Abstract
Ostrea chilensis (a flat oyster) is native to Chile and New Zealand. In Chile, this oyster has been cultured and harvested for at least 80 years. However, the culture of flat oysters has not developed like other aquaculture activities in Chile, mainly due to the inefficiency of the collectors (made of ribbed mussel shells) and the lack of spats produced. The objective of this study was to determine the capacity of spat collectors for the settlement of O. chilensis. For this purpose, field and laboratory experiments were carried out. Our results indicated that oyster larvae do not have a preference for either side of the shell (periostracum or nacreous) to settle on. However, after one year of growth in the field, juveniles were more abundant on the nacreous side of the shell (spat collector). Also, the oysters that settled on the nacreous side of the valve were larger. This was due to the fact that the periostracum had a greater number of epibionts, and they competed for space and resources with the settled oyster spats, causing a decrease in the abundance of oyster spats on that surface. Our findings raise the possibility that collectors could improve efficiency in the production of spats. Finally, we discuss the implications of these results for the Chilean oyster-farming industry.
1. Introduction
Oyster farming is conducted in several countries, and both native and introduced species are cultivated []. Of these, flat oysters (Ostrea spp.) have one of the highest market values []. As a result, the abundance of some natural populations of flat oysters has declined due to exploitation, and in some cases, they have become locally extinct (e.g., Ostrea edulis in the northeastern Atlantic; [,,]). Therefore, there has been a growing interest in restoring extinct populations (e.g., [,,]).
The culture of flat oysters depends to a great extent on the collection of spats (larvae) from the marine environment, although it is also performed artificially in hatcheries (e.g., []). This process is contingent upon the successful settlement and recruitment of larvae, which are closely related to the suitability and availability of the marine substrate [,,].
The Chilean flat oyster, Ostrea chilensis, is a commercially important species and is considered an excellent food based on its pleasant organoleptic characteristics []. This mollusc is native to Chile and New Zealand, with culture and fisheries being carried out in both countries [,]. In Chile, landings have increased in the last decade due to the high demand for this oyster (>500 tonnes per year: []). Furthermore, Chilean oyster cultivation is of particular interest, as the flat oyster cultivation worldwide has decreased due to overfishing and diseases such as Bonamiosis [].
The Chilean flat oyster is a protandrous sequential hermaphrodite mollusc with internal fertilization. The first gonadal maturation is as a male, and afterwards, it changes its sex to a female []. The average brooding female has an estimated fecundity of 60,000 larvae each season [,]. The larvae, which are inside the pallial cavity, are released into the water column after two to nine weeks of incubation [,,], where they swim for a short period of time (from a few minutes to hours; []). It is at this stage that the larvae are collected for aquaculture purposes.
Oyster culture begins with the collection of larvae from sites close to their natural beds using spat collectors. Spat collectors should be efficient because there is only one opportunity a year to collect the larvae [,,]. In addition, spat collectors must be cheap, easy to handle, and adaptable to the local area []. Oyster aquaculture depends on the success of the collection process, and hence, on the annual production of oysters. In Chile, spat collectors have been made using ribbed mussel (Aulacomya atra) shells, a low-cost material that is considered a suitable substrate for larval settlement []. The shells are arranged around a rope that measures approximately 1.5 m (see Figure S1). Each rope is attached to the centre line of the suspended system. In Chile, between 2000 and 10,000 collectors are installed every year during the oyster’s reproductive season []. Once the larvae have settled, they remain on the shell for approximately 12 months. Subsequently, the oysters are manually removed to be relocated to suspended growth systems (e.g., pearl nets or Japanese lanterns) until they reach a marketable size (50 mm) after 3–4 years [,]. The growth rate fluctuates between culture sites, as it depends on environmental conditions such as the quantity and quality of food, the temperature, and the salinity [,].
Knowledge about oyster spat collectors is scarce and has been generated mainly by oyster farmers. Even though spat collectors are used mainly due to their low cost (USD 1.5 for each collector in 2020; personal communication, Juan Pablo Fuentes, Quempillén center of the Universidad Austral de Chile), oyster farmers have stated disadvantages associated with the number of spats that settle on the shells, which can affect the collection efficiency and the annual production of oysters, which are subject to a greater demand each year.
The objective of this study was to analyse the collection of oyster spats (O. chilensis) on the shells of the ribbed mussel (A. atra) through laboratory and field culture experiments to determine the efficiency of the collectors with respect to the number of spats that settle, and their growth.
2. Materials and Methods
To evaluate the collection of oyster spats, two experiments were carried out, which are described below.
2.1. Laboratory Experiment: Spat Collection
Adult oysters were collected from the Quempillén estuary (Chiloé Island, Chile) (41°52′13.5” S; 73°46′02.5” W) (Figure 1) during a reproductive season and transferred to the Reproductive Biology Laboratory of the Centro de Investigación Marina Quintay (CIMARQ) of the Andrés Bello University, Chile. The oysters (200 = 53.98 to 55.53 mm maximum shell length) were kept in filtered seawater with constant aeration and fed ad libitum with the microalga Isochrysis galbana. During this period (25 days), the average temperature was 15.4 °C (±0.7). Four containers of 250 L each were placed in the laboratory, with 50 oysters (broodstock) on each unit to facilitate reproduction. Prior to the larval release, spat collectors (eight in total) were installed in each experimental container. Each spat collector contained 25 shells of the ribbed mussel (A. atra), which had an average length of 53.2 (± 2.2) mm. Each valve within the collector was separated by a piece (10 cm) of polyethylene hose (see Figure S1). The containers were constantly aerated, and the seawater was changed every three days. Thirty days after the release of the larvae, the number of oysters (spats) on both sides of the shell (nacreous and periostracum) of each collector was counted. During this period, the oysters were continuously fed with I. galbana at ca. 1.5 mg L−1 using a peristaltic pump.
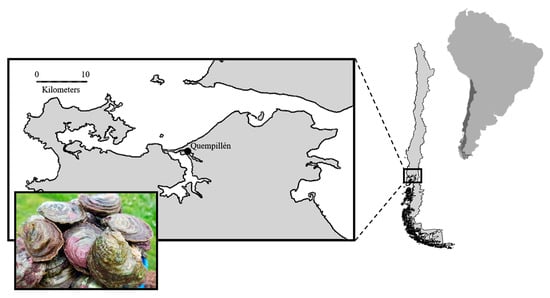
Figure 1.
Map of the collection site of Chilean flat oysters (Ostrea chilensis).
2.2. Field Experiment: Spat Collection
The experiment was carried out at the Quempillén estuary (Figure 1). At the end of November 2019, twenty spat collectors (described above; see Figure S1) were installed in the water column. These were located in areas where oyster spats have historically been collected. After 12 months in the water, the number of oyster spats that settled on both sides of the shell (periostracum and nacreous) were estimated, and the size of the oysters was measured (from the umbo to the farthest point on the posterior edge of the shell). The cover of shell epibionts on each collector surface was also estimated. To achieve this, we captured high-resolution digital photographs of each individual shell. Subsequently, the total area of each valve and the area of the epibionts on the valves were measured. In this way, the percentage of epibiont coverage on each side of the valves was calculated (see Figure S2). The ImageJ v.153a software [] was used to estimate the coverage areas using the ROI tool. The results were expressed in percentages.
2.3. Statistical Analysis
The results were expressed as the mean ± the standard error of the mean (SE). A completely randomized factorial analysis of variance (ANOVA) was performed to estimate the effects of the shell side (treatment = periostracum and nacreous) on the settlement of the oyster spats (dependent variable) in the laboratory experiment. In the variance analysis model, the following fixed factors were used: the treatment and the oyster spat collector (replica). The same statistical strategy was employed to assess the quantity of oyster seeds on collectors deployed in the sea. The fixed factors included the shell side, the orientation of the ribbed mussel valve with respect to the benthos, and the spat collectors. In the same way, the effect of the shell side of the collectors on the size of the settled oysters and on the epibiont cover was evaluated. Prior to the analyses, the assumptions of homoscedasticity and normality of the data were tested using the Levene and Kolmogorov–Smirnof tests, respectively. When there were significant differences, a Tukey post hoc test was performed. In all the analyses, a significance level of 0.05 with 95% confidence was used to determine statistically significant differences. The analyses were performed using the R package v.4.0.3 [].
3. Results
Spat collectors, laboratory experiment: An average of 248 ± 92 oysters were counted per valve, and each spat collector captured 6167 ± 92 oysters. Although there were significant differences among the oyster spat collectors (F7,382 = 28.698; p < 0.05), no differences were found in the number of settled oyster spats between the nacreous substrate and the periostracum of the shells (nacreous side = 119 ± 6; periostracum side = 128 ± 7; F1,382 = 1.211; p > 0.05) (see Figure 2).
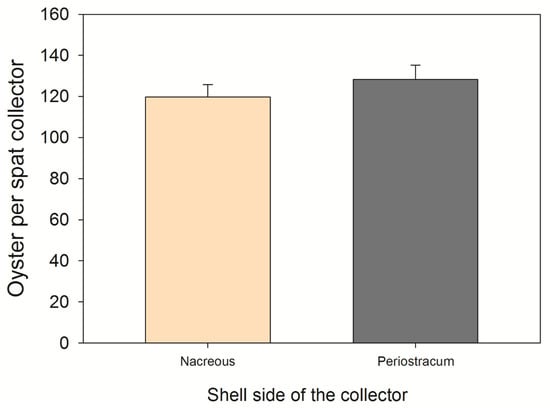
Figure 2.
The average (±SE) number of Chilean flat oysters (Ostrea chilensis) settled on each side of the ribbed mussel shells (spat collector) 30 days after the release of the larvae in the laboratory experiment.
Spat collectors, field experiment (Quempillén): No significant differences were found among the spat collectors located in the field (F19,538 =1.024; p > 0.05). After 12 months, there were 10 ± 6 spats per valve, and each collector contained 257 ± 6 oysters. However, the nacreous substrate showed a higher number of spats than the periostracum (F1,538 = 21.971; p < 0.05) (Figure 3). On the other hand, the orientation of the ribbed mussel valve with respect to the benthos had a significant effect on the number of settled oysters. In this sense, the side that was towards the bottom had more spats that settled (nacreous = 11.26 ± 0.59 oysters; periostracum = 7.34 ± 0.61 oysters) than the substrate oriented towards the surface of the water column (nacreous = 0.64 ± 0.15 oysters; periostracum = 0.49 ± 0.17 oysters) (Figure 3). However, the nacreous side of the shell presented an average of 35.08% more oyster spats than the periostracum side when both were oriented towards the benthos (F1,538 = 19.341; p < 0.05) (Figure 3).
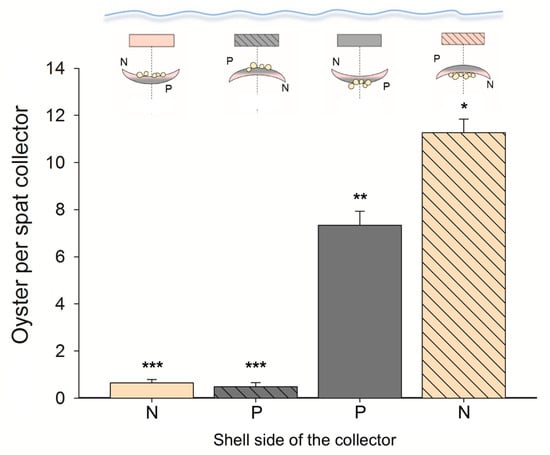
Figure 3.
The average (±SE) number of Chilean flat oysters (Ostrea chilensis) settled on each side of the ribbed mussel shells (spat collector; N = nacreous; P = periostracum) 12 months after the release of the larvae (field experiment). The diagram located above the bars represents the oyster spats counted in relation to the orientation of the valve with respect to the bottom. Statistically significant differences are marked with asterisks above the bars (p < 0.05).
Epibiont cover, field experiment (Quempillén): No significant differences in the epibiont cover were found among the oyster spat collectors (F19,486 = 0.697; p > 0.05). Therefore, the number of epibionts was the same among the collectors. However, there were 75.16% more epibionts on the periostracum than on the nacreous substrate of the ribbed mussel shells (collector) (F1,486 = 55.481; p <0.05) (Figure 4). The main epibionts found were barnacles, limpets, and polychaetes.
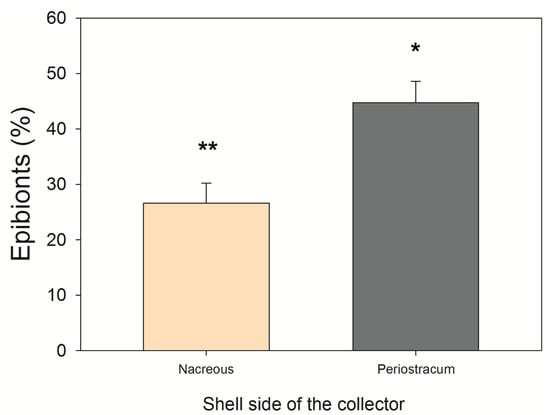
Figure 4.
The average (±SE) coverage of epibionts on each side of the ribbed mussel shells (spat collector—see Figure S2) after 12 months in the Quempillén estuary (Chiloé Island, Chile). Statistically significant differences are marked with asterisks above the bars (p < 0.05).
Oyster spat sizes, field experiment in the sea: The spat size of 12-month-old oysters was similar for all the experimental collectors (F1,597 = 0.495; p > 0.05). However, oysters on the nacreous side of the mussel shells were 18.6% larger than oysters attached to the periostracum (size of oysters on the nacreous side =15.94 ± 6.05 mm; size of oysters on the periostracum side = 12.97 ± 4.72 mm; F1,597 = 19.177; p < 0.05) (Figure 5).
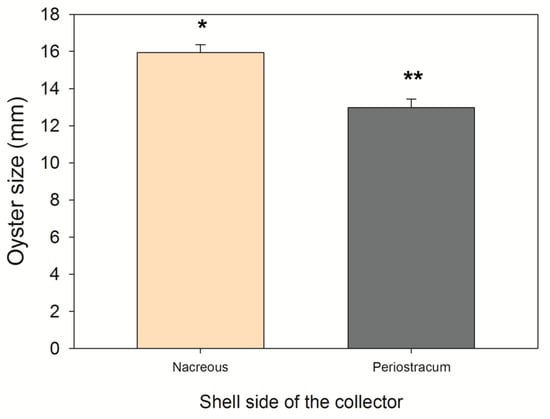
Figure 5.
The average size (±SE) of oysters (Ostrea chilensis) settled on each side of the ribbed mussel shells (spat collector) after 12 months in the Quempillén estuary (Chiloé Island, Chile). Statistically significant differences are marked with asterisks above the bars (p < 0.05).
4. Discussion
Laboratory and field experiments provided a better understanding of the settlement of oyster larvae of the Chilean flat oyster (O. chilensis). The experiments showed that collectors made from ribbed mussel shells were effective substrates for the settlement of O. chilensis. We demonstrated that larvae settled on the valves without a preference for the substrate type (periostracum vs. nacre). The air diffusers generated uniform circulation inside the experimental ponds so that the pediveliger larvae were free to settle randomly. For this reason, we deduced that the larvae were not able to detect or select between the two substrates during the settlement stage, despite the fact that the substrates differed considerably in terms of their structure and physical composition []. When an oyster pediveliger larva begins the settlement process, its foot is responsible for adhesion through the synthesis of polymers that permanently fix the oyster to a hard substrate [].
Alternatively, in the experiment carried out in the Quempillén estuary, it was apparent that oyster larvae were significantly more abundant on the nacre side, contrary to the results obtained in the experiment under controlled conditions in the laboratory. A study by Saoud et al. [] reported that, in the inner areas of the collector shells, there was a lower presence of epibionts and greater refuge, which may be a survival strategy for the larvae in nature. In addition, the concave shape of the valve generated a circular water movement that helped to group larvae in the underlying substrates [,], and this, together with the availability of space in the nacreous substrate, generated more favourable conditions for recruitment. Alternatively, the orientation of the shell with respect to the benthos had a significant effect on the number of oysters collected. That is, the side that was facing the seabed had more spats. We must consider that the larvae of O. chilensis are not good swimmers (like those of other flat oyster species such as Ostrea edulis); therefore, their dispersal depends mainly on two aspects: (i) the force with which the larvae are ejected from their parent, and (ii) the surrounding water currents [,]. In addition, the planktonic larval stage of O. chilensis can be restricted to a few hours, and in some cases, swimming times of minutes have been recorded [,]. Therefore, the larvae tend to settle on substrates close to the natural oyster beds. For the same reason, O. chilensis has a limited range of distribution [,]. This information reinforces the idea that the Chilean oyster fishery should be managed responsibly with clear sustainability objectives to adopt measures that avoid drastic declines in future populations, which has previously occurred for oyster species with similar biological characteristics (e.g., Ostrea edulis—[,]).
In the field experiment, we found that epibionts were more abundant on the periostracum of the collectors. One of the most likely reasons is that this surface is rougher. The periostracum accumulated a greater amount of particulate material that promoted the formation of bacterial biofilms, favouring the settlement of other marine invertebrates associated with the oyster culture [,,,,,,]. In contrast, on the nacreous surface, there was a lower presence of epibionts, and they were only abundant on the oyster spats (see Figure S2). The roughness of the oyster spats probably facilitated the settlement of epibionts.
The oysters on the nacreous side of the collector were 81.4% larger than those located on the periostracum, which may have been due to several reasons. For example, the most developed oyster larvae are the ones that manage to settle more easily on smooth surfaces such as nacre [], benefiting in the first phase of growth. As mentioned above, this occurs because of the circular movements of water. However, on the periostracum, increased competition for resources is generated between the oyster spats and the epibionts, which can directly damage the growth of the spats [,]. In this part of the collector, there is limited space between the oysters and the biota associated with the culture. Bacteria and diatoms colonize shell surfaces much faster than oyster larvae, whereas other sessile invertebrate species with higher growth rates (e.g., barnacles, limpets, and polychaetes) manage to settle and grow alongside them.
5. Conclusions
World oyster aquaculture currently registers an annual production of 6 million tonnes, which comes mainly from aquaculture activity (70% of total production) and which has a global market worth of USD 10 billion []. However, there are recognized gaps that hinder the sustainability of the industry, such as the following: (i) inefficiencies in spat collection that have made it difficult to increase the biomass of aquaculture production in a scenario of high demand, (ii) the absence of biotechnological tools that could improve growth rates and resistance to diseases (e.g., Bonamiosis), and (iii) a limited number of cultivable species (sensu []). In this context, O. chilensis can be described as a mollusc with a high potential because it has competitive advantages for farming. Furthermore, O. chilensis is a brooding bivalve (viviparous) that provides protection in the first larval stages; therefore, the larvae are protected against predation and adverse environmental fluctuations []. This species has also shown a greater resistance to some diseases [], which is beneficial to the production of healthy oysters with a rich nutritional content [].
In this study, we showed that mussel shells are excellent spat collectors because they are resistant to marine conditions, functionally efficient, cheap, and environmentally friendly to the ecosystem. However, in light of our results, we suggest making modifications that would increase their efficiency, such as removing the organic layer (periostracum) from the shells. In this way, oyster collectors would have a less desirable substrate for the fauna associated with the culture (e.g., sea squirts or mussels). We estimate that this modification could increase the collection of spats by up to 35%, and even by 19%, for the growth of oysters during the first year of cultivation, which is when they remain on the collectors. This modification could lead to significant progress in aquaculture activity, in terms of both an increase in the culture biomass and improvements experienced during the first growth phase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse12071148/s1, Figure S1: Oyster spat collector constructed from shells of the ribbed mussel (Aulacomya atra). Figure S2: Photograph of the nacreous side (left) and periostracum (right) of valves used in seed collectors after 1 year in the wild (Quempillén, Chiloé Island, Chile).
Author Contributions
P.A.O.: Conceptualization, methodology, and writing—review and editing. A.H.-C.: Formal analysis, validation, and visualization. G.S.: Investigation and visualization. J.M.E.: Investigation. G.R.-T.: Investigation and visualization. J.M.N.: Investigation and validation. J.E.T.: Investigation and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and financed by FONDEF ID19I10214 and FONDECYT 11220478 (P.A.O.).
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics committee of the Universidad Andrés Bello (protocol code 2627/2019 and 22 February 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Materials files).
Acknowledgments
We thank the Quempillén experimental station (UACh), and especially Juan Pablo Fuentes, Constanza Bustos, Victor Castañon, and Oscar Ramirez, for facilitating the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayne, B.L. Oysters and the ecosystem. Dev. Aquac. Fish. Sci. 2017, 41, 703–834. [Google Scholar]
- Pogoda, B.; Buck, B.H.; Hagen, W. Growth performance and condition of oysters (Crassostrea gigas and Ostrea edulis) farmed in an offshore environment (North Sea, Germany). Aquaculture 2011, 319, 484–492. [Google Scholar] [CrossRef]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Pogoda, B. Current status of European oyster decline and restoration in Germany. Humanities 2019, 8, 9. [Google Scholar] [CrossRef]
- Thurstan, R.H.; Hawkins, J.P.; Raby, L.; Roberts, C.M. Oyster (Ostrea edulis) extirpation and ecosystem transformation in the Firth of Forth, Scotland. J. Nat. Conserv. 2013, 21, 253–261. [Google Scholar] [CrossRef]
- Fariñas-Franco, J.M.; Pearce, B.; Mair, J.M.; Harries, D.B.; MacPherson, R.C.; Porter, J.S.; Reimer, P.J.; Sanderson, W.G. Missing native oyster (Ostrea edulis L.) beds in a European marine protected area: Should there be widespread restorative management? Biol. Conserv. 2018, 221, 293–311. [Google Scholar] [CrossRef]
- Smaal, A.C.; Kamermans, P.; Van der Have, T.M.; Engelsma, M.Y.; Sas, H. Feasibility of Flat Oyster (Ostrea edulis L.) Restoration in the Dutch Part of the North Sea; Report C028/15; IMARES: Yerseke, The Netherlands, 2015; Available online: https://edepot.wur.nl/335033 (accessed on 4 July 2024).
- Hernandis, S.; da Costa, F.; Hernández-Contreras, Á.; Albentosa, M. Hatchery seed production of flat oysters from the Mar Menor lagoon. Front. Mar. Sci. 2023, 10, 1231686. [Google Scholar] [CrossRef]
- Cowen, R.K.; Lwiza, K.M.M.; Sponaugle, S.; Paris, C.B.; Olson, D.B. Connectivity of marine populations: Open or closed? Science 2000, 287, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, S.A.; Castilla, J.C. Barnacle walls as mediators of intertidal mussel recruitment: Effects of patch size on the utilization of space. Mar. Ecol. Prog. Ser. 1990, 68, 113–119. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.; Sanderson, W.G.; Møller, L.F.; Henry, T.B.; James, M. Return to sender: The influence of larval behaviour on the distribution and settlement of the European oyster Ostrea edulis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2116–2132. [Google Scholar] [CrossRef]
- Oyarzún, P.A.; Toro, J.E.; Navarro, J.; Illesca, A.; Toledo, F. La explotación de los bancos naturales de ostra chilena (Ostrea chilensis) ¿Ha provocado un impacto en la diversidad genética y tasa de crecimiento? In Proceedings of the VII Congreso Nacional de Acuicultura, Arica, Chile, 11–14 September 2018.
- Brown, S.; Handley, S.; Michael, K.; Schiel, D. Annual pattern of brooding and settlement in a population of the flat oyster Ostrea chilensis from central New Zealand. N. Z. J. Mar. Freshw. Res. 2010, 44, 217–227. [Google Scholar] [CrossRef][Green Version]
- Toro, J.E.; Oyarzún, P.A.; Toledo, F.; Navarro, J.M.; Illesca, A.; Gardner, J.P.A. Genetic structure and diversity of the Chilean flat oyster Ostrea chilensis (Bivalvia: Ostreidae) along its natural distribution from natural beds subject to different fishing histories. Genet. Mol. Biology. 2022, 45, e20210214. [Google Scholar] [CrossRef] [PubMed]
- SERNAPESCA. Anuarios Estadisticos de Pesca y Acuicultura; SERNAPESCA: Valparaíso, Chile, 2021; 64p. [Google Scholar]
- Lane, H.; Webb, S.C.; Duncan, J. Bonamia ostreae in the New Zealand oyster Ostrea chilensis: A new host and geographic record for this haplosporidian parasite. Dis. Aquat. Org. 2016, 118, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, A.; Hickman, R. Reproductive activity in a pre-epizootic wild population of the Chilean oyster, Ostrea chilensis, from southern New Zealand. Aquaculture 2000, 183, 241–253. [Google Scholar] [CrossRef]
- Chaparro, O.; Mardones-Toledo, D.; Gray, M.; Cubillos, V.; Navarro, J.M.; Salas-Yanquin, L. Female-embryo relationships in Ostrea chilensis: Brooding, embryo, recognition, and larval hatching. Mar. Biol. 2019, 166, 1. [Google Scholar] [CrossRef]
- Toro, E.J.; Sanhueza, M.A.; Winter, J.E.; Senn, C.M.; Aguila, P.; Vergara, A.M. Environmental effects on the growth of the Chilean oyster Ostrea chilensis in five mariculture locations in the Chiloé Island, southern Chile. Aquaculture 1995, 136, 153–164. [Google Scholar] [CrossRef]
- Disalvo, L.H.; Alarcón, E.; Martínez, E. Induced spat production from Ostrea chilensis Philippi 1845 in mid-winter. Aquaculture 1983, 30, 357–362. [Google Scholar] [CrossRef]
- Toro, J.E.; Chaparro, O. Conocimiento Biologico de Ostrea chilensis Philippi 1845, impacto y perspectivas en el desarrollo de la ostricultura en Chile. In Cultivos de Moluscos en America Latina; Hernández, A., Ed.; CIID Canada: Bogota, Colombia, 1990; pp. 231–264. [Google Scholar]
- Gleisner, A. Ciclo Reproductivo y Desarrollo Larval de Ostrea chilensis Philippi, 1845 (Bivalvia, Ostreidae) en el Estuario Quempillén, Chiloé. Bachelor’s Thesis, Universidad Austral de Chile, Valdivia, Chile, 1981. [Google Scholar]
- Burke, K.; Bataller, E.; Miron, G. Spat collection of a non-native bivalve species (european oyster, Ostrea edulis) off the eastern Canadian coast. J. Shellfish Res. 2008, 27, 345–353. [Google Scholar] [CrossRef]
- Broekhuizen, N.C.; Lundquist, M.; Hadfield Brown, S. Dispersal of oyster (Ostrea chilensis) larvae in Tasman Bay inferred using a verified particle tracking model that incorporates larval behavior. J. Shellfish Res. 2011, 30, 643–658. [Google Scholar] [CrossRef]
- Stam, G. Estudio de la Eficiencia de los Colectores de Semilla de Ostra Chilena (Ostrea chilensis: Küster 1844) en Condiciones de Laboratorio. Bachelor’s Thesis, Universidad Andrés Bello, Santiago, Chile, 2020; p. 30. [Google Scholar]
- Navarro, J.M.; Oyarzún, P.A.; Hartmann, V.; Toro, J.E.; Garrido, C.; Valenzuela, A.; Pizarro, G. Feeding response and dynamic of intoxication and detoxification in two populations of the flat oyster Ostrea chilensis exposed to paralytic shellfish toxins (PST). Mar. Environ. Res. 2022, 177, 105634. [Google Scholar] [CrossRef]
- Zrnčić, S.; Oraić, D.; Mihaljević, Ž.; Zanella, D. Impact of varying cultivation depths on growth rate and survival of the European flat oyster Ostrea edulis, L. Aquac. Res. 2007, 38, 1305–1310. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010; Available online: http://www.r-project.org/ (accessed on 4 July 2024).
- Santana, P.; Aldana, D. Nacre morphology and chemical composition in Atlantic winged oyster Pteria colymbus (Röding, 1798). PeerJ 2021, 9, e11527. [Google Scholar] [CrossRef] [PubMed]
- Foulon, V.; Artiguad, S.; Buscaglia, M.; Bernay, B.; Fabioux, C.; Petton, B.; Elies, P.; Boukerma, K.; Hellio, C.; Guérard, F.; et al. Proteinaceous secretion of bioadhesive produced during crawling and settlement of Crassostrea gigas larvae. Sci. Rep. 2018, 8, 15298. [Google Scholar] [CrossRef] [PubMed]
- Saoud, I.; Rouse, D.; Wallace, R.; Howe, J.; Page, B. Oyster Crassostrea virginica spat settlement as it relates to the restoration of fish river reef in mobile Bay, Alabama. J. World Aquac. Soc. 2000, 31, 640–650. [Google Scholar] [CrossRef]
- Buitrago, E.; Alvarado, D. A highly efficient oyster spat collector made with recycled materials. Aquac. Eng. 2005, 33, 63–72. [Google Scholar] [CrossRef]
- Lok, A.; Acarli, S. Preliminary study of settlement of flat oyster spat (Ostrea edulis L.) on oyster and mussel shell collectors. Isr. J. Aquac. Bamidgeh 2006, 58, 105–115. [Google Scholar]
- Guo, X.-Z.; Wei, K.J.; Yan, R.-J.; Gardner, J.P.A. Pronounced Mitochondrial DNA population genetic structure in a brooding coastal marine invertebrate. Malacologia 2022, 65, 113–136. [Google Scholar] [CrossRef]
- Bell, C.; McQuaid, C.; Porri, F. Barnacle settlement on rocky shores: Substratum preference and epibiosis on mussels. J. Exp. Mar. Biol. Ecol. 2015, 473, 195–201. [Google Scholar] [CrossRef]
- Diez, M.; Vásquez, N.; da Cunha Lana, P.; Cremonte, F. Biogenic calcareous growth on the ribbed mussel Aulacomya atra (Bivalvia: Mytilidae) favours polydorid boring (Polychaeta: Spionida). Hydrobiologia 2015, 766, 349–355. [Google Scholar] [CrossRef]
- Scardino, A.J.; de Nys, R. Fouling Deterrence on the Bivalve shell Mytilus galloprovincialis: A Physical Phenomenon? Biofouling 2004, 20, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Whal, M. Epibiosis, Chapter 7. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-1-405-16926-4. [Google Scholar]
- Arakawa, K. Natural spat collecting in the pacific oyster Crassostrea gigas (Thunberg). Mar. Behav. Physiol. 1990, 17, 95–128. [Google Scholar] [CrossRef]
- Gascoigne, J.; Beadman, H.; Saurel, C.; Kaiser, M.J. Density dependence, spatial scale and patterning in sessile biota. Oecologia 2005, 145, 371–381. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture: Sustainability in Action; FAO: Rome, Italy, 2020; p. 224. [Google Scholar] [CrossRef]
- Metian, M.; Troeli, M.; Christensen, V.; Steenbeek, J.; Pouil, S. Mapping diversity of species in global aquaculture. Rev. Aquac. 2020, 12, 1090–1100. [Google Scholar] [CrossRef]
- Gray, M.; Chaparro, O.; Huebert, K.; Oneill, S.; Coutue, T.; Moreira, A.; Brady, D. Life history traits conferring larval resistance against ocean acidification: The case of brooding oysters of the genus Ostrea. J. Shellfish Res. 2019, 38, 751–761. [Google Scholar] [CrossRef]
- Lohrmann, K.B.; Hine, P.M.; Campalans, M. Ultrastructure of Bonamia sp. in Ostrea chilensis in Chile. Dis. Aquat. Org. 2009, 85, 199–208. [Google Scholar] [CrossRef]
- Valenzuela, A.; Oyarzún, P.A.; Toro, J.E.; Navarro, J.M.; Ramírez, O.; Farias, A. Proximal and fatty acid analysis in Ostrea chilensis, Crassostrea gigas and Mytilus chilensis (Bivalvia: Mollusca) from Southern Chile. PLoS ONE 2022, 17, e0270825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).