A Study of the EH36 Surface Sediment Layer under Joint Protection from Seawater Electrolysis Antifouling and Impressed Current Cathode Protection (ICCP) in a Marine Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Protected Body Samples
2.3. A Joint Protection Device for Seawater Electrolysis Antifouling and ICCP
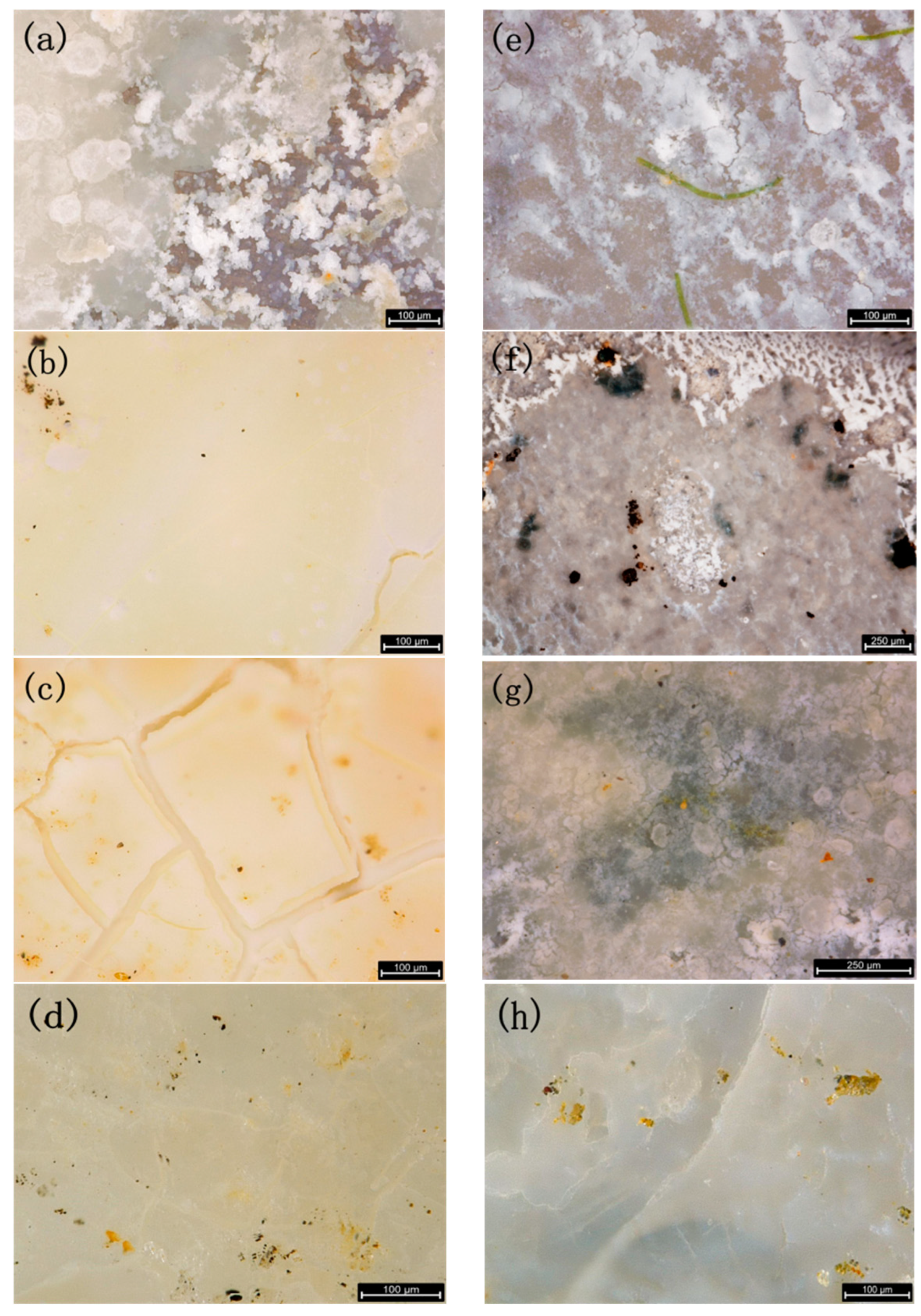
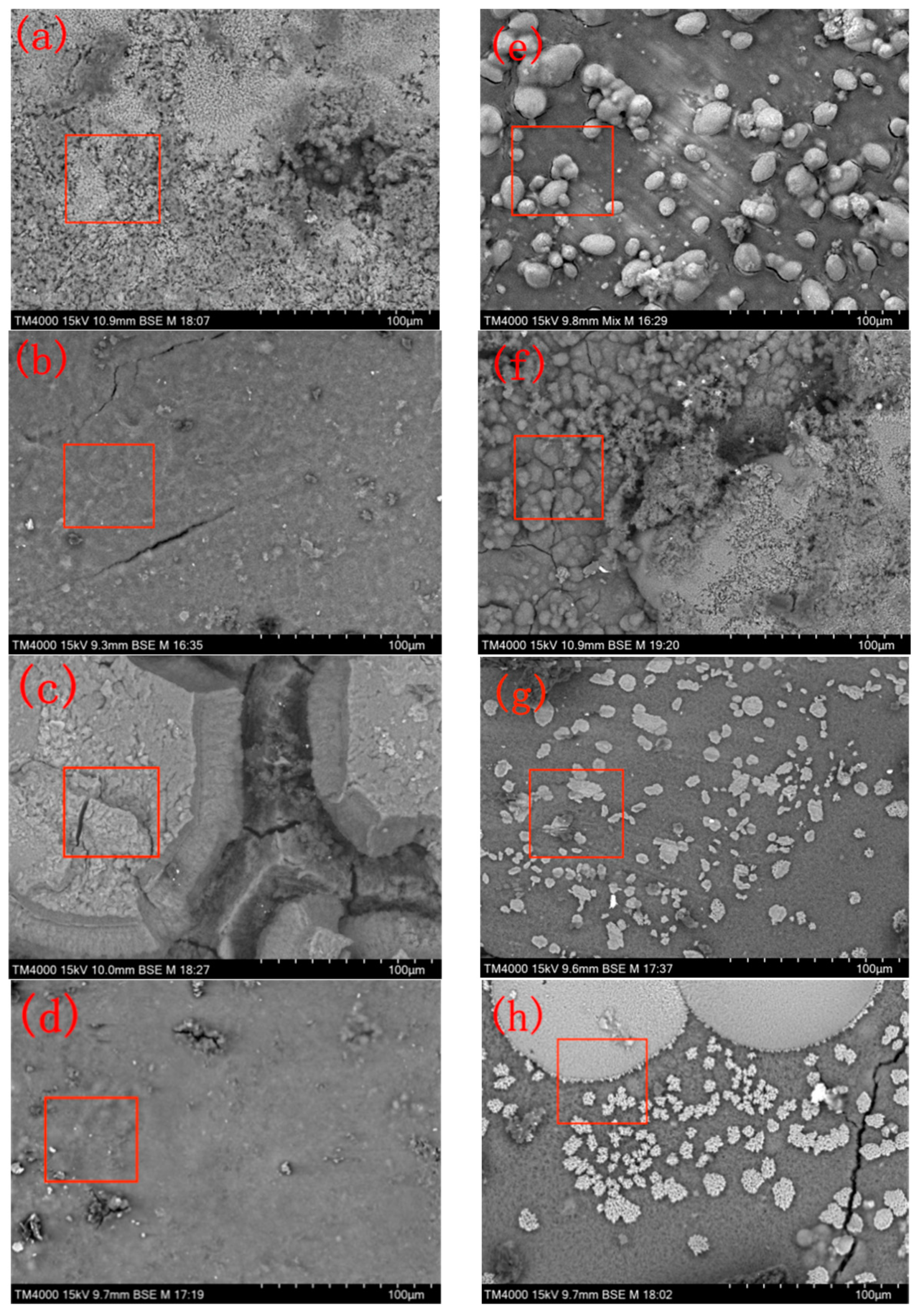
2.4. Analysis of the Sedimentary Layer Morphology
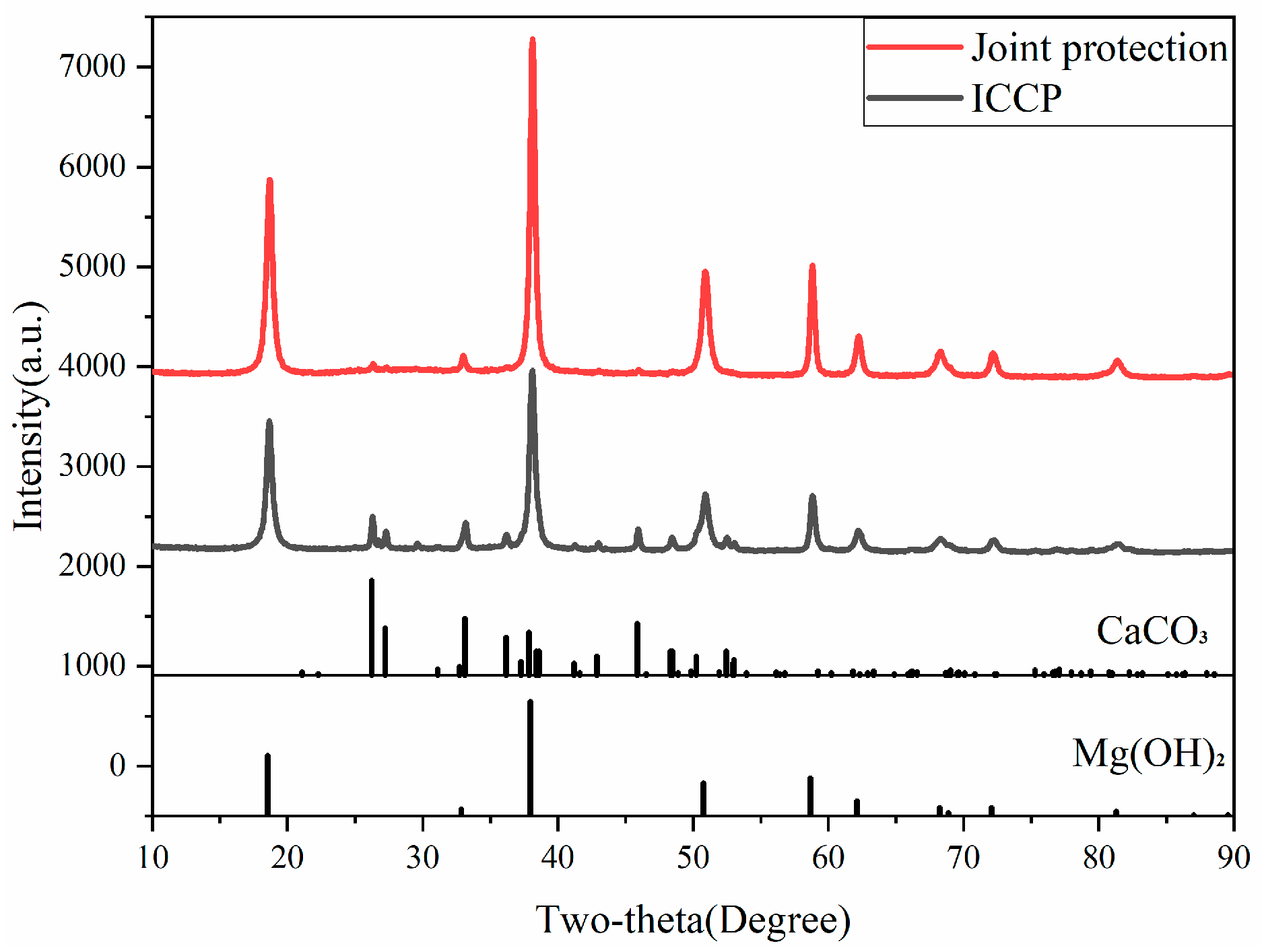
2.5. Analysis of Sedimentary Layer Components
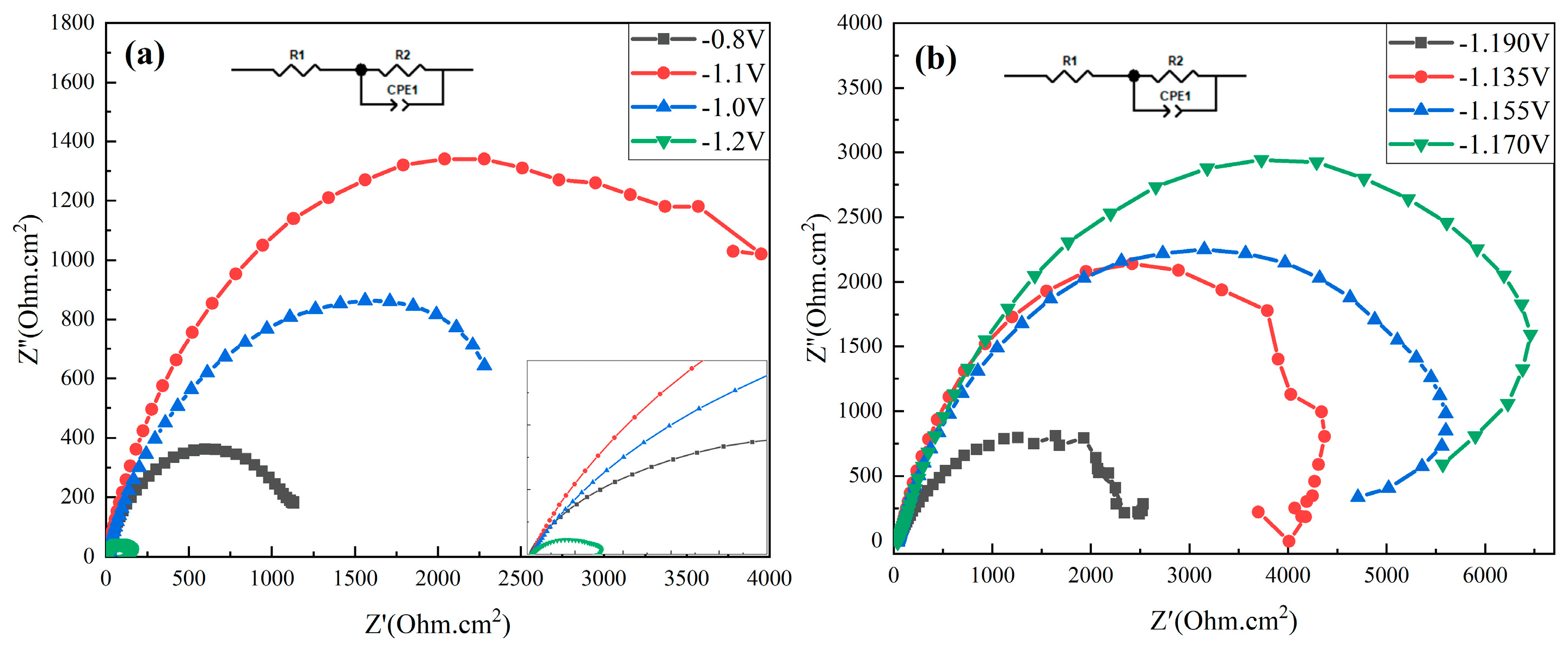
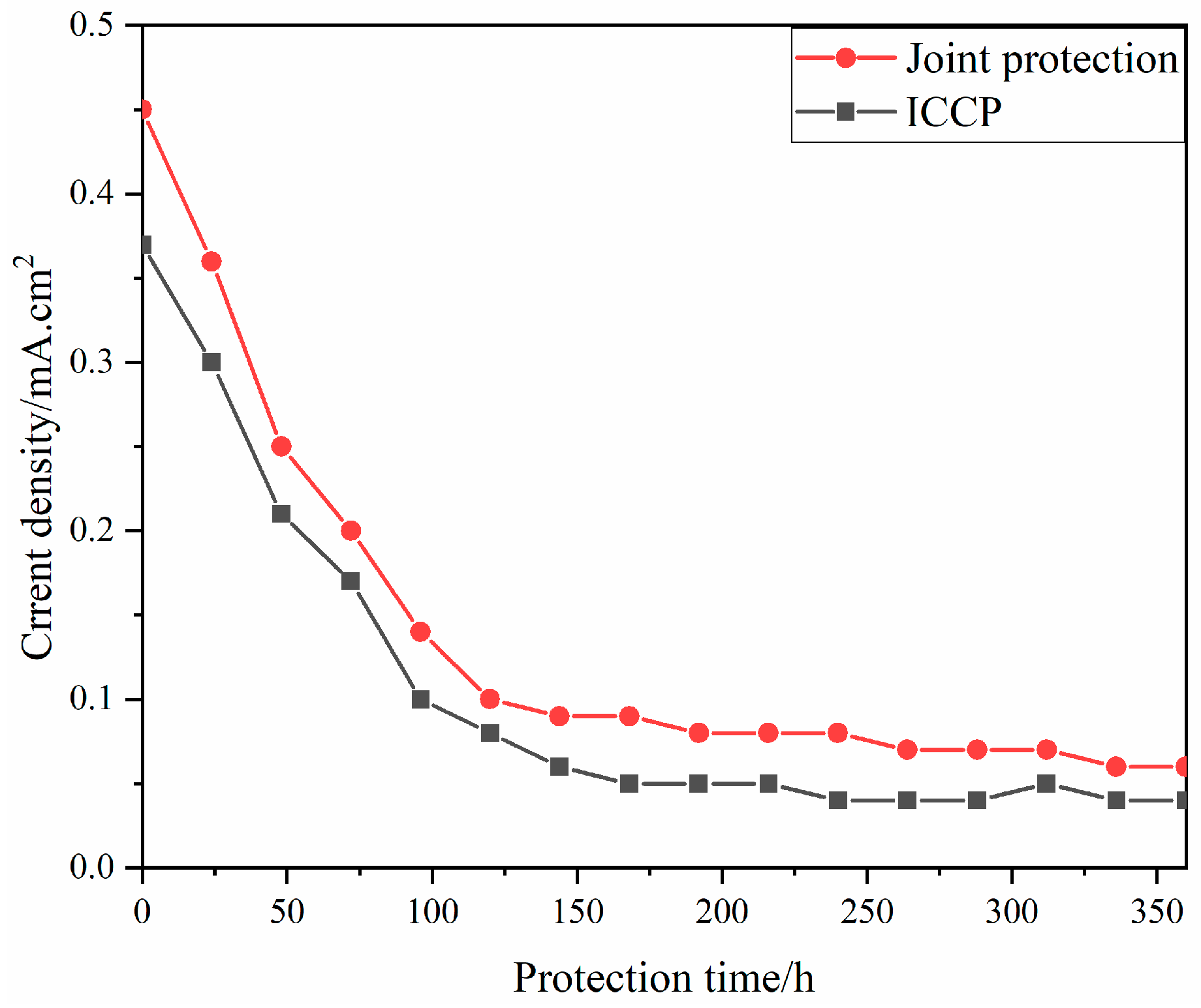
3. Results and Discussion
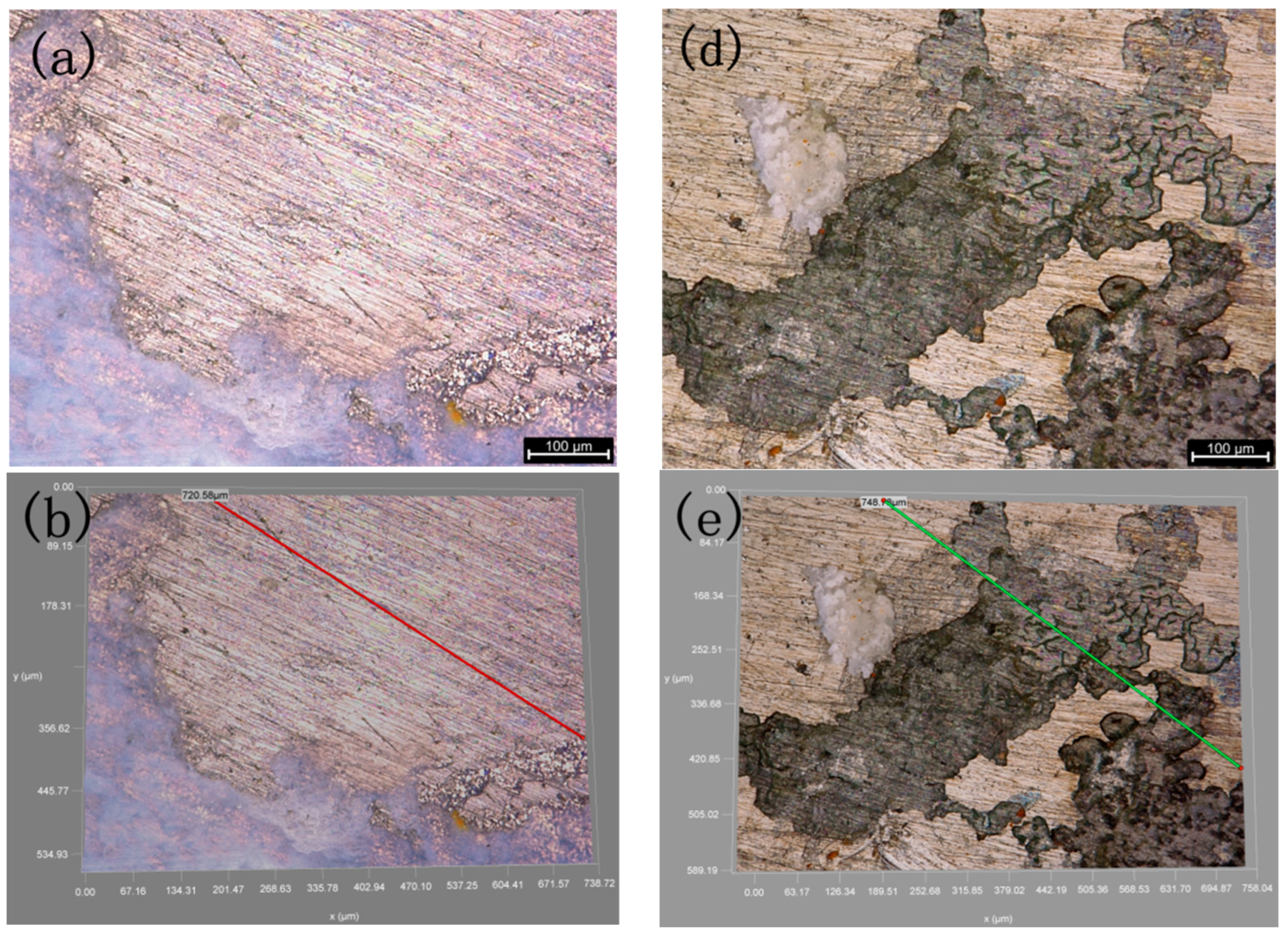
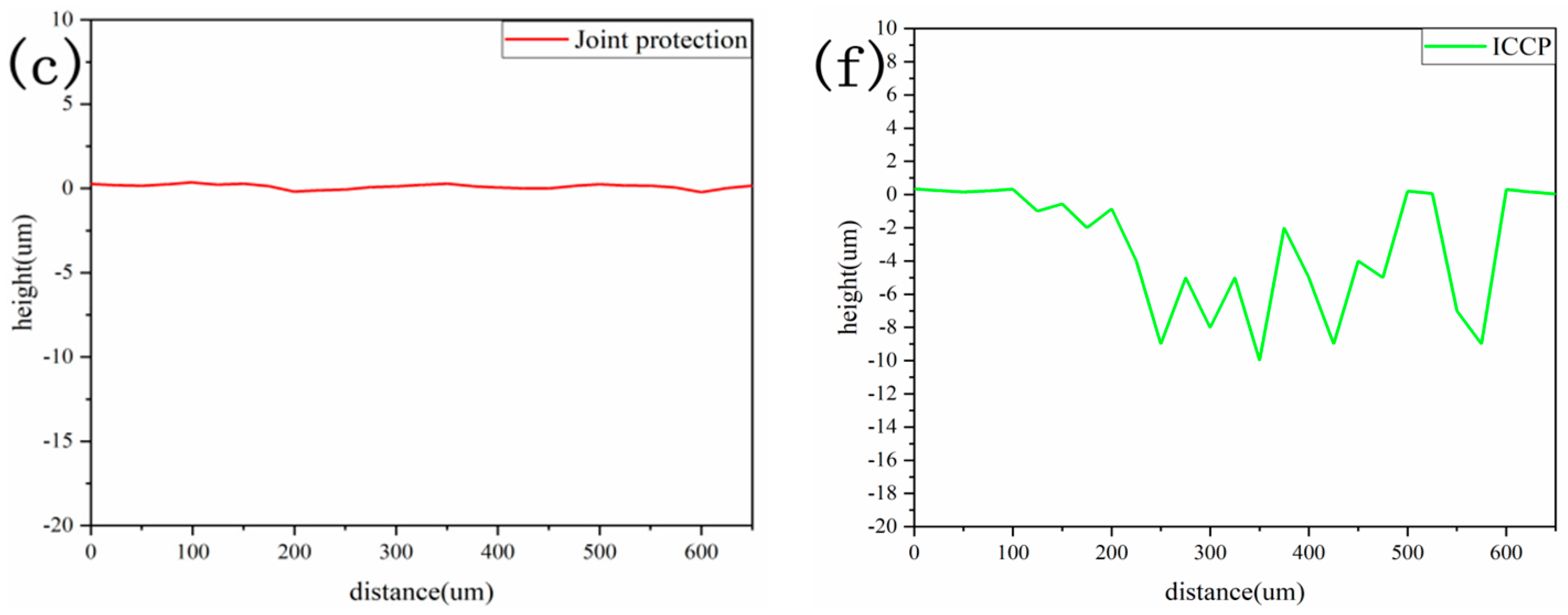
3.1. Comparison of the Sedimentary Layers’ Surface Morphology
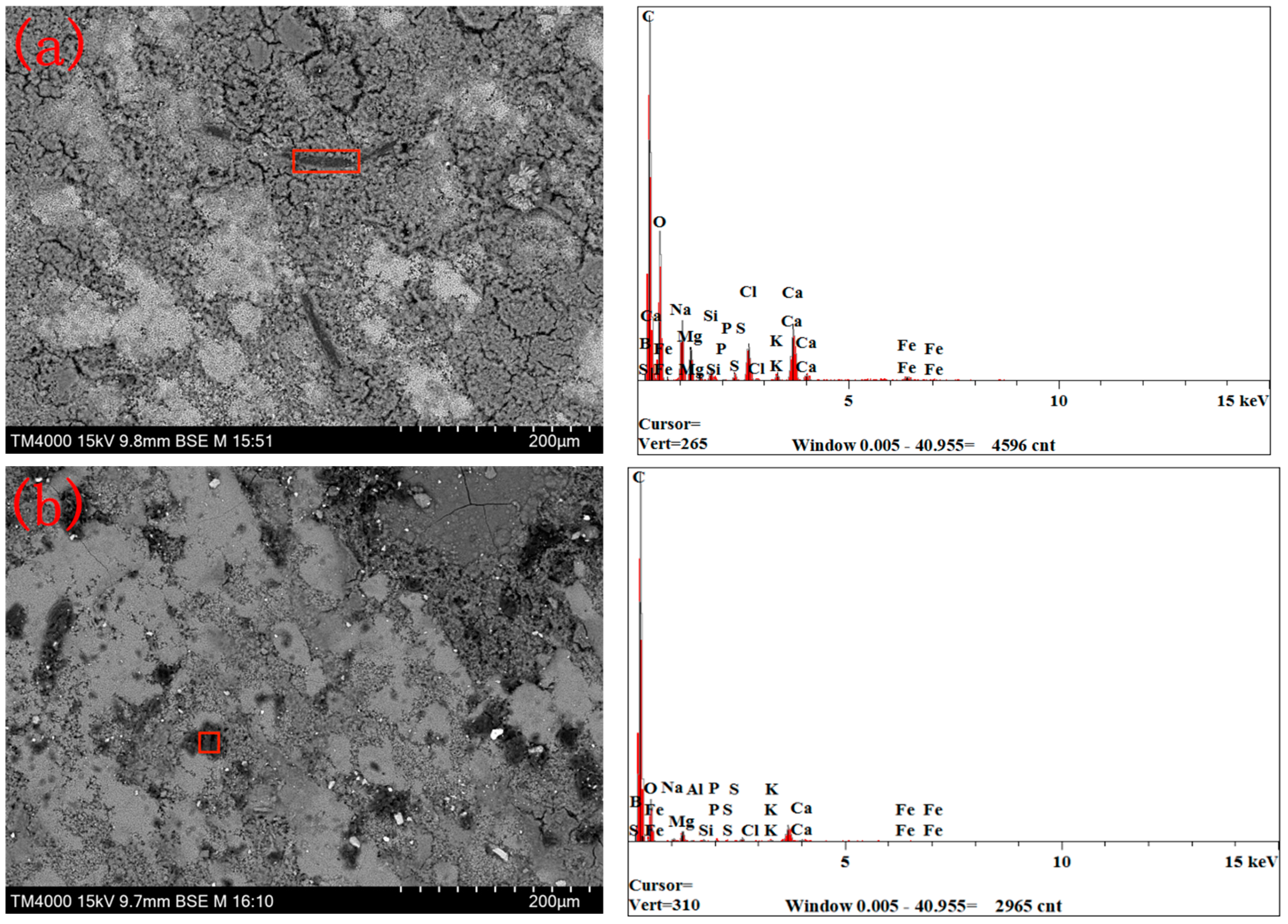
3.2. Composition of the Sedimentary Layer
3.3. Biological Fouling of Surface Sedimentary Layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsen, R.K. Designing and Managing an Offshore Cathodic Protection System. Mater. Perform. 2019, 58, 26–30. [Google Scholar]
- Hartt, H.W. 2012 Frank Newman Speller Award: Cathodic Protection of Offshore Structures-History and Current Status. Corros. J. Sci. Eng. 2012, 68, 1063–1075. [Google Scholar] [CrossRef]
- Szabó, S.; Bakos, I. Impressed current cathodic protection. Corros. Rev. 2006, 24, 39–62. [Google Scholar] [CrossRef]
- Barchiche, C.; Deslouis, C.; Festy, D.; Gil, O.; Refait, P.; Touzain, S.; Tribollet, B. Characterization of cal-careous deposits in artificial seawater by impedance techniques 3-Deposit of CaCO3 in the presence of Mg(II). Electrochim. Acta 2003, 48, 1645–1654. [Google Scholar] [CrossRef]
- Gabrielli, C.; Keddam, M.; Khalil, A.; Rosset, R.; Zidoune, M. Study of calcium carbonate scales by electro-chemical impedance spectroscopy. Electrochim. Acta 1997, 42, 1207–1218. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Wang, H.; Sun, X. Mixed Fouling Growth Process-Microbial and CaCO3 Fouling in Water Systems. J. Chem. Ind. Eng. 2002, 53, 1247–1252. [Google Scholar] [CrossRef]
- Li, F.; An, M.; Liu, G.; Duan, D. Effects of sulfidation of passive film in the presence of SRB on the pitting corrosion behaviors of stainless steels. Mater. Chem. Phys. 2008, 113, 971–976. [Google Scholar] [CrossRef]
- Christine, R.; PhilipS, S. Removal and inactivation of Staphylococcus epidermidis biofilms by electrolysis. Appl. Environ. Microbiol. 2006, 72, 6364–6366. [Google Scholar] [CrossRef]
- Quej-Ake, L.; Nava, N.; Espinosa-Medina, M.A.; Liu, H.B.; Alamilla, J.L.; Sosa, E. Characterisation of soil/pipe interface at a pipeline failure after 36 years of service under impressed current cathodic protection. Corros. Eng. Sci. Technol. 2015, 50, 311–319. [Google Scholar] [CrossRef]
- Deshpande, P.; Kolekar, A. Impressed Current Cathodic Protection of Low Carbon Steel in Conjunction with Conducting Polyaniline based Paint Coating. Prot. Met. Phys. Chem. Surf. 2019, 55, 1236–1241. [Google Scholar] [CrossRef]
- Pravin, D.; Aniket, K.; Abhijit, B.; Kalendova, A.; Kohl, M. Impressed current cathodic protection (ICCP) of mild steel in association with zinc based paint coating. Mater. Today Proc. 2022, 50, 1660–1665. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, C.; Du, J.; Shen, W.; Tang, S.; Ma, L.; He, M. A pipeline Was Protected By Impressed Current Cathodic Protection and Sacrificial Anode External Corrosion Factors and Control Measures. Compr. Corros. Control 2021, 35, 105–109. [Google Scholar] [CrossRef]
- Xiao, Y. A Case Study of Combined Use of Cathodic Protection of Sacrificial Anode and Impressed Current. Corros. Prot. 2021, 42, 66–70. [Google Scholar] [CrossRef]
- Liao, Z.; Li, H.; Luo, X.; Shi, Y.; Li, N.; Wang, C.; Liu, Y.; Yu, X.L. Joint cathodic protection of impressed current and sacrificial anode for long-distance pipelines under special conditions. Oil Gas Storage Transp. 2023, 42, 320–327. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Chi, M.; Yun, X.; Luo, B.; Guo, C.; Wang, S.; Min, D. Research progress on preparation and application of DSA electrode. Appl. Chem. Ind. 2021, 50, 498–503. [Google Scholar] [CrossRef]
- Liu, X.; Geng, J.; Liao, D.; Bai, B. Electrocatalytic chlorine cvolution performance of Ti/RuO2-IrO2 mesh anode. Chem. Eng. (China) 2023, 51, 61–65. [Google Scholar] [CrossRef]
- Qi, H.; Gao, K.; You, J.; Sun, W.; Wang, L.; Liu, G. Preparation and properties of highly active low iridium-doping Ti/IrRuSnSbOx electrode for chloride evolution. Mod. Chem. Ind. 2023, 43, 94–98. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wang, J.; Duan, J.; Hou, B. Effects of Sulfate Reducing Bacteria Adhesion Formation of Calcareous Deposits under Method Protection Condition And Its Protection for Metals. Corros. Prot. 2018, 39, 265–269. [Google Scholar] [CrossRef]
- Luo, J.; Lee, R.; Chen, T.; Hartt, W.; Smith, S. Formation of Calcareous Deposits under Different Modes of Cathodic Polarization. Corrosion 1991, 47, 189–196. [Google Scholar] [CrossRef]
- Yang, Y.; Scantlebury, J.; Koroleva, E. A Study of Calcareous Deposits on Cathodically Protected Mild Steel in Artificial Seawater. Met. Open Access Metall. J. 2015, 5, 439–456. [Google Scholar] [CrossRef]
- Wen, G.; Zheng, F. The formation and application of calcium deposits during cathodic protection in seawater. Corros. Prot. 1995, 16, 50–53. [Google Scholar]
- Karoui, H.; Riffault, B.; Jeannin, M.; Kahoul, A.; Gil, O.; Amor, M.B.; Tlili, M.M. Electrochemical scaling of stainless steel in artificial seawater: Role of experimental conditions on CaCO3 and Mg(OH)2 formation. Desalination 2013, 311, 234–240. [Google Scholar] [CrossRef]
- Du, M.; Sun, Z. Study on the Cathodic Polarization Behavior of Stainless Steel 410 in Seawater. Period. Ocean Univ. China 2010, 40, 91–95. [Google Scholar] [CrossRef]
- Lee, R.U.; Ambrose, J.R. Influence of Cathodic Protection Parameters on Calcareous Deposit Formation. Corrosion 2012, 44, 887–891. [Google Scholar] [CrossRef]
- Sharker, T.; Simonsen, K.R.; Margheritini, L.; Kucheryavskiy, S.V.; Simonsen, M.E. Optimisation of electrochemical deposition of calcareous material during cathodic protection by implementing response surface methodology (RSM). Electrochim. Acta 2023, 444, 141960. [Google Scholar] [CrossRef]
- Wang, X.; Bai, S.F.; Guo, Y.F.; Huang, Z.H.; Li, X.B.; Hou, J.; Zhang, H.X. Study on cathodic protection behavior of high-strength stainless steel in seawater environment. Equip. Environ. Eng. 2023, 20, 45–52. [Google Scholar]
- Perme, S.; Lau, K.; Boan, M.; Tansel, B.; ASCE, F.; Duncan, M. Cathodic Polarization Behavior of Steel with Different Marine Fouling Morphologies on Submerged Bridge Elements with Cathodic Protection. J. Mater. Civ. Eng. 2020, 32, 04020184. [Google Scholar] [CrossRef]
- Vitor, L.; Mariana, G.; Simone, B.; Servulo, E. SRB-mediated corrosion of marine submerged AISI 1020 steel under impressed current cathodic protection. Colloids Surf. B Biointerfaces 2021, 202, 111701. [Google Scholar] [CrossRef]

| Time (day) | C | O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 1.98% | 45.13% | 4.68% | 36.55% | - | 3.47% | - | - | - | - | 1.47% | 6.72% |
| 15 d | 2.60% | 52.74% | - | 42.16% | 0.01% | 1.20% | - | - | 0.78% | 0.25% | - | 0.25% |
| 30 d | - | 48.74% | 2.43% | 34.42% | 0.05% | 4.80% | - | 0.96% | 3.69% | 0.76% | 0.71% | 3.43% |
| 60 d | - | 47.54% | 1% | 46.27% | 0.39% | 0.06% | 0.16% | 2.74% | - | - | 1.39% | 0.45% |
| Time (day) | C | O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | - | 50.67% | 1.50% | 26.65% | 0.04% | 2.76% | 0.21% | 0.42% | 6.36% | 0.64% | 10.75% | - |
| 15 d | 1.10% | 55.79% | 0.84% | 25.54% | 0.01% | 2.27% | 0.17% | 0.26% | 1.56% | 0.14% | 11.87% | 0.45% |
| 30 d | 5.98% | 50.10% | 0.81% | 23.50% | - | 0.01% | 0.21% | 0.21% | 1.44% | - | 17.59% | 0.15% |
| 60 d | 8.75% | 53.16% | 1.05% | 6.36% | 0.32% | 0.34% | 0.01% | 0.10% | 0.45% | - | 29.38% | 0.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, P.; Deng, P.; Geng, B.; Zeng, J.; Hu, X. A Study of the EH36 Surface Sediment Layer under Joint Protection from Seawater Electrolysis Antifouling and Impressed Current Cathode Protection (ICCP) in a Marine Environment. J. Mar. Sci. Eng. 2024, 12, 1155. https://doi.org/10.3390/jmse12071155
Hu J, Wang P, Deng P, Geng B, Zeng J, Hu X. A Study of the EH36 Surface Sediment Layer under Joint Protection from Seawater Electrolysis Antifouling and Impressed Current Cathode Protection (ICCP) in a Marine Environment. Journal of Marine Science and Engineering. 2024; 12(7):1155. https://doi.org/10.3390/jmse12071155
Chicago/Turabian StyleHu, Jiezhen, Peilin Wang, Peichang Deng, Baoyu Geng, Junhao Zeng, and Xin Hu. 2024. "A Study of the EH36 Surface Sediment Layer under Joint Protection from Seawater Electrolysis Antifouling and Impressed Current Cathode Protection (ICCP) in a Marine Environment" Journal of Marine Science and Engineering 12, no. 7: 1155. https://doi.org/10.3390/jmse12071155
APA StyleHu, J., Wang, P., Deng, P., Geng, B., Zeng, J., & Hu, X. (2024). A Study of the EH36 Surface Sediment Layer under Joint Protection from Seawater Electrolysis Antifouling and Impressed Current Cathode Protection (ICCP) in a Marine Environment. Journal of Marine Science and Engineering, 12(7), 1155. https://doi.org/10.3390/jmse12071155






