Yellow Sea Coast, China | Nd–20.7 | [102] |
| Sarafloxacin | Yellow Sea Coast, China | Nd–14.6 | [102] |
| Mediterranean Sea, Tunisia | Nd–5300 | [97] | |
| Sparfloxacin | Yellow Sea Coast, China | Nd–0.79 | [102] |
| Gulf of Cadiz, Southwestern Spain | Nd–14.9 | [78] | |
| Lincomycin | Korean seawater | Nd–438 | [101] |
| Gulf of Cadiz, Southwestern Spain | Nd–6.1 | [78] | |
| Cefalexin | Southwestern Taiwan | Nd–9.19 | [81] |
| Hong Kong coastal waters | Nd–182 | [98] | |
| Cefaclor | Gulf of Cadiz, Southwestern Spain | Nd–9.4 | [78] |
| Cefdinir | Gulf of Cadiz, Southwestern Spain | Nd–15.8 | [78] |
| Cefquinone | Gulf of Cadiz, Southwestern Spain | Nd–44.9 | [78] |
| Ceftiotur | Gulf of Cadiz, Southwestern Spain | Nd–1.7 | [78] |
| Sulfadiazine | Eastern Mediterranean Sea, Greece | <0.1–2 | [77] |
| Gulf of Cadiz, Southwestern Spain | Nd–1.8 | [78] | |
| Mediterranean Sea, Tunisia | Nd–29,100 | [97] | |
| Mahdia Coasta, Tunisia | 6–11 | [76] | |
| Dalian Coast, China | Nd–2 | [106] | |
| Yellow Sea, North China | 0.24 | [96] | |
| Yellow Sea Coast, China | Nd–3.0 | [102] | |
| Bohai Bay, China | Nd–41 | [99] | |
| Bohai Sea and Yellow Sea, China | Nd–0.36 | [95] | |
| Laizhou Bay, China | Nd–0.43 | [94] | |
| Sulfamerazine | Mediterranean Sea, Tunisia | Nd–4500 | [97] |
| Southern Baltic Sea, Polish coastal zone | Nd | [88] | |
| Sulfamoxole | Mediterranean Sea, Tunisia | Nd–800 | [97] |
| Sulfamethoxazole | Eastern Mediterranean Sea, Greece | <0.1–6 | [77] |
| Aegean Sea and Dardanelles, Greece and Turkey | 11 | [79] | |
| Northern Adriatic Sea, Italy | 4.1 | [79] | |
| Mediterranean Sea, Tunisia | Nd–2400 | [97] | |
| Gulf of Cadiz, Southwestern Spain | Nd–99 | [78] | |
| Mediterranean coastal lagoon, Spain | 94 | [82] | |
| Baltic Sea, Germany | 42 | [79] | |
| Southern Baltic Sea, Polish coastal zone | Nd–20.0 | [88] | |
| Baltic Sea, Poland | Nd–10.8 | [88] | |
| Mahdia Coast, Tunisia | 2–6 | [76] | |
| Red Sea, Saudi Arabian coastal waters | 63 | [86] | |
| Dalian Coast, China | Nd–2.2 | [106] | |
| Yellow Sea Coast, China | Nd–212 | [102] | |
| Bohai Bay, China | Nd–140 | [99] | |
| Bohai Sea and Yellow Sea, China | Nd | [95] | |
| Yellow Sea, North China | 50.4 | [96] | |
| Laizhou Bay, China | 1.5–82 | [94] | |
| Korean seawater | Nd–2.20 | [101] | |
| San Francisco Bay, USA | 61 | [79] | |
| Pacific Ocean, USA | 6.4 | [79] | |
| Southern California Bight, USA | Nd–3.4 | [85] | |
| German Baltic Sea | 1.5 | [107] | |
| Sulfathiazole | Mahdia Coast, Tunisia | Nd–3 | [76] |
| Bohai Sea and Yellow Sea, China | Nd–0.17 | [95] | |
| Dalian Coast, China | Nd–1.2 | [106] | |
| Korean seawater | 7.01–18.6 | [101] | |
| Sulfaphenazole | Mediterranean Sea, Tunisia | Nd–600 | [97] |
| Sulfamethizole | Mahdia Coast, Tunisia | 4–11 | [76] |
| Mediterranean Sea, Tunisia | Nd–2800 | [97] | |
| Dalian Coast, China | Nd–13 | [106] | |
| Gulf of Cadiz, Southwestern Spain | Nd–67.1 | [78] | |
| Metronidazole | Mediterranean Sea, Southeast Spain | 13.4 | [104] |
| Gulf of Cadiz, Southwestern Spain | Nd–2.3 | [78] | |
| Nitrofurantoin | Gulf of Cadiz, Southwestern Spain | Nd–21.7 | [78] |
| Ornidazole | Gulf of Cadiz, Southwestern Spain | Nd–1.9 | [78] |
| Sulfamethazine | Mahdia Coast, Tunisia | Nd–3 | [76] |
| Dalian Coast, China | Nd–2.8 | [106] | |
| Yellow Sea Coast, China | Nd–37 | [102] | |
| Bohai Bay, China | Nd–130 | [99] | |
| Laizhou Bay, China | Nd–1.5 | [94] | |
| Gulf of Cadiz, Southwestern Spain | Nd–9.1 | [78] | |
| Southern Baltic Sea, Polish coastal zone | Nd | [88] | |
| Sulfadimidine | Bohai Sea and Yellow Sea, China | Nd–0.16 | [95] |
| Yellow Sea, North China | 0.35 | [96] | |
| Mediterranean Sea, Tunisia | Nd–1800 | [97] | |
| Sulfaquinoxaline | Mediterranean Sea, Tunisia | Nd–1900 | [97] |
| Sulfaguanidine | Mediterranean Sea, Tunisia | Nd–200 | [97] |
| Sulfamethoxyrpyridazine | Mahdia Coast, Tunisia | Nd–5 | [76] |
| Sulfacetamide | Dalian Coast, China | Nd–1.5 | [106] |
| Yellow Sea Coast, China | Nd–4.3 | [102] | |
| Bohai Sea and Yellow Sea, China | Nd–0.12 | [95] | |
| Sulfameter | Dalian Coast, China | Nd–1.9 | [106] |
| Yellow Sea Coast, China | Nd–1.2 | [102] | |
| Sulfamonomethoxine | Dalian Coast, China | Nd–2.3 | [106] |
| Yellow Sea Coast, China | Nd–4.6 | [102] | |
| Sulfadimethoxine | Dalian Coast, China | Nd–1.9 | [106] |
| Yellow Sea Coast, China | Nd–1.9 | [102] | |
| Gulf of Cadiz, Southwestern Spain | Nd–0.9 | [78] | |
| Southern Baltic Sea, Polish coastal zone | Nd–1.0 | [88] | |
| Baltic Sea, Poland | Nd–0.8 | [88] | |
| Sulfapyridine | Mediterranean Sea, Tunisia | Nd–400 | [97] |
| Southern Baltic Sea, Polish coastal zone | Nd–33.2 | [88] | |
| Chloramphenicol | Dalian Coast, China | Nd–1.4 | [106] |
| Gulf of Cadiz, Southwestern Spain | Nd–8.1 | [78] | |
| Timulin | Gulf of Cadiz, Southwestern Spain | Nd–0.8 | [78] |
| Florophenicol | Dalian Coast, China | Nd–2.3 | [106] |
| Oxytetracycline | Dalian Coast, China | 1.1–6.3 | [106] |
| Yellow Sea Coast, China | Nd–13.0 | [102] | |
| Bohai Bay, China | Nd–270 | [99] | |
| Gulf of Cadiz, Southwestern Spain | Nd–25.1 | [78] | |
| Doxycycline | Dalian Coast, China | Nd–1.6 | [106] |
| Yellow Sea Coast, China | Nd–3.2 | [102] | |
| Gulf of Cadiz, Southwestern Spain | Nd–10.3 | [78] | |
| Tetracycline | Dalian Coast, China | Nd–3.8 | [106] |
| Yellow Sea Coast, China | Nd–5.3 | [102] | |
| Bohai Bay, China | Nd–30 | [99] | |
| Hong Kong coastal waters | Nd–122 | [98] | |
| Gulf of Cadiz, Southwestern Spain | Nd–63.3 | [78] | |
| Sulfisoxazole | Yellow Sea Coast, China | Nd–16.5 | [102] |
| Mediterranean Sea, Tunisia | 100–700 | [97] | |
| Sulfachloropyridazine | Yellow Sea Coast, China | Nd–5.9 | [102] |
| Oxolinic acid | Yellow Sea Coast, China | 29–105 | [102] |
| Pyrrole acid | Yellow Sea Coast, China | 0.95–17.5 | [102] |
| Nalidixic acid | Yellow Sea Coast, China | Nd–28.9 | [102] |
| Mediterranean Sea, Tunisia | Nd–16,700 | [97] | |
| Pefloxacic | Yellow Sea Coast, China | Nd–14.6 | [102] |
| Flumequine | Yellow Sea Coast, China | Nd–7.0 | [102] |
| Mediterranean Sea, Southeast Spain | 0.13 | [104] | |
| Gulf of Cadiz, Southwestern Spain | Nd–3.6 | [78] | |
| Dapsone | Mediterranean Sea, Tunisia | Nd–2800 | [97] |
| Antidepressant Drug | Environment Studied | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| Carbamazepine | Eastern Mediterranean Sea, Greece | <1.4 | [77] |
| French coast on the Mediterranean Sea | 0.05–0.71 | [108] | |
| Mediterranean coastal lagoon, Spain | 4.9 | [82] | |
| Mediterranean Sea, Israel | 8.8 | [79] | |
| Gulf of Cadiz, Southwestern Spain | Nd–31.1 | [78] | |
| North Seawater, Germany | 2 | [109] | |
| Baltic Sea, Germany | 157 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 22 | [79] | |
| Northern Adriatic Sea, Italy | 3.1 | [79] | |
| Southwestern Taiwan | Nd–3.83 | [81] | |
| Red Sea, Saudi Arabian coastal waters | 110 | [86] | |
| San Francisco Bay, USA | 13 | [79] | |
| Southern California Bight, USA | Nd–0.9 | [85] | |
| Californian Coast, USA | Nd–21 | [110] | |
| Mahdia Coast, Tunisia | Nd–0.5 | [76] | |
| Korean seawater | 4.58–38.6 | [101] | |
| Seawater from Singapore | <0.3–11 | [90] | |
| Norvenlafaxine | Eastern Mediterranean Sea, Greece | <0.01–2 | [77] |
| Venlafaxine | Rías Baixas coastline, Northwestern Spain | Nd–291 | [111] |
| Citalopram | Eastern Mediterranean Sea, Greece | <0.06–8 | [77] |
| Rías Baixas coastline, Northwestern Spain | Nd–92.5 | [111] | |
| Mediterranean Sea, Israel | 4.3 | [79] | |
| Pacific Ocean, USA | 27 | [79] | |
| Fluoxetine | Mahdia Coast, Tunisia | Nd–41 | [76] |
| Gulf of Cadiz, Southwestern Spain | Nd–0.6 | [78] | |
| Rías Baixas coastline, Northwestern Spain | Nd–10.6 | [111] | |
| Pacific Ocean, USA | 90 | [79] | |
| Amitriptyline | Mahdia Coast, Tunisia | Nd–10 | [76] |
| Gulf of Cadiz, Southwestern Spain | Nd–0.4 | [78] | |
| Hydroxyzine | Rías Baixas coastline, Northwestern Spain | Nd–0.57 | [111] |
| Sertraline | Rías Baixas coastline, Northwestern Spain | Nd–15.3 | [111] |
| Cardiovascular Drug | Environment Studied | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| Timolol | Mahdia Coast, Tunisia | Nd–0.3 | [76] |
| Gulf of Cadiz, Southwestern Spain | Nd–1.1 | [78] | |
| Nadolol | Mahdia Coast, Tunisia | Nd–0.8 | [76] |
| Gulf of Cadiz, Southwestern Spain | Nd–1.6 | [78] | |
| Atenolol | Southern California Bight, USA | Nd–11 | [85] |
| Korean seawater | Nd–85.7 | [101] | |
| Gulf of Cadiz, Southwestern Spain | 0.4–138.9 | [78] | |
| Baltic Sea, Germany | 13 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 194 | [79] | |
| San Francisco Bay, USA | 57 | [79] | |
| Santos Bay, Brazil | Nd | [87] | |
| Propanolol | Korean seawater | Nd–11.9 | [101] |
| Mediterranean coastal lagoon, Spain | 0.5 | [82] | |
| Gulf of Cadiz, Southwestern Spain | Nd–5.9 | [78] | |
| Metoprolol | Mediterranean coastal lagoon, Spain | 0.73 | [82] |
| Gulf of Cadiz, Southwestern Spain | Nd–5.1 | [78] | |
| Baltic Sea, Germany | 158 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 6 | [79] | |
| San Francisco Bay, USA | 32 | [79] | |
| Mediterranean Sea, Israel | 6.7 | [79] | |
| Sotalol | Mediterranean coastal lagoon, Spain | 0.8 | [82] |
| Baltic Sea, Germany | 65 | [79] | |
| Aegean and Dardanelles, Greece and Turkey | 67 | [79] | |
| San Francisco Bay, USA | 12 | [79] | |
| Pindolol | Gulf of Cadiz, Southwestern Spain | Nd–0.7 | [78] |
| Lipid Regulator | Environment Studied | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| Fenofibrate | Mahdia Coast, Tunisia | Nd–14 | [76] |
| Gulf of Cadiz, Southwestern Spain | Nd–1.1 | [78] | |
| Bezafibrate | Gulf of Cadiz, Southwestern Spain | Nd–0.5 | [78] |
| Aegean and Dardanelles, Greece and Turkey | 3.5 | [79] | |
| Mediterranean Sea, Israel | 3.8 | [79] | |
| Gemfibrozil | Seawater from Singapore | <0.09–20 | [90] |
| Marina Bay, Singapore | 1–9 | [91] | |
| Pacific Ocean, USA | 6.2 | [79] | |
| San Francisco Bay, USA | 43 | [79] | |
| Southern California Bight, USA | Nd–13 | [85] | |
| Southwestern Taiwan | Nd–3.67 | [81] | |
| Mediterranean coastal lagoon, Spain | 3.3 | [82] | |
| Gulf of Cadiz, Southwestern Spain | Nd–5.7 | [78] | |
| Aegean and Dardanelles, Greece and Turkey | 18 | [79] | |
| Atorvastatin | Southern California Bight, USA | Nd–0.4 | [85] |
| Estrogen | Environment Studies | Concentration in Water Sample (ng/L) | Reference |
|---|---|---|---|
| 17α ethynylestradiol (EE2) | Aegean Sea, Greece | Nd | [112] |
| Singapore | Nd | [90] | |
| Baltic Sea | 1.7–8.0 | [113] | |
| Southeastern Australia | <0.20 | [114] | |
| Suruga Bay, Japan | <3 | [115] | |
| Sein River, France | Nd | [116] | |
| Bahia, Brazil | Nd | [117] | |
| Yangtze Estuary, China | Nd | [118] | |
| Scheldt Estuary, Belgium | Nd | [119] | |
| Venice Lagoon, Italy | <5–75 | [120] | |
| Venice Lagoon, Italy | <0.8–34 | [121] | |
| Douro River Estuary, Portugal | Nd–83.1 | [122] | |
| Mondego River Estuary, Portugal | Nd | [123] | |
| Halifax Harbour, Canada | Nd–0.14 | [124] | |
| Mondego River, Portugal | 4 | [125] | |
| Ria de Aveiro, Portugal | 20.7–33.2 | [126] | |
| Sado River Estuary, Portugal | 1.1–3.3 | [125] | |
| Dublin Bay, Ireland | Nd | [127] | |
| Yangtze River Estuary, China | Nd–0.11 | [128] | |
| Rio de la Plata Estuary, Argentina | Nd–43 | [129] | |
| Hong Kong and Japan | Nd | [130] | |
| Yundang Lagoon, China | Nd–0.43 | [131] |
2.2. Occurrence of Pharmaceuticals in the Marine Environment
3. Biological Effects of Pharmaceuticals on Marine Fishes
3.1. Non-Steroidal Anti-Inflammatory Drugs
3.2. Antibiotics
3.3. Antidepressant Drugs
3.4. Cardiovascular Drugs
3.5. Lipid Regulators
3.6. Steroid Hormones and Estrogens
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the Aquatic Environments: Evidence of Emerged Threat and Future Challenges for Marine Organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef]
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public. Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the Marine Environment: What Are the Present Challenges in Their Monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef]
- Mezzelani, M.; Regoli, F. The Biological Effects of Pharmaceuticals in the Marine Environment. Annu. Rev. Mar. Sci. 2022, 14, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Gauri, D.; Swati, U.; Tejal, B.; Somkuwar, A.P.; Sonal, D.; More, G.V.; Alka, S.; Limsay, R.P. Pharmaceutical Waste a Global Challenge to Ecosystem. Chron. Aquat. Sci. 2024, 10, 53–64. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Maletz, S.; Floehr, T.; Beier, S.; Klümper, C.; Brouwer, A.; Behnisch, P.; Higley, E.; Giesy, J.P.; Hecker, M.; Gebhardt, W.; et al. In Vitro Characterization of the Effectiveness of Enhanced Sewage Treatment Processes to Eliminate Endocrine Activity of Hospital Effluents. Water Res. 2013, 47, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ruhoy, I.S. The Afterlife of Drugs and the Role of PharmEcovigilance. Drug Saf. 2008, 31, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Calisto, V.; Esteves, V.I. Psychiatric Pharmaceuticals in the Environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Klatte, S.; Schaefer, H.-C.; Hempel, M. Pharmaceuticals in the Environment—A Short Review on Options to Minimize the Exposure of Humans, Animals and Ecosystems. Sustain. Chem. Pharm. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Wille, K.; Noppe, H.; Verheyden, K.; Vanden Bussche, J.; De Wulf, E.; Van Caeter, P.; Janssen, C.R.; De Brabander, H.F.; Vanhaecke, L. Validation and Application of an LC-MS/MS Method for the Simultaneous Quantification of 13 Pharmaceuticals in Seawater. Anal. Bioanal. Chem. 2010, 397, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Hallgren, P.; Wallberg, P.; Pyhälä, M.; Zandaryaa, S. Baltic Marine Environment Protection Commission. Pharmaceuticals in the Aquatic Environment of the Baltic Sea Region: A Status Report; CCB Report: Pharmaceutical Pollution in the Baltic Sea Region; UNESCO Publishing: Uppsala, Sweden, 2017; Volume 1. [Google Scholar]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S.; Abdulghani, M. The Occurrence of Human Pharmaceuticals in Wastewater Effluents and Surface Water of Langat River and Its Tributaries, Malaysia. Int. J. Environ. Anal. Chem. 2013, 93, 245–264. [Google Scholar] [CrossRef]
- Bandala, E.R.; Kruger, B.R.; Cesarino, I.; Leao, A.L.; Wijesiri, B.; Goonetilleke, A. Impacts of COVID-19 Pandemic on the Wastewater Pathway into Surface Water: A Review. Sci. Total Environ. 2021, 774, 145586. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Fang, C.; Deng, Y.; Xu, Z. Intensified Disinfection Amid COVID-19 Pandemic Poses Potential Risks to Water Quality and Safety. Environ. Sci. Technol. 2021, 55, 4084–4086. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and Personal Care Products (PPCPs): A Review on Environmental Contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral Drugs in Aquatic Environment and Wastewater Treatment Plants: A Review on Occurrence, Fate, Removal and Ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and Elimination of Antibiotics at Four Sewage Treatment Plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Kummrow, F. What Do We Know about the Ecotoxicology of Pharmaceutical and Personal Care Product Mixtures? A Critical Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1453–1496. [Google Scholar] [CrossRef]
- Huang, C.; Jin, B.; Han, M.; Yu, Y.; Zhang, G.; Arp, H.P.H. The Distribution of Persistent, Mobile and Toxic (PMT) Pharmaceuticals and Personal Care Products Monitored across Chinese Water Resources. J. Hazard. Mater. Lett. 2021, 2, 100026. [Google Scholar] [CrossRef]
- Aus Der Beek, T.; Weber, F.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment—Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Arpin-Pont, L.; Bueno, M.J.M.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the Marine Environment: A Review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, Impacts and Trends of Pharmaceuticals in the Marine and Coastal Environment. Phil. Trans. R. Soc. B 2014, 369, 20130572. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Arpin-Pont, L.; Gomez, E.; Fenet, H.; Courant, F. Metabolic Profiling Identification of Metabolites Formed in Mediterranean Mussels (Mytilus galloprovincialis) after Diclofenac Exposure. Sci. Total Environ. 2017, 583, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and Ecotoxicological Assessment of Pharmaceuticals: Is There a Risk for the Mediterranean Aquatic Environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn, R.; Brorström-Lundén, E.; Reiersen, L.-O.; Wilson, S. Pharmaceuticals and Personal Care Products (PPCPs) in Arctic Environments: Indicator Contaminants for Assessing Local and Remote Anthropogenic Sources in a Pristine Ecosystem in Change. Environ. Sci. Pollut. Res. 2018, 25, 33001–33013. [Google Scholar] [CrossRef]
- Colzani, L.; Forni, C.; Clerici, L.; Barreca, S.; Dellavedova, P. Determination of Pollutants, Antibiotics, and Drugs in Surface Water in Italy as Required by the Third EU Water Framework Directive Watch List: Method Development, Validation, and Assessment. Environ. Sci. Pollut. Res. 2024, 31, 14791–14803. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Tavazzi, S.; Paracchini, B.; Canuti, E.; Weissteiner, C. Analysis of Polar Organic Contaminants in Surface Water of the Northern Adriatic Sea by Solid-Phase Extraction Followed by Ultrahigh-Pressure Liquid Chromatography–QTRAP® MS Using a Hybrid Triple-Quadrupole Linear Ion Trap Instrument. Anal. Bioanal. Chem. 2013, 405, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C.V. Understanding and Managing Human Threats to the Coastal Marine Environment. Ann. N. Y. Acad. Sci. 2009, 1162, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Arculeo, M.; Arizza, V.; Pace, A.; Melfi, R.; Caracappa, S.; Caracappa, G.; Vullo, C.; Cambera, I.; Visconti, G.; et al. Impact of Heavy Metals in Eggs and Tissues of C. Caretta along the Sicilian Coast (Mediterranean Sea). Environments 2022, 9, 88. [Google Scholar] [CrossRef]
- Savoca, D.; Lo Coco, R.; Melfi, R.; Pace, A. Uptake and Photoinduced Degradation of Phthalic Acid Esters (PAEs) in Ulva Lactuca Highlight Its Potential Application in Environmental Bioremediation. Environ. Sci. Pollut. Res. 2022, 29, 90887–90897. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A.; Arizza, V.; Arculeo, M.; Melfi, R. Controlled Uptake of PFOA in Adult Specimens of Paracentrotus Lividus and Evaluation of Gene Expression in Their Gonads and Embryos. Environ. Sci. Pollut. Res. 2022, 30, 26094–26106. [Google Scholar] [CrossRef] [PubMed]
- Cordero, H.; Mauro, M.; Cuesta, A.; Cammarata, M.; Esteban, M.Á. In Vitro Cytokine Profile Revealed Differences from Dorsal and Ventral Skin Susceptibility to Pathogen-Probiotic Interaction in Gilthead Seabream. Fish Shellfish Immunol. 2016, 56, 188–191. [Google Scholar] [CrossRef]
- Celi, M.; Filiciotto, F.; Vazzana, M.; Arizza, V.; Maccarrone, V.; Ceraulo, M.; Mazzola, S.; Buscaino, G. Shipping Noise Affecting Immune Responses of European Spiny Lobster (Palinurus elephas). Can. J. Zool. 2015, 93, 113–121. [Google Scholar] [CrossRef]
- Celi, M.; Filiciotto, F.; Parrinello, D.; Buscaino, G.; Damiano, A.; Cuttitta, A.; D’Angelo, S.; Mazzola, S.; Vazzana, M. Physiological and Agonistic Behavioural Response of Procambarus clarkii to an Acoustic Stimulus. J. Exp. Biol. 2012, 216, jeb.078865. [Google Scholar] [CrossRef]
- Nicosia, A.; Celi, M.; Vazzana, M.; Damiano, M.A.; Parrinello, N.; D’Agostino, F.; Avellone, G.; Indelicato, S.; Mazzola, S.; Cuttitta, A. Profiling the Physiological and Molecular Response to Sulfonamidic Drug in Procambarus Clarkii. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature Increases, Hypoxia, and Changes in Food Availability Affect Immunological Biomarkers in the Marine Mussel Mytilus Galloprovincialis. J. Comp. Physiol. B 2017, 187, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Pérez-Arjona, I.; Perez, E.J.B.; Ceraulo, M.; Bou-Cabo, M.; Benson, T.; Espinosa, V.; Beltrame, F.; Mazzola, S.; Vazzana, M.; et al. The Effect of Low Frequency Noise on the Behaviour of Juvenile Sparus aurata. J. Acoust. Soc. Am. 2020, 147, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.; Arizza, V.; La Rosa, S.; Queiroz, V.; Mauro, M.; Vazzana, M.; Inguglia, L. Allograft Inflammatory Factor AIF-1: Early Immune Response in the Mediterranean Sea Urchin Paracentrotus Lividus. Zoology 2020, 142, 125815. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Ceraulo, M.; Mauro, M.; Papale, E.; Dioguardi, M.; Mazzola, S.; Arizza, V.; Chiaramonte, M.; Buscaino, G. Effects of Acoustic Stimulation on Biochemical Parameters in the Digestive Gland of Mediterranean Mussel Mytilus Galloprovincialis (Lamarck, 1819). J. Acoust. Soc. Am. 2020, 147, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Mauro, M.; Ceraulo, M.; Dioguardi, M.; Papale, E.; Mazzola, S.; Arizza, V.; Beltrame, F.; Inguglia, L.; Buscaino, G. Underwater High Frequency Noise: Biological Responses in Sea Urchin Arbacia Lixula (Linnaeus, 1758). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110650. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, R.T.; Hinton, D.E. The Toxicology of Fishes; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Cerveny, D.; Grabic, R.; Grabicová, K.; Randák, T.; Larsson, D.G.J.; Johnson, A.C.; Jürgens, M.D.; Tysklind, M.; Lindberg, R.H.; Fick, J. Neuroactive Drugs and Other Pharmaceuticals Found in Blood Plasma of Wild European Fish. Environ. Int. 2021, 146, 106188. [Google Scholar] [CrossRef]
- Ramirez, A.J.; Mottaleb, M.A.; Brooks, B.W.; Chambliss, C.K. Analysis of Pharmaceuticals in Fish Using Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2007, 79, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Painter, M.M.; Bartell, S.E.; Logue, A.; Furlong, E.T.; Werner, S.L.; Schoenfuss, H.L. Selective Uptake and Biological Consequences of Environmentally Relevant Antidepressant Pharmaceutical Exposures on Male Fathead Minnows. Aquat. Toxicol. 2011, 104, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodríguez-Mozaz, S.; Barceló, D. Pharmaceuticals in Biota in the Aquatic Environment: Analytical Methods and Environmental Implications. Anal. Bioanal. Chem. 2012, 404, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodriguez-Mozaz, S.; Lazorchak, J.; Barcelo, D.; Batt, A.; Wathen, J.; Stahl, L. Presence of Pharmaceuticals in Fish Collected from Urban Rivers in the U.S. EPA 2008–2009 National Rivers and Streams Assessment. Sci. Total Environ. 2018, 634, 542–549. [Google Scholar] [CrossRef]
- Subedi, B.; Du, B.; Chambliss, C.K.; Koschorreck, J.; Rüdel, H.; Quack, M.; Brooks, B.W.; Usenko, S. Occurrence of Pharmaceuticals and Personal Care Products in German Fish Tissue: A National Study. Environ. Sci. Technol. 2012, 46, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Haddad, S.P.; Luek, A.; Scott, W.C.; Saari, G.N.; Kristofco, L.A.; Connors, K.A.; Rash, C.; Rasmussen, J.B.; Chambliss, C.K.; et al. Bioaccumulation and Trophic Dilution of Human Pharmaceuticals across Trophic Positions of an Effluent-Dependent Wadeable Stream. Philos.Trans. R. Soc. B 2014, 369, 20140058. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, R.; Nomiyama, K.; Nakamura, H.; Kim, J.-W.; Isobe, T.; Shinohara, R.; Kunisue, T.; Tanabe, S. Uptake and Tissue Distribution of Pharmaceuticals and Personal Care Products in Wild Fish from Treated-Wastewater-Impacted Streams. Environ. Sci. Technol. 2015, 49, 11649–11658. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of Psychoactive Pharmaceuticals in Fish in an Effluent Dominated Stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef]
- Muir, D.; Simmons, D.; Wang, X.; Peart, T.; Villella, M.; Miller, J.; Sherry, J. Bioaccumulation of Pharmaceuticals and Personal Care Product Chemicals in Fish Exposed to Wastewater Effluent in an Urban Wetland. Sci. Rep. 2017, 7, 16999. [Google Scholar] [CrossRef] [PubMed]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and Biomagnification Potential of Pharmaceuticals with a Focus to the Aquatic Environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A Review of the Pharmaceutical Exposome in Aquatic Fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Caçador, I. Ecotoxicology of Marine Organisms, 1st ed.; Duarte, B., Caçador, I., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon-on-Thames, UK, 2019; ISBN 1138035491. [Google Scholar]
- Peake, B.M.; Braund, R.; Tong, A.Y.C.; Tremblay, L.A. The Life-Cycle of Pharmaceuticals in the Environment; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-1-907568-25-1. [Google Scholar]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of Pharmaceuticals, Cosmetics and Hormones in a Sewage Treatment Plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- García Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Removal of Sulfonamide Antibiotics upon Conventional Activated Sludge and Advanced Membrane Bioreactor Treatment. Anal. Bioanal. Chem. 2012, 404, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Toja, Y.; Vázquez-Rowe, I.; Chenel, S.; Marín-Navarro, D.; Moreira, M.T.; Feijoo, G. Eco-Efficiency Analysis of Spanish WWTPs Using the LCA + DEA Method. Water Res. 2015, 68, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Sala-Garrido, R.; Hernández-Sancho, F.; Molinos-Senante, M. Assessing the Efficiency of Wastewater Treatment Plants in an Uncertain Context: A DEA with Tolerances Approach. Environ. Sci. Policy 2012, 18, 34–44. [Google Scholar] [CrossRef]
- Oliveira, P.; Almeida, Â.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and Biochemical Alterations Induced in the Mussel Mytilus Galloprovincialis after Short and Long-Term Exposure to Carbamazepine. Water Res. 2017, 117, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy Use of Prophylactic Antibiotics in Aquaculture: A Growing Problem for Human and Animal Health and for the Environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical Use in Salmon Aquaculture: A Review of Current Practices and Possible Environmental Effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Seo, C.-K.; Kim, T.-H.; Myung, S.-W. Occurrence and Ecological Hazard Assessment of Selected Veterinary Medicines in Livestock Wastewater Treatment Plants. J. Environ. Sci. Health Part B 2013, 48, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Kim, S.-C.; Abd El-Azeem, S.A.M.; Kim, K.-H.; Kim, K.-R.; Kim, K.; Jeon, C.; Lee, S.S.; Ok, Y.S. Veterinary Antibiotics Contamination in Water, Sediment, and Soil near a Swine Manure Composting Facility. Environ. Earth Sci. 2014, 71, 1433–1440. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of Pharmaceuticals in the Lis River (Portugal): Sources, Fate and Seasonal Variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products in the Groundwater: A Review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Afsa, S.; Hamden, K.; Lara Martin, P.A.; Mansour, H.B. Occurrence of 40 Pharmaceutically Active Compounds in Hospital and Urban Wastewaters and Their Contribution to Mahdia Coastal Seawater Contamination. Environ. Sci. Pollut. Res. 2020, 27, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, Distribution and Environmental Risk of Pharmaceutically Active Compounds (PhACs) in Coastal and Ocean Waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Voutsa, D.; Licha, T. Polar Organic Micropollutants in the Coastal Environment of Different Marine Systems. Mar. Pollut. Bull. 2014, 85, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lolić, A.; Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of Non-Steroidal Anti-Inflammatory and Analgesic Pharmaceuticals in Seawaters of North of Portugal: Occurrence and Environmental Risk. Sci. Total Environ. 2015, 508, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-J.; Lee, C.-L.; Fang, M.-D. Emerging Organic Contaminants in Coastal Waters: Anthropogenic Impact, Environmental Release and Ecological Risk. Mar. Pollut. Bull. 2014, 85, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; Rodriguez-Mozaz, S.; Gros, M.; Barceló, D.; León, V.M. Seasonal Distribution of Pharmaceuticals in Marine Water and Sediment from a Mediterranean Coastal Lagoon (SE Spain). Environ. Res. 2015, 138, 326–344. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Torres-Padrón, M.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Assessment of the Presence of Pharmaceutical Compounds in Seawater Samples from Coastal Area of Gran Canaria Island (Spain). Antibiotics 2013, 2, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and Personal Care Products in Municipal Wastewater and the Marine Receiving Environment Near Victoria Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef]
- Vidal-Dorsch, D.E.; Bay, S.M.; Maruya, K.; Snyder, S.A.; Trenholm, R.A.; Vanderford, B.J. Contaminants of Emerging Concern in Municipal Wastewater Effluents and Marine Receiving Water. Environ. Toxicol. Chem. 2012, 31, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of Pharmaceuticals and Personal Care Products in Effluent-Dominated Saudi Arabian Coastal Waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental Impact of Pharmaceuticals from Portuguese Wastewaters: Geographical and Seasonal Occurrence, Removal and Risk Assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Borecka, M.; Białk-Bielińska, A.; Siedlewicz, G.; Kornowska, K.; Kumirska, J.; Stepnowski, P.; Pazdro, K. A New Approach for the Estimation of Expanded Uncertainty of Results of an Analytical Method Developed for Determining Antibiotics in Seawater Using Solid-Phase Extraction Disks and Liquid Chromatography Coupled with Tandem Mass Spectrometry Technique. J. Chromatogr. A 2013, 1304, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.-H.; Nan, F.-H.; Chin, T.-S.; Feng, H.-M. The Occurrence and Distribution of Pharmaceutical Compounds in the Effluents of a Major Sewage Treatment Plant in Northern Taiwan and the Receiving Coastal Waters. Mar. Pollut. Bull. 2012, 64, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Zhang, H.; Desai, M.M.; Ooi, S.K.; Kelly, B.C. Occurrence and Distribution of Pharmaceutically Active and Endocrine Disrupting Compounds in Singapore’s Marine Environment: Influence of Hydrodynamics and Physical–Chemical Properties. Environ. Pollut. 2013, 182, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qian, X.; Yang, Z.; Zhang, L. Study on the Matrix Effect in the Determination of Selected Pharmaceutical Residues in Seawater by Solid-Phase Extraction and Ultra-High-Performance Liquid Chromatography–Electrospray Ionization Low-Energy Collision-Induced Dissociation Tandem Mass Spectrometry. J. Chromatogr. A 2010, 1217, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Ngubane, N.P.; Naicker, D.; Ncube, S.; Chimuka, L.; Madikizela, L.M. Determination of Naproxen, Diclofenac and Ibuprofen in Umgeni Estuary and Seawater: A Case of Northern Durban in KwaZulu–Natal Province of South Africa. Reg. Stud. Mar. Sci. 2019, 29, 100675. [Google Scholar] [CrossRef]
- Weigel, S.; Berger, U.; Jensen, E.; Kallenborn, R.; Thoresen, H.; Hühnerfuss, H. Determination of Selected Pharmaceuticals and Caffeine in Sewage and Seawater from Tromsø/Norway with Emphasis on Ibuprofen and Its Metabolites. Chemosphere 2004, 56, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, G.; Zheng, Q.; Tang, J.; Chen, Y.; Xu, W.; Zou, Y.; Chen, X. Occurrence and Risks of Antibiotics in the Laizhou Bay, China: Impacts of River Discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, J.; Li, J.; Zheng, Q.; Liu, D.; Chen, Y.; Zou, Y.; Chen, X.; Luo, C.; Zhang, G. Antibiotics in the Offshore Waters of the Bohai Sea and the Yellow Sea in China: Occurrence, Distribution and Ecological Risks. Environ. Pollut. 2013, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, J.; Li, J.; Cheng, Z.; Chaemfa, C.; Liu, D.; Zheng, Q.; Song, M.; Luo, C.; Zhang, G. Occurrence and Risks of Antibiotics in the Coastal Aquatic Environment of the Yellow Sea, North China. Sci. Total Environ. 2013, 450–451, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, L.; Van Loco, J.; Anthonissen, R.; Verschaeve, L.; Ben Mansour, H.; Reyns, T. Identification and Risk Assessment of Human and Veterinary Antibiotics in the Wastewater Treatment Plants and the Adjacent Sea in Tunisia. Water Sci. Technol. 2017, 76, 3000–3021. [Google Scholar] [CrossRef] [PubMed]
- Gulkowska, A.; He, Y.; So, M.K.; Yeung, L.W.Y.; Leung, H.W.; Giesy, J.P.; Lam, P.K.S.; Martin, M.; Richardson, B.J. The Occurrence of Selected Antibiotics in Hong Kong Coastal Waters. Mar. Pollut. Bull. 2007, 54, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and Distribution of Antibiotics in Coastal Water of the Bohai Bay, China: Impacts of River Discharge and Aquaculture Activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Zou, S.; Li, X.; Liu, Y. Determination of Selected Antibiotics in the Victoria Harbour and the Pearl River, South China Using High-Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Lee, I.-S.; Oh, J.-E. Human and Veterinary Pharmaceuticals in the Marine Environment Including Fish Farms in Korea. Sci. Total Environ. 2017, 579, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Gu, J.; Ge, L.; Zhang, P.; Wang, Z.; Liu, C.; Zhang, L. Detection of 36 Antibiotics in Coastal Waters Using High Performance Liquid Chromatography-Tandem Mass Spectrometry. Chin. J. Ocean. Limnol. 2011, 29, 1093–1102. [Google Scholar] [CrossRef]
- Hernández, F.; Calısto-Ulloa, N.; Gómez-Fuentes, C.; Gómez, M.; Ferrer, J.; González-Rocha, G.; Bello-Toledo, H.; Botero-Coy, A.M.; Boıx, C.; Ibáñez, M.; et al. Occurrence of Antibiotics and Bacterial Resistance in Wastewater and Sea Water from the Antarctic. J. Hazard. Mater. 2019, 363, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Martínez Bueno, M.J.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Application of Passive Sampling Devices for Screening of Micro-Pollutants in Marine Aquaculture Using LC–MS/MS. Talanta 2009, 77, 1518–1527. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhou, J.L. Simultaneous Determination of Various Pharmaceutical Compounds in Water by Solid-Phase Extraction–Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Fang, X.; Cai, Y.; Ge, L.; Zong, H.; Yuan, X.; Yao, Z.; Zhang, Z. Occurrence, Distribution, and Bioaccumulation of Antibiotics in Coastal Environment of Dalian, China. Mar. Pollut. Bull. 2013, 69, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.; Waniek, J.J.; Schulz-Bull, D.E. Occurrence of Pharmaceuticals and UV-Filters in Riverine Run-Offs and Waters of the German Baltic Sea. Mar. Pollut. Bull. 2017, 124, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Martínez Bueno, M.J.; Herrera, S.; Munaron, D.; Boillot, C.; Fenet, H.; Chiron, S.; Gómez, E. POCIS Passive Samplers as a Monitoring Tool for Pharmaceutical Residues and Their Transformation Products in Marine Environment. Environ. Sci. Pollut. Res. 2016, 23, 5019–5029. [Google Scholar] [CrossRef] [PubMed]
- Weigel, S.; Kuhlmann, J.; Hühnerfuss, H. Drugs and Personal Care Products as Ubiquitous Pollutants: Occurrence and Distribution of Clofibric Acid, Caffeine and DEET in the North Sea. Sci. Total Environ. 2002, 295, 131–141. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Maruya, K.A.; Dodder, N.G.; Lao, W.; Furlong, E.T.; Smalling, K.L. Occurrence of Contaminants of Emerging Concern along the California Coast (2009–2010) Using Passive Sampling Devices. Mar. Pollut. Bull. 2014, 81, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, J.; Rodríguez-Gil, J.L.; Postigo, C.; Mastroianni, N.; López De Alda, M.; Barceló, D.; Valcárcel, Y. Psychoactive Pharmaceuticals and Illicit Drugs in Coastal Waters of North-Western Spain: Environmental Exposure and Risk Assessment. Chemosphere 2019, 224, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Arditsoglou, A.; Voutsa, D. Occurrence and Partitioning of Endocrine-Disrupting Compounds in the Marine Environment of Thermaikos Gulf, Northern Aegean Sea, Greece. Mar. Pollut. Bull. 2012, 64, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.-C.; Bruhn, R.; Gandrass, J.; Ruck, W. Liquid Chromatography–Tandem Mass Spectrometry Analysis of Estrogenic Compounds in Coastal Surface Water of the Baltic Sea. J. Chromatogr. A 2005, 1090, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Allinson, M.; Allinson, G.; Swearer, S.E.; Hassell, K.L. Fluctuations in Natural and Synthetic Estrogen Concentrations in a Tidal Estuary in South-Eastern Australia. Water Res. 2013, 47, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Ueda, Y.; Kurihara, R.; Shiraishi, F. Comparison of the Estrogenic Activities of Seawater Extracts from Suruga Bay, Japan, Based on Chemical Analysis or Bioassay. Environ. Toxicol. Chem. 2007, 26, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Labadie, P.; Budzinski, H. Development of an Analytical Procedure for Determination of Selected Estrogens and Progestagens in Water Samples. Anal. Bioanal. Chem. 2005, 381, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, N.S.; Fahning, C.S.; Cotrim, G.; Dos Anjos, J.P.; De Andrade, J.B.; Hatje, V.; Da Rocha, G.O. A Simple and Sensitive UFLC-Fluorescence Method for Endocrine Disrupters Determination in Marine Waters. Talanta 2013, 117, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yan, C.; Dong, W.; Liu, M.; Zhou, J.; Yang, Y. Occurrence, Distribution and Risk Assessment of Estrogens in Surface Water, Suspended Particulate Matter, and Sediments of the Yangtze Estuary. Chemosphere 2015, 127, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Noppe, H.; Verslycke, T.; De Wulf, E.; Verheyden, K.; Monteyne, E.; Van Caeter, P.; Janssen, C.R.; De Brabander, H.F. Occurrence of Estrogens in the Scheldt Estuary: A 2-Year Survey. Ecotoxicol. Environ. Saf. 2007, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pojana, G.; Bonfà, A.; Busetti, F.; Collarin, A.; Marcomini, A. Estrogenic Potential of the Venice, Italy, Lagoon Waters. Environ. Toxicol. Chem. 2004, 23, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Pojana, G.; Gomiero, A.; Jonkers, N.; Marcomini, A. Natural and Synthetic Endocrine Disrupting Compounds (EDCs) in Water, Sediment and Biota of a Coastal Lagoon. Environ. Int. 2007, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Tiritan, M.E.; Rocha, E.; Rocha, M.J. Seasonal and Spatial Distribution of Several Endocrine-Disrupting Compounds in the Douro River Estuary, Portugal. Arch. Environ. Contam. Toxicol. 2009, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Pardal, M.Â.; Martinho, F.; Margalho, R.; Tiritan, M.E.; Rocha, E.; Rocha, M.J. Distribution of Endocrine Disruptors in the Mondego River Estuary, Portugal. Environ. Monit. Assess. 2009, 149, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.J.; Hui, J.P.M.; Soo, E.C.; Hellou, J. Estrogenic Compounds in Seawater and Sediment from Halifax Harbour, Nova Scotia, Canada. Environ. Toxicol. Chem. 2009, 28, 18–25. [Google Scholar] [CrossRef]
- Rocha, M.J.; Cruzeiro, C.; Reis, M.; Rocha, E.; Pardal, M.A. Determination of 17 Endocrine Disruptor Compounds and Their Spatial and Seasonal Distribution in the Sado River Estuary (Portugal). Toxicol. Environ. Chem. 2013, 95, 237–253. [Google Scholar] [CrossRef]
- Rocha, M.J.; Cruzeiro, C.; Reis, M.; Pardal, M.Â.; Rocha, E. Spatial and Seasonal Distribution of 17 Endocrine Disruptor Compounds in an Urban Estuary (Mondego River, Portugal): Evaluation of the Estrogenic Load of the Area. Environ. Monit. Assess. 2014, 186, 3337–3350. [Google Scholar] [CrossRef] [PubMed]
- Ronan, J.M.; McHugh, B. A Sensitive Liquid Chromatography/Tandem Mass Spectrometry Method for the Determination of Natural and Synthetic Steroid Estrogens in Seawater and Marine Biota, with a Focus on Proposed Water Framework Directive Environmental Quality Standards. Rapid Comm. Mass Spectrom. 2013, 27, 738–746. [Google Scholar] [CrossRef]
- Shi, J.; Liu, X.; Chen, Q.; Zhang, H. Spatial and Seasonal Distributions of Estrogens and Bisphenol A in the Yangtze River Estuary and the Adjacent East China Sea. Chemosphere 2014, 111, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Marino, D.J.; Wunderlin, D.A.; Somoza, G.M.; Ronco, A.E.; Carriquiriborde, P. Screening Concentration of E1, E2 and EE2 in Sewage Effluents and Surface Waters of the “Pampas” Region and the “Río de La Plata” Estuary (Argentina). Bull. Environ. Contam. Toxicol. 2015, 94, 29–33. [Google Scholar] [CrossRef]
- Xu, E.G.; Ho, P.W.-L.; Tse, Z.; Ho, S.-L.; Leung, K.M.Y. Revealing Ecological Risks of Priority Endocrine Disrupting Chemicals in Four Marine Protected Areas in Hong Kong through an Integrative Approach. Environ. Pollut. 2016, 215, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Li, Q.; Li, G.; Guo, Q.; Yan, C. Estrogenic Compounds and Estrogenicity in Surface Water, Sediments, and Organisms from Yundang Lagoon in Xiamen, China. Arch. Environ. Contam. Toxicol. 2011, 61, 93–100. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Kolpin, D.; Furlong, E.; Focazio, M. Environmental Presence and Persistence of Pharmaceuticals an Overview. In Fate of Pharmaceuticals in the Environment and in Water Treatment Systems; Aga, D., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 3–51. ISBN 978-1-4200-5232-9. [Google Scholar]
- Ramirez, A.J.; Brain, R.A.; Usenko, S.; Mottaleb, M.A.; O’Donnell, J.G.; Stahl, L.L.; Wathen, J.B.; Snyder, B.D.; Pitt, J.L.; Perez-Hurtado, P.; et al. Occurrence of Pharmaceuticals and Personal Care Products in Fish: Results of a National Pilot Study in the United States. Environ. Toxicol. Chem. 2009, 28, 2587–2597. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Degradation Kinetics of Pharmaceuticals and Personal Care Products in Surface Waters: Photolysis vs Biodegradation. Sci. Total Environ. 2017, 590–591, 643–654. [Google Scholar] [CrossRef]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. A Year-Long Study of the Spatial Occurrence and Relative Distribution of Pharmaceutical Residues in Sewage Effluent, Receiving Marine Waters and Marine Bivalves. Sci. Total Environ. 2014, 476–477, 317–326. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals in the Marine Environment: A Review. Environ. Rev. 2019, 27, 151–165. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Multi-Residue Analysis of Pharmaceutical Compounds in Aqueous Samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Kaklamanos, G.; Nicolau, A.I.; Stolker, A.A.M. (Linda) High Sensitive Multiresidue Analysis of Pharmaceuticals and Antifungals in Surface Water Using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube River Basin on the Romanian Territory. Sci. Total Environ. 2015, 532, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Nassef, M.; Matsumoto, S.; Seki, M.; Khalil, F.; Kang, I.J.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Acute Effects of Triclosan, Diclofenac and Carbamazepine on Feeding Performance of Japanese Medaka Fish (Oryzias latipes). Chemosphere 2010, 80, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Ribalta, C.; Solé, M. In Vitro Interaction of Emerging Contaminants with the Cytochrome P450 System of Mediterranean Deep-Sea Fish. Environ. Sci. Technol. 2014, 48, 12327–12335. [Google Scholar] [CrossRef]
- Solé, M.; Sanchez-Hernandez, J.C. An in Vitro Screening with Emerging Contaminants Reveals Inhibition of Carboxylesterase Activity in Aquatic Organisms. Aquat. Toxicol. 2015, 169, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Solé, M. The Use of Juvenile Solea Solea as Sentinel in the Marine Platform of the Ebre Delta: In Vitro Interaction of Emerging Contaminants with the Liver Detoxification System. Environ. Sci. Pollut. Res. 2016, 23, 19229–19236. [Google Scholar] [CrossRef] [PubMed]
- Morando, M.B.; Medeiros, L.R.; McDonald, M.D. Fluoxetine Treatment Affects Nitrogen Waste Excretion and Osmoregulation in a Marine Teleost Fish. Aquat. Toxicol. 2009, 93, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature Rise and Microplastics Interact with the Toxicity of the Antibiotic Cefalexin to Juveniles of the Common Goby (Pomatoschistus microp): Post-Exposure Predatory Behaviour, Acetylcholinesterase Activity and Lipid Peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.; Fierro-Castro, C.; Na-Phatthalung, P.; Tvarijonaviciute, A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Evaluation of Gemfibrozil Effects on a Marine Fish (Sparus aurata) Combining Gene Expression with Conventional Endocrine and Biochemical Endpoints. J. Hazard. Mater. 2016, 318, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Díaz-Garduño, B.; Martín-Díaz, M.L. The Impact of Propranolol, 17α-Ethinylestradiol, and Gemfibrozil on Early Life Stages of Marine Organisms: Effects and Risk Assessment. Environ. Sci. Pollut. Res. 2018, 25, 32196–32209. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, C.; Woodhouse, A.J.; Miao, X.-S.; Metcalfe, C.D.; Moon, T.W.; Trudeau, V.L. The Human Lipid Regulator, Gemfibrozil Bioconcentrates and Reduces Testosterone in the Goldfish, Carassius Auratus. Aquat. Toxicol. 2005, 73, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Barreto, A.; Luis, L.G.; Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C.; Soares, A.M.V.M.; Hylland, K.; Loureiro, S.; Oliveira, M. A Multibiomarker Approach Highlights Effects Induced by the Human Pharmaceutical Gemfibrozil to Gilthead Seabream Sparus aurata. Aquat. Toxicol. 2018, 200, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Key, P.B.; Hoguet, J.; Chung, K.W.; Venturella, J.J.; Pennington, P.L.; Fulton, M.H. Lethal and Sublethal Effects of Simvastatin, Irgarol, and PBDE-47 on the Estuarine Fish, Fundulus Heteroclitus. J. Environ. Sci. Health Part B 2009, 44, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Chaves-Pozo, E.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. The Effect of 17α-Ethynylestradiol on Steroidogenesis and Gonadal Cytokine Gene Expression Is Related to the Reproductive Stage in Marine Hermaphrodite Fish. Mar. Drugs 2013, 11, 4973–4992. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-Y.; Lin, C.-Y.; Chen, T.-H. Environmentally Relevant Exposure of 17α-Ethinylestradiol Impairs Spawning and Reproductive Behavior in the Brackish Medaka Oryzias Melastigma. Mar. Pollut. Bull. 2014, 85, 338–343. [Google Scholar] [CrossRef]
- Qin, X.; Lin, H.; Cao, Y.; Wu, R.S.S.; Lai, K.P.; Kong, R.Y.C. Embryo Developmental Toxicity in Marine Medaka (Oryzias melastigma) Due to Parental and Embryonic 17α-Ethinylestradiol Exposure. Sci. Total Environ. 2023, 861, 160594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Gao, M.; Li, X.; Zhao, L.; Ru, S. Polystyrene Microplastics Increase Estrogenic Effects of 17α-Ethynylestradiol on Male Marine Medaka (Oryzias melastigma). Chemosphere 2022, 287, 132312. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.R.; Peterson, D.R.; Kitamura, S.-I.; Segner, H.; Seemann, F.; Au, D.W.T. Sex-Specific Immunomodulatory Action of the Environmental Estrogen 17α-Ethynylestradiol alongside with Reproductive Impairment in Fish. Aquat. Toxicol. 2018, 203, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Abril, S.I.M.; Pin, A.O.; Schonemann, A.M.; Bellot, M.; Gómez-Canela, C.; Beiras, R. Evaluating the Alterations of the Estrogen-Responsive Genes in Cyprinodon Variegatus Larvae for Biomonitoring the Impacts of Estrogenic Endocrine Disruptors (EEDs). Environ. Toxicol. Pharmacol. 2023, 97, 104042. [Google Scholar] [CrossRef] [PubMed]
- Schönemann, A.M.; Beiras, R.; Diz, A.P. Widespread Alterations upon Exposure to the Estrogenic Endocrine Disruptor Ethinyl Estradiol in the Liver Proteome of the Marine Male Fish Cyprinodon variegatus. Aquat. Toxicol. 2022, 248, 106189. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.; Flanagan, S.P.; Jones, A.G. The Effects of Synthetic Estrogen Exposure on the Sexually Dimorphic Liver Transcriptome of the Sex-Role-Reversed Gulf Pipefish. PLoS ONE 2015, 10, e0139401. [Google Scholar] [CrossRef]
- Seemann, F.; Knigge, T.; Rocher, B.; Minier, C.; Monsinjon, T. 17β-Estradiol Induces Changes in Cytokine Levels in Head Kidney and Blood of Juvenile Sea Bass (Dicentrarchus labrax, L., 1758). Mar. Environ. Res. 2013, 87–88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Soloperto, S.; Olivier, S.; Poret, A.; Minier, C.; Halm-Lemeille, M.; Jozet-Alves, C.; Aroua, S. Effects of 17α-Ethinylestradiol on the Neuroendocrine Gonadotropic System and Behavior of European Sea Bass Larvae (Dicentrarchus labrax). J. Toxicol. Environ. Health Part A 2023, 86, 198–215. [Google Scholar] [CrossRef]
- Meek, I.L.; Van De Laar, M.A.F.J.; Vonkeman, H.E. Non-Steroidal Anti-Inflammatory Drugs: An Overview of Cardiovascular Risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A.; Provini, A. Chronic Effects Induced by Ibuprofen on the Freshwater Bivalve Dreissena Polymorpha. Ecotoxicol. Environ. Saf. 2011, 74, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.L.; Van Der Kraak, G. An Investigation into the Role of Prostaglandins in Zebrafish Oocyte Maturation and Ovulation. Gen. Comp. Endocrinol. 2008, 159, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Flippin, J.L.; Huggett, D.; Foran, C.M. Changes in the Timing of Reproduction Following Chronic Exposure to Ibuprofen in Japanese Medaka, Oryzias Latipes. Aquat. Toxicol. 2007, 81, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; Rodríguez-Mozaz, S.; Huerta, B.; Barceló, D.; León, V.M. Do Pharmaceuticals Bioaccumulate in Marine Molluscs and Fish from a Coastal Lagoon? Environ. Res. 2016, 146, 282–298. [Google Scholar] [CrossRef]
- Nassef, M.; Matsumoto, S.; Seki, M.; Kang, I.J.; Moroishi, J.; Shimasaki, Y.; Oshima, Y. Pharmaceuticals and Personal Care Products Toxicity to Japanese Medaka Fish (Oryzias latipes). J. Fac. Agric. Kyushu Univ. 2009, 54, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.; Kim, S.G.; Seki, M.; Kang, I.J.; Hano, T.; Shimasaki, Y.; Oshima, Y. In Ovo Nanoinjection of Triclosan, Diclofenac and Carbamazepine Affects Embryonic Development of Medaka Fish (Oryzias latipes). Chemosphere 2010, 79, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the Aquatic Environment: A Critical Review of the Evidence for Health Effects in Fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Gambino, D.; Savoca, D.; Sucato, A.; Gargano, V.; Gentile, A.; Pantano, L.; Vicari, D.; Alduina, R. Occurrence of Antibiotic Resistance in the Mediterranean Sea. Antibiotics 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Sucato, A.; Vecchioni, L.; Savoca, D.; Presentato, A.; Arculeo, M.; Alduina, R. A Comparative Analysis of Aquatic and Polyethylene-Associated Antibiotic-Resistant Microbiota in the Mediterranean Sea. Biology 2021, 10, 200. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta Caretta a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective Serotonin Re-Uptake Inhibitors (SSRIs) in the Aquatic Environment: An Ecopharmacovigilance Approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Duarte, I.A.; Reis-Santos, P.; Fick, J.; Cabral, H.N.; Duarte, B.; Fonseca, V.F. Neuroactive Pharmaceuticals in Estuaries: Occurrence and Tissue-Specific Bioaccumulation in Multiple Fish Species. Environ. Pollut. 2023, 316, 120531. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Härtter, S. Pharmacokinetics of Selective Serotonin Reuptake Inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef]
- Minagh, E.; Hernan, R.; O’Rourke, K.; Lyng, F.M.; Davoren, M. Aquatic Ecotoxicity of the Selective Serotonin Reuptake Inhibitor Sertraline Hydrochloride in a Battery of Freshwater Test Species. Ecotoxicol. Environ. Saf. 2009, 72, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Caamaño-Tubío, R.I.; Pérez, J.; Ferreiro, S.; Aldegunde, M. Peripheral Serotonin Dynamics in the Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Lado, W.E.; Zamora, J.M.; Duarte-Guterman, P.; Langlois, V.S.; Metcalfe, C.D.; Chang, J.P.; Moon, T.W.; Trudeau, V.L. Waterborne Fluoxetine Disrupts the Reproductive Axis in Sexually Mature Male Goldfish, Carassius Auratus. Aquat. Toxicol. 2010, 100, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Sassine, J.; Trudeau, V.L.; Moon, T.W. Waterborne Fluoxetine Disrupts Feeding and Energy Metabolism in the Goldfish Carassius Auratus. Aquat. Toxicol. 2010, 100, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klaper, R. Environmental Concentrations of the Selective Serotonin Reuptake Inhibitor Fluoxetine Impact Specific Behaviors Involved in Reproduction, Feeding and Predator Avoidance in the Fish Pimephales promelas (Fathead Minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ashiwaju, B.I.; Uzougbo, C.G.; Orikpete, O.F. Environmental Impact of Pharmaceuticals: A Comprehensive Review. Matrix Sci. Pharma 2023, 7, 85–94. [Google Scholar] [CrossRef]
- Winder, V.L.; Pennington, P.L.; Hurd, M.W.; Wirth, E.F. Fluoxetine Effects on Sheepshead Minnow (Cyprinodon variegatus) Locomotor Activity. J. Environ. Sci. Health Part B 2012, 47, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Oakeshott, J.G.; Sanchez-Hernandez, J.; Wheelock, C. Carboxylesterases in the Metabolism and Toxicity of Pesticides. Anticholinesterase Pesticides: Metabolism, Neurotoxicity and Epidemiology; John Wily & Sons: Hoboken, NJ, USA, 2010; pp. 57–76. ISBN 9780470640500. [Google Scholar]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP Effluents and Their Solar Photodegradation in Aquatic Environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Calamari, D. Assessment of Persistent and Bioaccumulating Chemicals in the Aquatic Environment. Toxicology 2002, 181–182, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Fanelli, R.; Calamari, D.; Bagnati, R.; Zuccato, E. Methodological Approaches for Studying Pharmaceuticals in the Environment by Comparing Predicted and Measured Concentrations in River Po, Italy. Regul. Toxicol. Pharmacol. 2004, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of Therapeutic Drugs in the Environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A Tool for Removal of Pharmaceuticals, Contrast Media and Musk Fragrances from Wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxēaus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Environmental Risk Assessment of Six Human Pharmaceuticals: Are the Current Environmental Risk Assessment Procedures Sufficient for the Protection of the Aquatic Environment? Environ. Toxicol. Chem. 2004, 23, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.J.; Thomas, K.V. Determination of Selected Human Pharmaceutical Compounds in Effluent and Surface Water Samples by High-Performance Liquid Chromatography–Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2003, 1015, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the Environmental Transport of Human Pharmaceuticals to Streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment-A Review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lortie, M.B.; Moon, T.W. The Rainbow Trout Skeletal Muscle β-Adrenergic System: Characterization and Signaling. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R689–R697. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, J.G.; Dugan, S.G.; Drouin, G.; Perry, S.F.; Moon, T.W. Activity of the Unique β-Adrenergic Na +/H + Exchanger in Trout Erythrocytes Is Controlled by a Novel β3-AR Subtype. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R526–R535. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Giltrow, E.; Huggett, D.; Hutchinson, T.; Saye, J.; Winter, M.; Sumpter, J. Comparative Physiology, Pharmacology and Toxicology of β-Blockers: Mammals versus Fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome Proliferator-Activated Receptors: Nuclear Control of Metabolism*. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Torra, I.P.; Fruchart, J.-C.; Staels, B. Regulation of Lipid and Lipoprotein Metabolism by PPAR Activators. Clin. Chem. Lab. Med. (CCLM) 2000, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, B.; Andersen, Ø.; Dehli, A.; Östlund Farrants, A.-K.; Gjøen, T.; Thomassen, M.S. Peroxisome Proliferator Activated Receptors in Atlantic Salmon (Salmo salar): Effects on PPAR Transcription and Acyl-CoA Oxidase Activity in Hepatocytes by Peroxisome Proliferators and Fatty Acids. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1997, 1348, 331–338. [Google Scholar] [CrossRef]
- Scarano, L.J.; Calabrese, E.J.; Kostecki, P.T.; Baldwin, L.A.; Leonard, D.A. Evaluation of a Rodent Peroxisome Proliferator in Two Species of Freshwater Fish: Rainbow Trout (Onchorynchus mykiss) and Japanese Medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 1994, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Prindiville, J.S.; Mennigen, J.A.; Zamora, J.M.; Moon, T.W.; Weber, J.-M. The Fibrate Drug Gemfibrozil Disrupts Lipoprotein Metabolism in Rainbow Trout. Toxicol. Appl. Pharmacol. 2011, 251, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Raldúa, D.; André, M.; Babin, P.J. Clofibrate and Gemfibrozil Induce an Embryonic Malabsorption Syndrome in Zebrafish. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Almeida, Â.; Silva, M.G.; Soares, A.M.V.M.; Freitas, R. Concentrations Levels and Effects of 17alpha-Ethinylestradiol in Freshwater and Marine Waters and Bivalves: A Review. Environ. Res. 2020, 185, 109316. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-Ethynylestradiol (EE2) in the Environment and Effect on Exposed Biota: A Review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex Male Production in Finfish as Exemplified by Tilapia: Applications, Problems, and Prospects. In Reproductive Biotechnology in Finfish Aquaculture; Elsevier: Amsterdam, The Netherlands, 2001; pp. 283–301. ISBN 978-0-444-50913-0. [Google Scholar]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, Bioaccumulation and Risk Assessment of Lipophilic Pharmaceutically Active Compounds in the Downstream Rivers of Sewage Treatment Plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 116, pp. 135–170. ISBN 978-0-12-815561-5. [Google Scholar]
- Milla, S.; Depiereux, S.; Kestemont, P. The Effects of Estrogenic and Androgenic Endocrine Disruptors on the Immune System of Fish: A Review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen Signaling Multiple Pathways to Impact Gene Transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W. A Life-Long Search for the Molecular Pathways of Steroid Hormone Action. Mol. Endocrinol. 2005, 19, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A.Y. A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. Int. J. Endocrinol. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paut Kusturica, M.; Jevtic, M.; Ristovski, J.T. Minimizing the Environmental Impact of Unused Pharmaceuticals: Review Focused on Prevention. Front. Environ. Sci. 2022, 10, 1077974. [Google Scholar] [CrossRef]
- Roy, B.; Basak, R.; Rai, U. Impact of Xenoestrogens on Sex Differentiation and Reproduction in Teleosts. Aquac. Fish. 2022, 7, 562–571. [Google Scholar] [CrossRef]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; Van Aerle, R.; Santos, E.; Brighty, G. Predicted Exposures to Steroid Estrogens in U.K. Rivers Correlate with Widespread Sexual Disruption in Wild Fish Populations. Environ. Health Perspect. 2006, 114, 32–39. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Johnson, A.C.; Runnalls, T.J. Pharmaceuticals in the Aquatic Environment: No Answers Yet to the Major Questions. Environ. Toxicol. Chem. 2024, 43, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Notch, E.G.; Miniutti, D.M.; Mayer, G.D. 17α-Ethinylestradiol Decreases Expression of Multiple Hepatic Nucleotide Excision Repair Genes in Zebrafish (Danio rerio). Aquat. Toxicol. 2007, 84, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, M.; Craft, J.A.; Lehtonen, K.K.; Lindström, K. An Endocrine Disrupting Chemical Changes Courtship and Parental Care in the Sand Goby. Aquat. Toxicol. 2010, 97, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Partridge, C.; Boettcher, A.; Jones, A.G. Short-Term Exposure to a Synthetic Estrogen Disrupts Mating Dynamics in a Pipefish. Horm. Behav. 2010, 58, 800–807. [Google Scholar] [CrossRef]
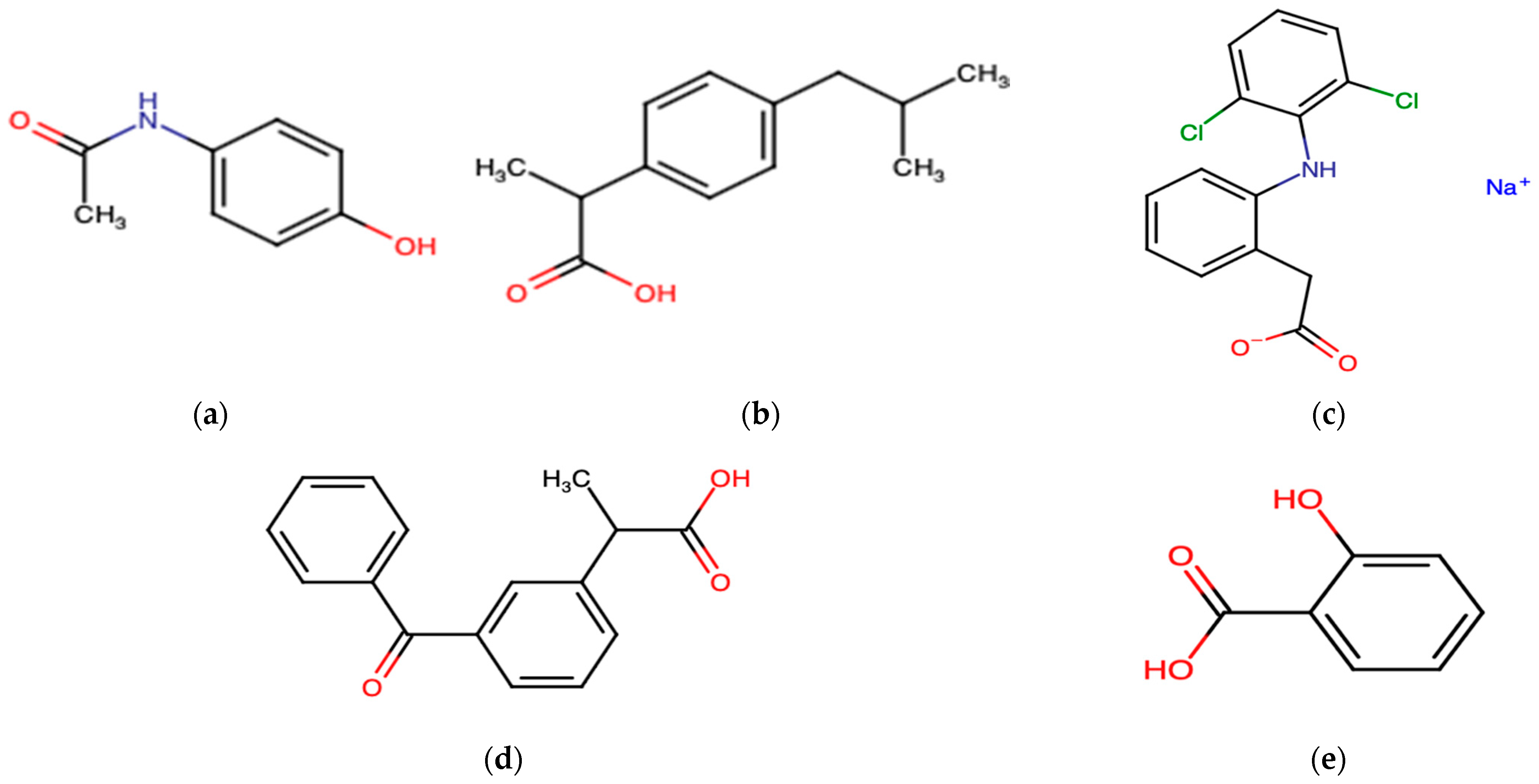
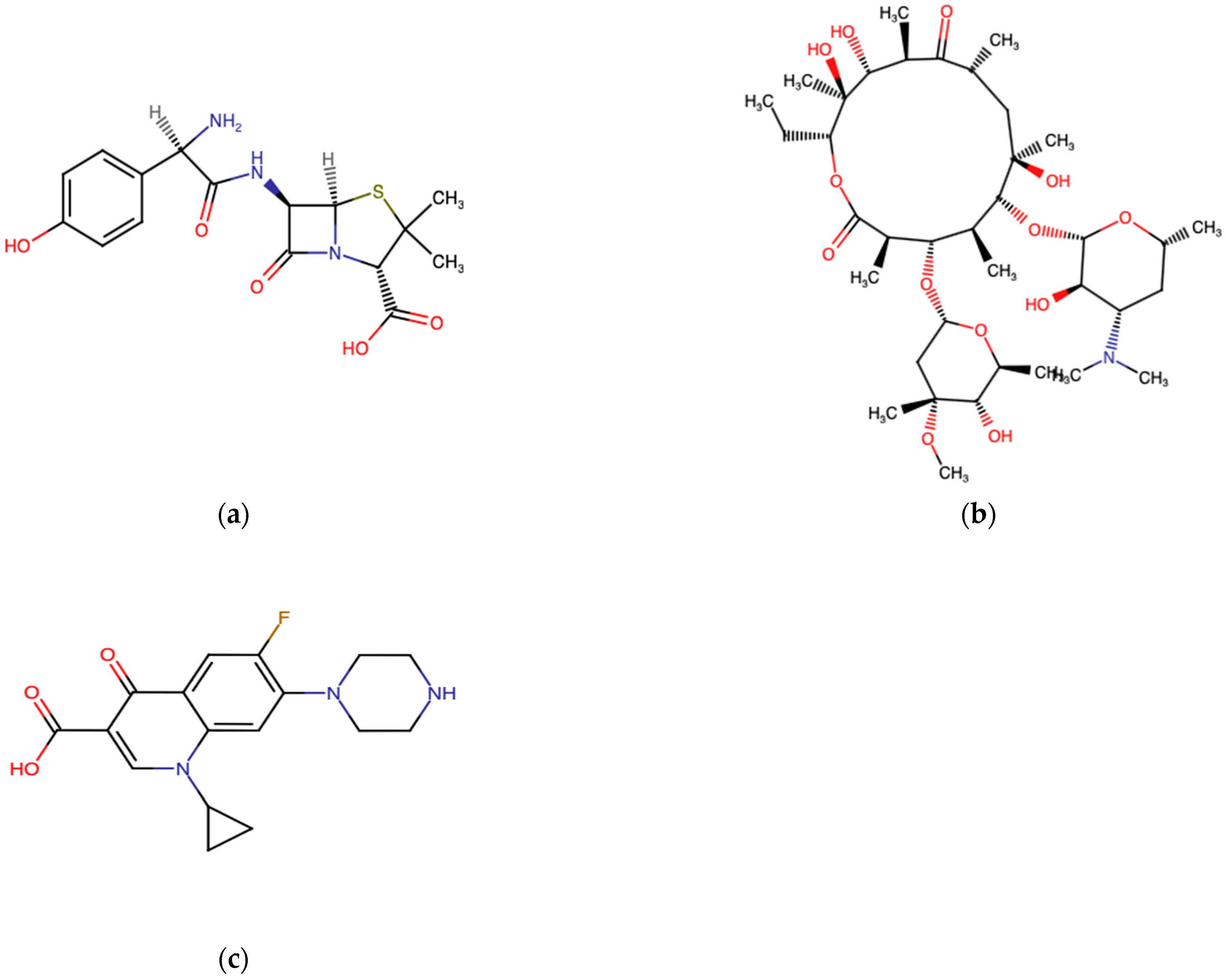
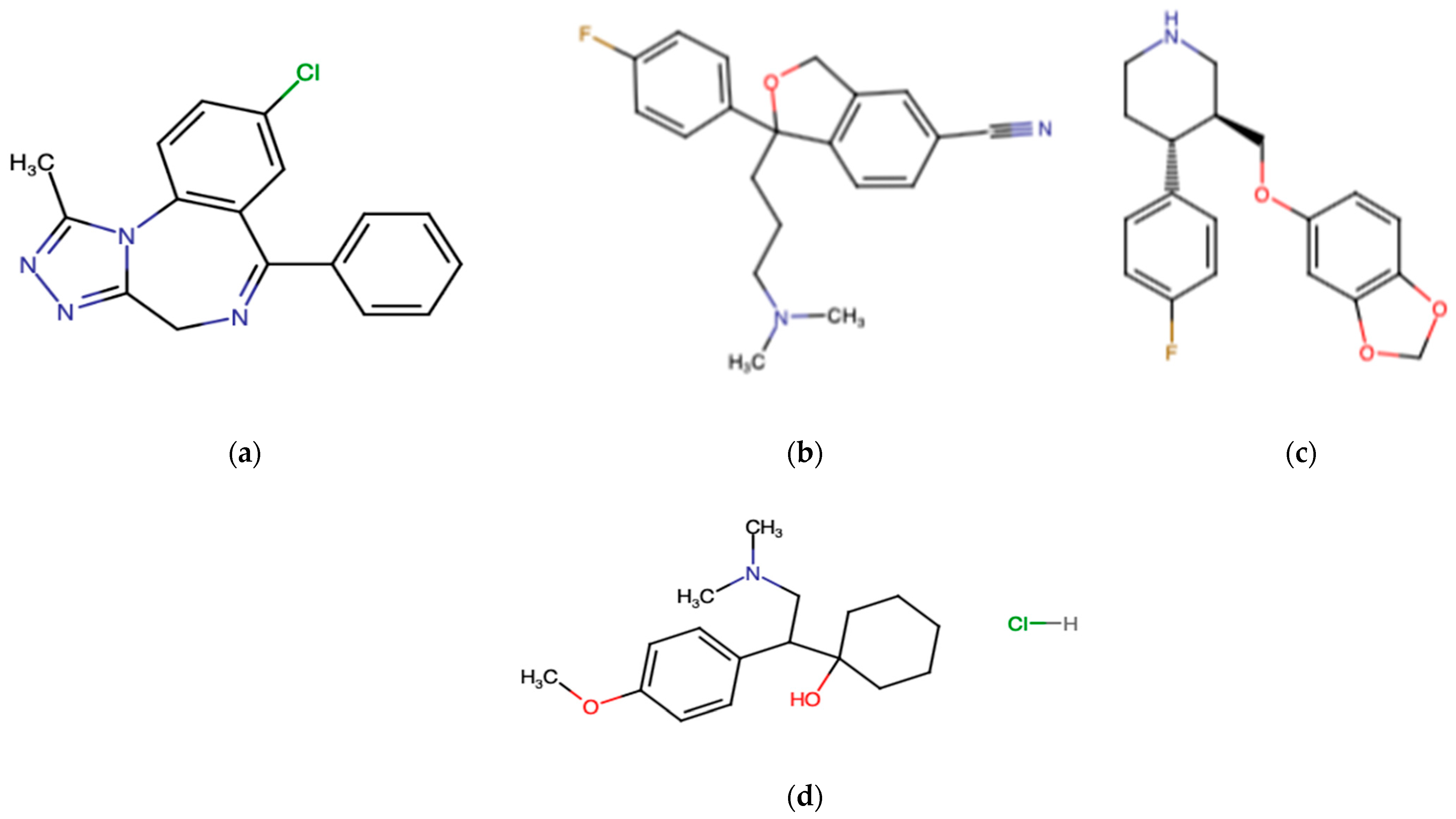
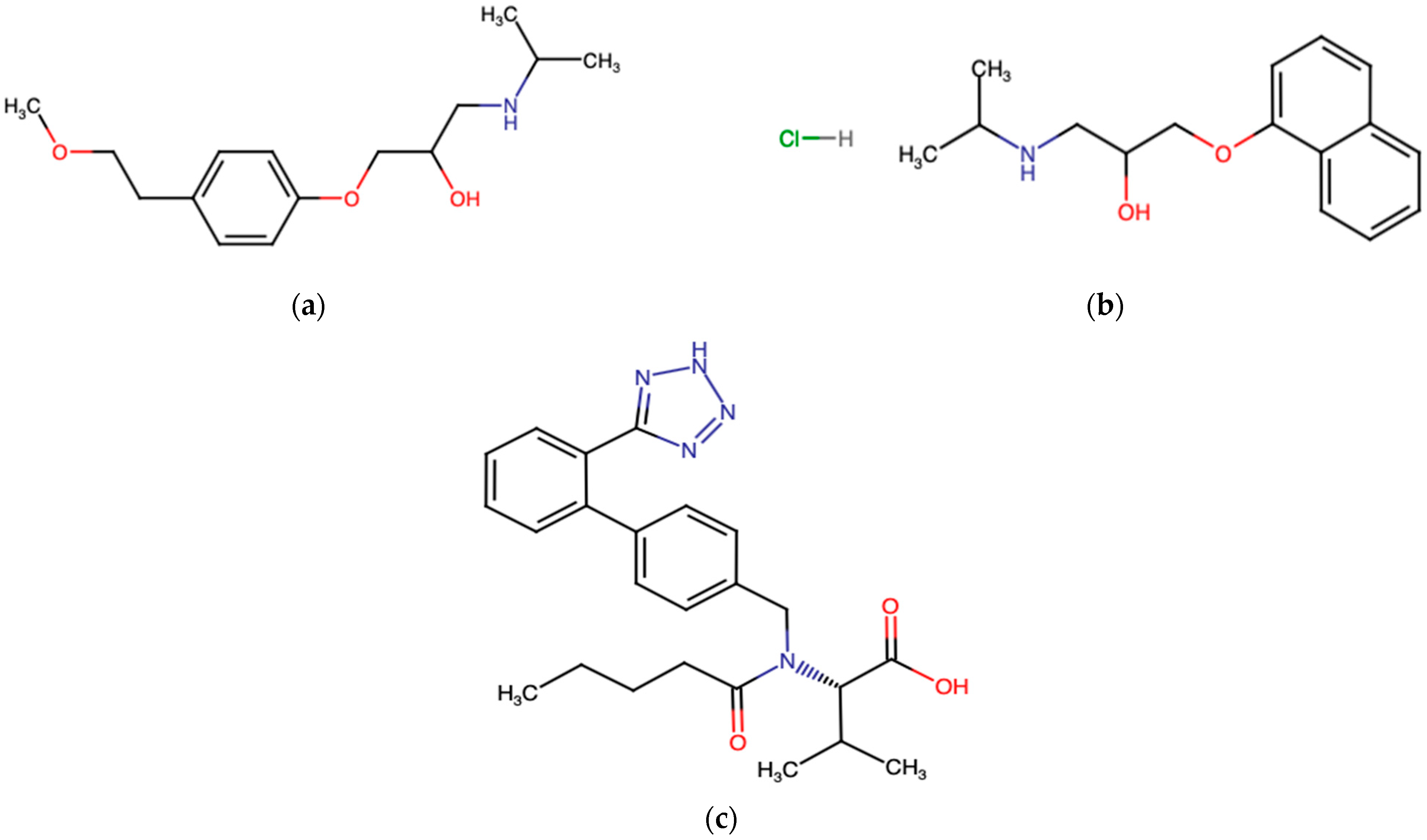

| Therapeutical Class | Species | Molecule | Exposure Doses | Effects | Reference |
|---|---|---|---|---|---|
| Non-steroidal anti-inflammatory drugs | Oryzias latipes | Diclofenac | 1.0 mg/L; 12–15 ng | Change in swimming; decrease in the number of eggs | [140] |
| Trachyrincus scabrus | Diclofenac | 100 μM | Interference with the metabolism of fish (CYP1A and CYP3A metabolism) | [141] | |
| Alocephalus rostratus | Diclofenac | 100 μM | Inhibition of CE activity | [142] | |
| Solea solea | Ibuprofen | 100 μM | Inhibition of the activity of the CYP3A4 enzyme | [143] | |
| Dicentrarchus labrax, Alocephalus rostratus, Cataetix laticeps | Ibuprofen | 100 μM | Moderate inhibition of CE activity | [142] | |
| Solea solea, Dicentrarchus labrax, Alocephalus rostratus, Cataetix laticeps | Acetaminophen | 100 μM | No change in CE activity | [142] | |
| Antidepressant drugs | Solea solea, Dicentrarchus labrax, Alocephalus rostratus, Cataetix laticeps | Fluoxetine | 100 μM | No change in CE activity | [142] |
| Alocephalus rostratus, Mora moro | Fluoxetine | 100 μM | Significant inhibition on BFCOD activity | [141] | |
| Opsanus beta | Fluoxetine | 25 μg/g | Significant influence on branchial ureal excretion | [144] | |
| Antibiotics | Pomatoschistus microps | Cefalexin | 1.3–10 mg/L | Changes in feeding, behavior, biomarker responses, and predation performance | [145] |
| Cardiovascular drugs | Dicentrarchus labrax, Cataetix laticeps | Propanolol | 100 μM | Inhibition of CE activity | [142] |
| Lipid regulators | Solea senegalensis | Gemfibrozil | 1 mg/kg | Inhibition of antioxidant defences, induction of the activity of CYP-related and Phase II biotransformation enzymes | [142] |
| Sparus aurata | Gemfibrozil | 150 μg/L | Upregulated transcription of PPAR-related genes Increased cortisol levels | [146] | |
| Sparus aurata | Gemfibrozil | 500 ng/L | Influence on the survival of larvae | [147] | |
| Carassius auratus | Gemfibrozil | 1.5 μg/L | Reduced testosterone levels | [148] | |
| Sparus aurata | Gemfibrozil | 1.5–15,000 μg/L | Increase in CAT and GR in the gills. Decrease in GPx and GR in the liver | [149] | |
| Dicentrarchus labrax, Trachyrincus scabrus, Alocephalus rostratus, Cataetix laticeps | Simvastatin | 100 μM | Inhibition of CE activity | [142] | |
| Fundulus heteroclitus | Simvastatin | 1.25 mg/L | Decrease in AChE levels | [150] | |
| Solea solea | Simvastatin | 100 μM | Influence on CbE activity | [143] | |
| Dicentrarchus labrax, Solea solea, Trachyrincus scabrus, Alocephalus rostratus, Cataetix laticeps | Fenofibrate | 100 μM | Inhibition of CE activity | [142] | |
| Solea solea | Fenofibrate | 100 μM | Influence on CbE activity | [143] |
| Molecule | Species | Exposure Doses | Effects | Reference |
|---|---|---|---|---|
| EE2 | Sparus aurata | 5 μg/g | Sperm motility and reduction in seminal liquid; changes in the levels of sex steroidal hormones and the gene expression profiles of various enzymes involved in the production of steroids; increases in the expression profile of the hepatic vtg gene; modulation in the expression of testicular protein and some hormone receptor genes in the gonads | [151] |
| EE2 | Sparus aurata | 50 and 500 ng/L | Decrease in the survival of fish larvae | [147] |
| EE2 | Oryzyas melastigma | 50–100 ng/L | Reduction in spawning and reproductive behavior | [152] |
| EE2 | Oryzyas melastigma | 90 ng/L | Growth retardation, reduction in embryos’ heart rate, decrease in the hatching rate, impaired larval locomotion, delays in the appearance of eye pigmentation | [153] |
| EE2 co-exposure to microplastics | Oryzyas melastigma | 20–200 μg/L | Reductions in gonadosomatic and hepatosomatic growth and indices; increases in transcription levels of estrogen biomarker and estrogen receptor genes | [154] |
| EE2 | Oryzyas melastigma | 113 ng/L | Effects on immune function and reproduction of females | [155] |
| EE2 | Cyprinodon variegatus | 1000 ng/L | Alterations in the expression of estrogen genes | [156] |
| EE2 | Cyprinodon variegatus | 100 ng/L | Alterations in proteomic metabolism. | [157] |
| Syngnathus scovelli | 5 ng/L | Upregulation of estrogen biomarker genes | [158] | |
| EE2 | Dicentrarchus labrax | 0.2–200 ng/L | Alterations in cytokine levels | [159] |
| EE2 | Dicentrarchus labrax | 0.5–50 nM | Alterations in the neuroendocrine gonadotropic system | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punginelli, D.; Maccotta, A.; Savoca, D. Biological and Environmental Impact of Pharmaceuticals on Marine Fishes: A Review. J. Mar. Sci. Eng. 2024, 12, 1133. https://doi.org/10.3390/jmse12071133
Punginelli D, Maccotta A, Savoca D. Biological and Environmental Impact of Pharmaceuticals on Marine Fishes: A Review. Journal of Marine Science and Engineering. 2024; 12(7):1133. https://doi.org/10.3390/jmse12071133
Chicago/Turabian StylePunginelli, Diletta, Antonella Maccotta, and Dario Savoca. 2024. "Biological and Environmental Impact of Pharmaceuticals on Marine Fishes: A Review" Journal of Marine Science and Engineering 12, no. 7: 1133. https://doi.org/10.3390/jmse12071133
APA StylePunginelli, D., Maccotta, A., & Savoca, D. (2024). Biological and Environmental Impact of Pharmaceuticals on Marine Fishes: A Review. Journal of Marine Science and Engineering, 12(7), 1133. https://doi.org/10.3390/jmse12071133







