Development and Succession of Non-Indigenous and Cryptogenic Species over Two Different Substrates in the Port of Alicante (Western Mediterranean)
Abstract
1. Introduction
2. Materials and Methods
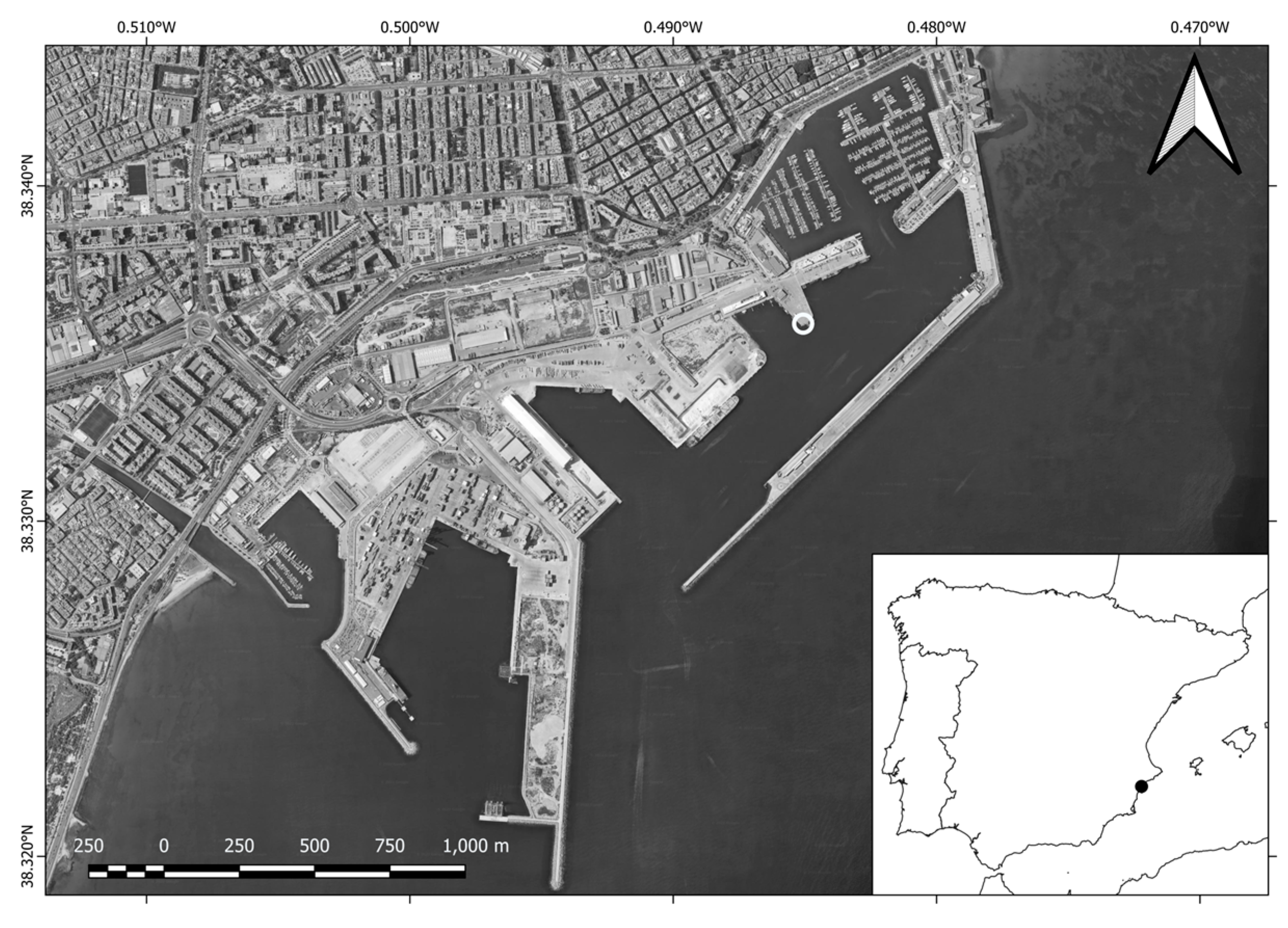
2.1. Study Site
2.2. Sampling
2.3. Data Treatment
3. Results
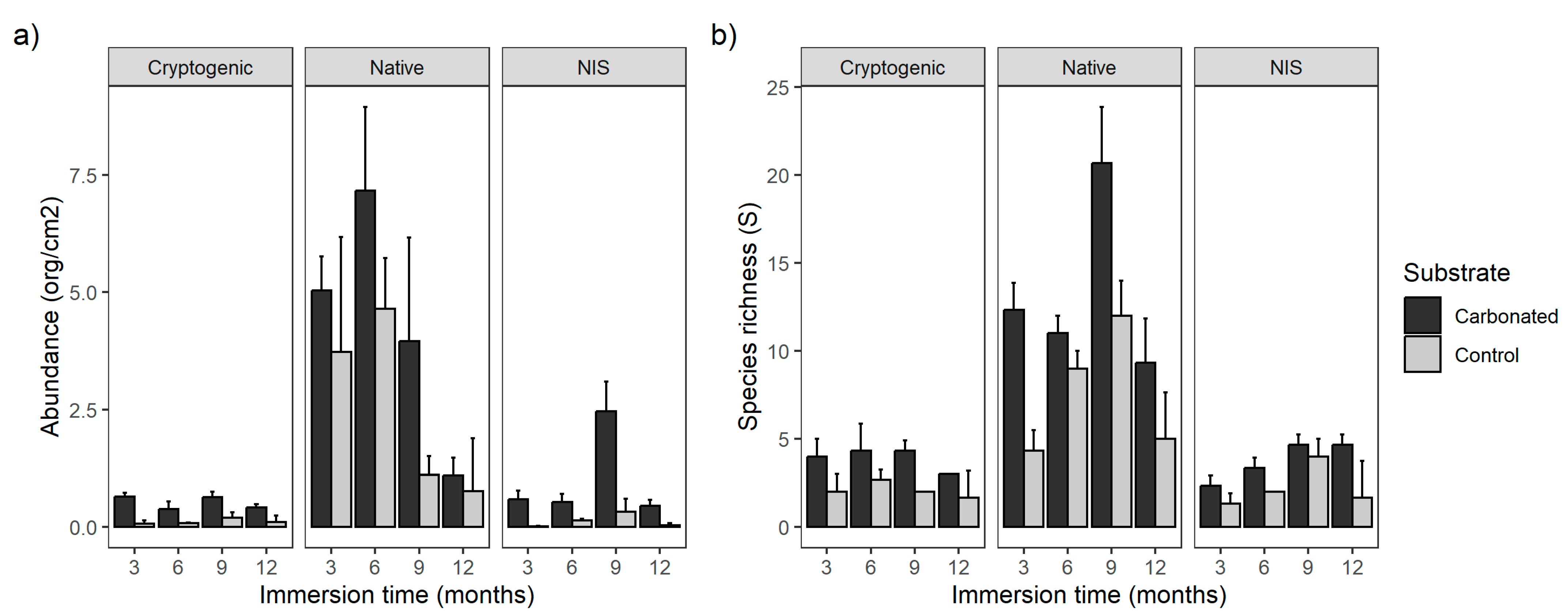
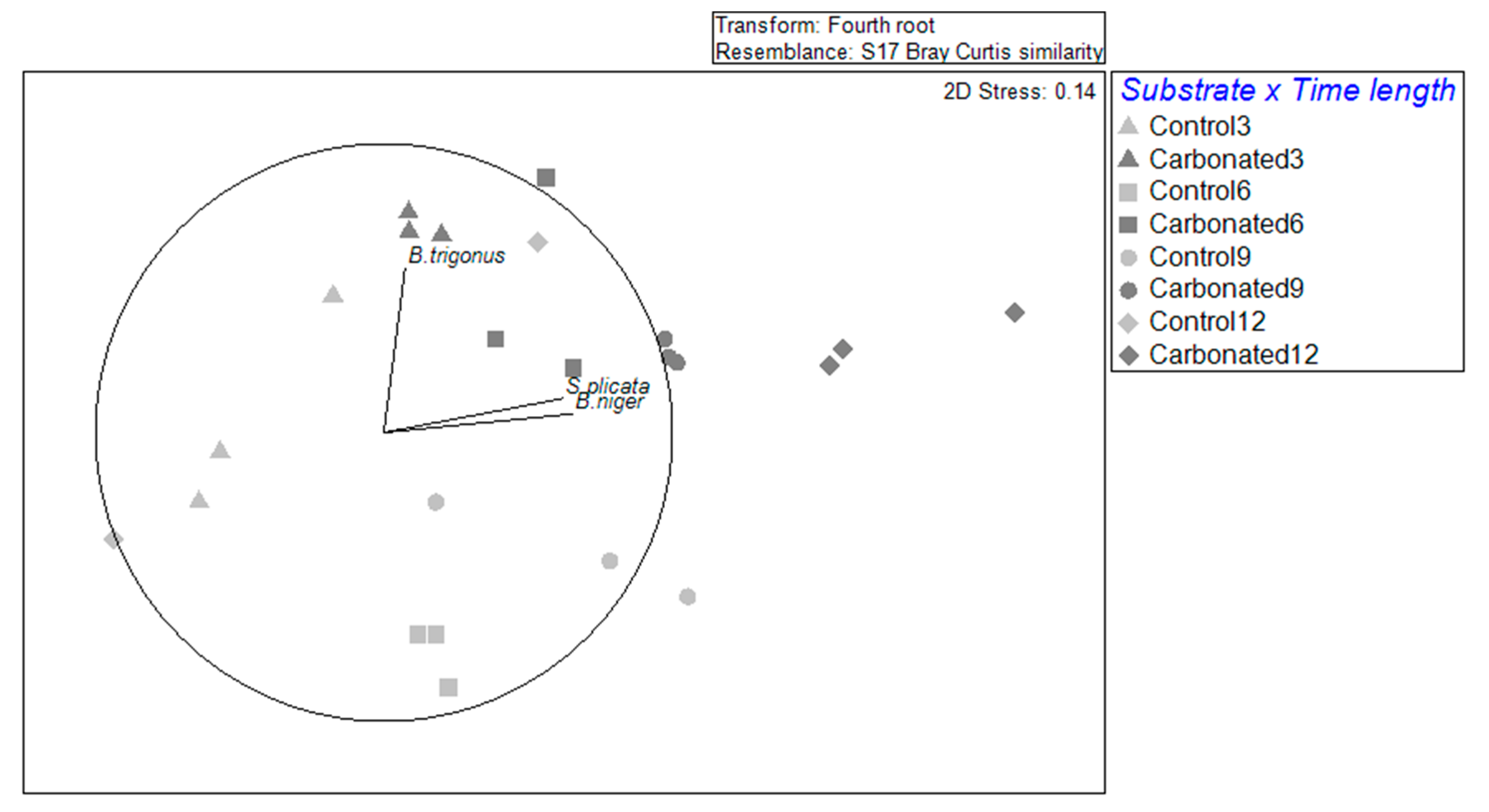
3.1. Diversity
3.2. Non-Indigenous and Cryptogenic Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catford, J.A.; Bode, M.; Tilman, D. Introduced Species That Overcome Life History Tradeoffs Can Cause Native Extinctions. Nat. Commun. 2018, 9, 2131. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, J.; Viard, F.; González Sepúlveda, E.; Díaz, C.; Neira Hinojosa, J.; Pérez Araneda, K.; Silva, F.; Brante, A. Habitat Type Drives the Distribution of Non-indigenous Species in Fouling Communities Regardless of Associated Maritime Traffic. Divers Distrib. 2020, 26, 62–75. [Google Scholar] [CrossRef]
- Chaudhary, C.; Richardson, A.J.; Schoeman, D.S.; Costello, M.J. Global Warming Is Causing a More Pronounced Dip in Marine Species Richness around the Equator. Proc. Natl. Acad. Sci. USA 2021, 118, e2015094118. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, L.; Bulleri, F. Anthropogenic Disturbance Can Determine the Magnitude of Opportunistic Species Responses on Marine Urban Infrastructures. PLoS ONE 2011, 6, e22985. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Geraldi, N.R.; Lovelock, C.E.; Apostolaki, E.T.; Bennett, S.; Cebrian, J.; Krause-Jensen, D.; Marbà, N.; Martinetto, P.; Pandolfi, J.M.; et al. Global Ecological Impacts of Marine Exotic Species. Nat. Ecol. Evol. 2019, 3, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Griffiths, C.; McQuaid, C.; Rius, M. Marine Alien Species of South Africa—Status and Impacts. Afr. J. Mar. Sci. 2005, 27, 297–306. [Google Scholar] [CrossRef]
- Smith, C.R.; Grange, L.J.; Honig, D.L.; Naudts, L.; Huber, B.; Guidi, L.; Domack, E. A Large Population of King Crabs in Palmer Deep on the West Antarctic Peninsula Shelf and Potential Invasive Impacts. Proc. R. Soc. B Biol. Sci. 2012, 279, 1017–1026. [Google Scholar] [CrossRef]
- Albano, M.J.; Obenat, S.M. Fouling Assemblages of Native, Non-Indigenous and Cryptogenic Species on Artificial Structures, Depths and Temporal Variation. J. Sea Res. 2019, 144, 1–15. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The Enlargement of the Suez Canal—Erythraean Introductions and Management Challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López García, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established Non-Indigenous Species Increased by 40% in 11 Years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Terwin, J.R.; Whitlatch, R.B.; Osman, R.W. Linking Climate Change and Biological Invasions: Ocean Warming Facilitates Nonindigenous Species Invasions. Proc. Natl. Acad. Sci. USA 2002, 99, 15497–15500. [Google Scholar] [CrossRef] [PubMed]
- Lezzi, M.; Del Pasqua, M.; Pierri, C.; Giangrande, A. Seasonal Non-Indigenous Species Succession in a Marine Macrofouling Invertebrate Community. Biol. Invasions 2018, 20, 937–961. [Google Scholar] [CrossRef]
- Levine, J.M. Species Diversity and Biological Invasions: Relating Local Process to Community Pattern. Science 2000, 288, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.E. Impact of Non-indigenous Species on Natives Enhanced by Anthropogenic Alteration of Selection Regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The Dispersal of Alien Species Redefines Biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, M.; Ashton, G.; Brown, C.; Chang, A.; Ruiz, G. Settlement Plates as Monitoring Devices for Non-Indigenous Species in Marine Fouling Communities. Manag. Biol. Invasions 2017, 8, 559–566. [Google Scholar] [CrossRef]
- Outinen, O.; Puntila-Dodd, R.; Barda, I.; Brzana, R.; Hegele-Drywa, J.; Kalnina, M.; Kostanda, M.; Lindqvist, A.; Normant-Saremba, M.; Ścibik, M.; et al. The Role of Marinas in the Establishment and Spread of Non-Indigenous Species in Baltic Sea Fouling Communities. Biofouling 2021, 37, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J. Epifaunal Colonization of the Loch Linnhe Artificial Reef: Influence of Substratum on Epifaunal Assemblage Structure. Biofouling 2005, 21, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Pinto, F.; Torrontegi, O.; Prestes, A.C.L.; Álvaro, N.V.; Neto, A.I.; Martins, G.M. Invasion Success and Development of Benthic Assemblages: Effect of Timing, Duration of Submersion and Substrate Type. Mar. Environ. Res. 2014, 94, 72–79. [Google Scholar] [CrossRef]
- Chase, A.L.; Dijkstra, J.A.; Harris, L.G. The Influence of Substrate Material on Ascidian Larval Settlement. Mar. Pollut. Bull. 2016, 106, 35–42. [Google Scholar] [CrossRef]
- Airoldi, L.; Turon, X.; Perkol-Finkel, S.; Rius, M. Corridors for Aliens but Not for Natives: Effects of Marine Urban Sprawl at a Regional Scale. Divers Distrib. 2015, 21, 755–768. [Google Scholar] [CrossRef]
- Megina, C.; González-Duarte, M.M.; López-González, P.J. Benthic Assemblages, Biodiversity and Invasiveness in Marinas and Commercial Harbours: An Investigation Using a Bioindicator Group. Biofouling 2016, 32, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Glasby, T.M.; Connell, S.D.; Holloway, M.G.; Hewitt, C.L. Nonindigenous Biota on Artificial Structures: Could Habitat Creation Facilitate Biological Invasions? Mar. Biol. 2007, 151, 887–895. [Google Scholar] [CrossRef]
- Tyrrell, M.C.; Byers, J.E. Do Artificial Substrates Favor Nonindigenous Fouling Species over Native Species? J. Exp. Mar. Biol. Ecol. 2007, 342, 54–60. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Glasby, T.M.; Johnston, E.L. Comparing the Invasibility of Experimental “Reefs” with Field Observations of Natural Reefs and Artificial Structures. PLoS ONE 2012, 7, e38124. [Google Scholar] [CrossRef] [PubMed]
- Cangussu, L.C.; Altvater, L.; Haddad, M.A.; Cabral, A.C.; Heyse, H.L.; Rocha, R.M. Substrate Type as a Selective Tool against Colonization by Non-Native Sessile Invertebrates. Braz. J. Oceanogr. 2010, 58, 219–231. [Google Scholar] [CrossRef]
- Farfan, G.A.; Apprill, A.; Cohen, A.; Decarlo, T.M.; Post, J.E.; Waller, R.G.; Hansel, C.M. Crystallographic and Chemical Signatures in Coral Skeletal Aragonite. Coral. Reefs 2022, 41, 19–34. [Google Scholar] [CrossRef]
- Carmona-Rodríguez, A.; Antón, C.; Climent, M.-Á.; Garcés, P.; Montiel, V.; Ramos-Esplá, A.A. Sessile Biofouling on Electrolytic Carbonated Structures: Stages of Colonization and Succession. J. Mar. Sci. Eng. 2024, 12, 443. [Google Scholar] [CrossRef]
- Hilbertz, W.H. Electrodeposition of Minerals in Sea Water: Experiments and Applications. IEEE J. Ocean. Eng. 1979, 4, 94–113. [Google Scholar] [CrossRef]
- Antón, C.; Carmona, A.; Climent, M.Á.; Garcés, P.; Montiel, V.; Ramos-Esplá, A.Á. Sistema Para La Formación de Arrecifes Marinos Artificiales y Estructuras Submarinas Con Recubrimiento Calcáreo Inducido Por Electrólisis. Rev. Digit. Del Cedex 2024, 203, 33–42. [Google Scholar]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and Validation of Alien Species Catalogues: The Mediterranean as an Example. Estuar. Coast Shelf. Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.E.; García Raso, J.E.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien Species in the Mediterranean Sea by 2010. A Contribution to the Application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial Distribution. Mediterr. Mar. Sci. 2010, 11, 381. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Cinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azurro, E.; et al. Alien Species in the Mediterranean Sea by 2012. A Contribution to the Application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction Trends and Pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhpinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A Massive Update of Non-Indigenous Species Records in Mediterranean Marinas. PeerJ 2017, 5, e3954. [Google Scholar] [CrossRef] [PubMed]
- McArdle, B.H.; Anderson, M.J. Fitting Multivariate Models to Community Data: A Comment on Distance-based Redundancy Analysis. Ecology 2001, 8, 290–297. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Clarke, K.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Auckland, New Zealand, 2014. [Google Scholar]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; Sheahart, W.A., Wilks, S.S., Eds.; John Wiley & Sons: New York, NY, USA, 1973. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Ltd.: Auckland, New Zealand, 2008; pp. 1–224. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Dailianis, T.; Akyol, O.; Babali, N.; Bariche, M.; Crocetta, F.; Gerovasileau, V.; Chanem, R.; Gökoglu, M.; Hasiotis, T.; Izquierdo-Muñoz, A.; et al. New Mediterranean Biodiversity Records (July 2016). Mediterr. Mar. Sci. 2016, 17, 608. [Google Scholar] [CrossRef]
- Zibrowius, H.; Ramos, A.A. Oculina Patagonica, Scléractiniaire Exotiqueen Méditerranée Nouvelles Observations Dans Le Sud-Est de l’Espagne. Rep. Int. Comm. Mediterr. Sea 1983, 28. [Google Scholar]
- Del-Pilar-Ruso, Y.; San Martín, G.; Giménez-Casalduero, F.; López, E.; de-la-Ossa-Carretero, J.A.; Ramos-Esplá, A.A.; Sánchez-Lizaso, J.L. Interesting Polychaeta Species in Alicante Bay (W Mediterranean): Syllidae and Sabellidae. In Proceedings of the 11th International Polychaete Conference, Sidney, Australia, 4–9 August 2013. [Google Scholar]
- Ulman, A. Recreational Boating as a Major Vector of Spread of Non-Indigenous Species around the Mediterranean. Ph.D. Thesis, University of Sorbonne and University of Pavia, Paris, France, 2018. [Google Scholar]
- Katsanevakis, S. Unpublished Mediterranean Records of Marine Alien and Cryptogenic Species. Bioinvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Zibrowius, H. Ongoing Modification of the Mediterranean Marine Fauna and Flora by the Establishment of Exotic Species. Mésogée 1991, 5, 83–107. [Google Scholar]
- Ramos-Esplá, A.A. Ascidias Litorales Del Mediterráneo Ibérico: Faunística, Ecología y Biogeografía; Universidad de Barcelona: Barcelona, Spain, 1988. [Google Scholar]
- Cornet, C.; Ramos-Esplá, A.A. Ascidiacea. In Estudios Sistemático y Ecológico de las Esponjas y Ascidias del Mediterráneo Español; Fundación Juan March: Madrid, Spain, 1980; pp. 362–499. [Google Scholar]
- Cifuentes, M.; Krueger, I.; Dumont, C.P.; Lenz, M.; Thiel, M. Does Primary Colonization or Community Structure Determine the Succession of Fouling Communities? J. Exp. Mar. Biol. Ecol. 2010, 395, 10–20. [Google Scholar] [CrossRef]
- Lara-Romero, C.; de la Cruz, M.; Escribano-Ávila, G.; García-Fernández, A.; Iriondo, J.M. What Causes Conspecific Plant Aggregation? Disentangling the Role of Dispersal, Habitat Heterogeneity and Plant–Plant Interactions. Oikos 2016, 125, 1304–1313. [Google Scholar] [CrossRef]
- Lu, L.; Wu, R.S.S. Seasonal Effects on Recolonization of Macrobenthos in Defaunated Sediment: A Series of Field Experiments. J. Exp. Mar. Biol. Ecol. 2007, 351, 199–210. [Google Scholar] [CrossRef]
- Fortič, A.; Mavrič, B.; Pitacco, V.; Lipej, L. Temporal Changes of a Fouling Community: Colonization Patterns of the Benthic Epifauna in the Shallow Northern Adriatic Sea. Reg. Stud. Mar. Sci. 2021, 45, 101818. [Google Scholar] [CrossRef]
- Fargione, J.; Brown, C.S.; Tilman, D. Community Assembly and Invasion: An Experimental Test of Neutral versus Niche Processes. Proc. Natl. Acad. Sci. USA 2003, 100, 8916–8920. [Google Scholar] [CrossRef] [PubMed]
- Tempesti, J.; Langeneck, J.; Maltagliati, F.; Castelli, A. Macrobenthic Fouling Assemblages and NIS Success in a Mediterranean Port: The Role of Use Destination. Mar. Pollut. Bull. 2020, 150, 110768. [Google Scholar] [CrossRef] [PubMed]
- Palero, F.; Torrado, H.; Perry, O.; Kupriyanova, E.; Ulman, A.; Genis-Armero, R.; Ten Hove, H.A.; Capaccioni-Azzati, R. Following the Phoenician Example: Western Mediterranean Colonization by Spirobranchus Cf. Tetraceros(Annelida: Serpulidae). Sci. Mar. 2020, 84, 83. [Google Scholar] [CrossRef]
- Zibrowius, H. Remarques Sur Trois Espèces de Serpulidae Acclimatées En Méditerranée: Hydroides Dianthus (Verrill, 1873), Hydroides Dirampha Mörch, 1863, et Hydroides Elegans (Haswell, 1883). Rep. Int. Comm. Mediterr. Sea 1973, 21, 683–686. [Google Scholar]
- Gollasch, S.; Haydar, D.; Minchin, D.; Wolff, W.J.; Reise, K. Introduced Aquatic Species of the North Sea Coasts and Adjacent Brackish Waters. In Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives; Springer: Berlin/Heidelberg, Germany, 2009; pp. 507–528. [Google Scholar]
- Castilla, J.C.; Lagos, N.A.; Cerda, M. Marine Ecosystem Engineering by the Alien Ascidian Pyura Praeputialis on a Mid-Intertidal Rocky Shore. Mar. Ecol. Prog. Ser. 2004, 268, 119–130. [Google Scholar] [CrossRef]
- Giachetti, C.B.; Battini, N.; Castro, K.L.; Schwindt, E. Invasive Ascidians: How Predators Reduce Their Dominance in Artificial Structures in Cold Temperate Areas. J. Exp. Mar. Biol. Ecol. 2020, 533, 151459. [Google Scholar] [CrossRef]
| Species | Status | First Record | Source |
|---|---|---|---|
| Porifera | |||
| Paraleucilla magna Klautau et al. 2004 | NIS | 2016 | [41] |
| Anthozoa | |||
| Oculina patagonica de Angelis D’Ossat, 1908 | NIS | 1973 | [42] |
| Polychaeta | |||
| Branchiomma bairdi (McIntosh, 1885) | NIS | 2013 (Alicante Bay); 2016 | [43,44]; This study |
| Ficopomatus enigmaticus (Fauvel, 1923) | NIS | 2016 | [44] |
| Hydroides dirampha Mörch, 1863 | NIS | 2020 | This study |
| Hydroides elegans (Haswell, 1883) | NIS | 2016 | [44]; This study |
| Spirobranchus cf. tetraceros (Schmarda, 1861) | NIS | 2020 | This study |
| Cirripedia | |||
| Amphibalanus amphitrite (Darwin, 1854) | Cryp | 1972 | [45]; This study |
| Balanus trigonus Darwin, 1854 | NIS | 2020 | This study |
| Gastropoda | |||
| Bostrycapulus odites Collin, 2005 | NIS | 1973 | [46] (1) |
| Bryozoa | |||
| Amathia verticillata (delle Chiaje, 1822) | NIS | 1972 | [45] |
| Bugulina fulva (Ryland, 1960) | Cryp | 2020 | This study |
| Bugulina stolonifera (Ryland, 1960) | Cryp | 2020 | This study |
| Bugula neritina (Linnaeus, 1758) | Cryp | 1972 | [45]; This study |
| Celleporaria brunnea (Hincks, 1884) | NIS | 2016 | [34] |
| Savignyella lafontii (Audouin, 1826) | Cryp | 2020 | This study |
| Schizoporella errata (Waters, 1878) | Cryp | 1972 | [45]; This study |
| Watersipora arcuata Banta, 1969 | NIS | 2016 | [34] |
| Watersipora subtorquata (d’Orbigny, 1852) | Cryp | 2020 | This study |
| Ascidiacea | |||
| Botrylloides cf. niger Herdman, 1886 | NIS | 2020 | This study |
| Botryllus shclosseri (Pallas, 1766) | Cryp | 1980 | [47] |
| Ciona intestinalis (Linnaeus, 1767) | Cryp | 1980 | [47]; This study |
| Clavelina lepadiformis (Müller, 1776) | Cryp | 1979 | [48] |
| Diplosoma listerianum (Milne Edwards, 1841) | Cryp | 1980 | [47]; This study |
| Microcosmus squamiger Michaelsen, 1972 | Cryp | 1980 | [47] (2) |
| Styela canopus (Savigny, 1816) | Cryp | 1980 | [47]; This study |
| Styela plicata (Lesueur, 1823) | NIS | 1978 | [48]; This study |
| Source | df | MS | Pseudo-F | P(perm) | Post Hoc Test |
|---|---|---|---|---|---|
| Native | |||||
| S | 1 | 18.364 | 8.4719 | 0.0094 | C>I |
| T | 3 | 84.974 | 13.067 | 0.0004 | 6 = 3>9>12 |
| S × T | 3 | 6.0315 | 0.9275 | 0.4482 | |
| Residuals | 16 | 34.682 | |||
| Total | 23 | ||||
| NIS | |||||
| S | 1 | 4.6712 | 67.627 | 0.0002 | C>I |
| T | 3 | 5.4685 | 26.39 | 0.0002 | 9>3 = 6 = 12 |
| S × T | 3 | 3.2067 | 15.475 | 0.0002 | 3: C>I; 6: C>I; 9: C>I; 12: C>I |
| Residuals | 16 | 1.1052 | |||
| Total | 23 | ||||
| Cryptogenic | |||||
| S | 1 | 0.9791 | 87.844 | 0.0002 | C>I |
| T | 3 | 0.0456 | 4.0917 | 0.0228 | 3 = 9>6 = 12 |
| S × T | 3 | 0.0273 | 2.4563 | 0.099 | |
| Residuals | 16 | 0.0115 | |||
| Total | 23 |
| Source | df | MS | Pseudo-F | P(perm) | Post Hoc Test |
|---|---|---|---|---|---|
| Native | |||||
| S | 1 | 198.38 | 46.223 | 0.0004 | C>S |
| T | 3 | 96.931 | 22.586 | 0.0002 | 9>6>3 = 12 |
| S × T | 3 | 11.264 | 2.6246 | 0.0814 | |
| Residuals | 16 | 4.2917 | |||
| Total | 23 | ||||
| NIS | |||||
| S | 1 | 13.5 | 15.429 | 0.0016 | C>S |
| T | 3 | 6.5556 | 7.4921 | 0.0028 | 9>12 = 6>3 |
| S × T | 3 | 1.6111 | 1.8413 | 0.1766 | |
| Residuals | 16 | 0.875 | |||
| Total | 23 | ||||
| Cryptogenic | |||||
| S | 1 | 20.167 | 18.615 | 0.001 | C>S |
| T | 3 | 1 | 0.9231 | 0.469 | |
| S × T | 3 | 0.9444 | 0.8718 | 0.483 | |
| Residuals | 16 | 1.0833 | |||
| Total | 23 |
| Source | df | MS | Pseudo-F | P(perm) | Post Hoc Test |
|---|---|---|---|---|---|
| S | 1 | 8541.5 | 28.975 | 0.0002 | C≠S |
| T | 3 | 3845.8 | 13.045 | 0.0002 | 3≠6≠9≠12 |
| S × T | 3 | 1230.6 | 4.1745 | 0.001 | 3: C≠S; 6: C≠S; 9: C≠S; 12: C≠S |
| Residuals | 16 | 294.8 | |||
| Total | 23 |
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| H. elegams | 1 | 1 | |||||
| S. cf. tetraceros | 2 | 0.86 * | 1 | ||||
| B. trigonus | 3 | −0.02 | −0.13 | 1 | |||
| H. dirampha | 4 | 0.80 * | 0.52 | −0.09 | 1 | ||
| B. bairdi | 5 | 0.75 * | 0.84 * | −0.29 | 0.61 * | 1 | |
| S. plicata | 6 | 0.88 * | 0.81 * | 0.22 | 0.8 * | 0.72 * | 1 |
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| H. elegans | 1 | 1 | |||||
| B. cf. niger | 2 | −0.57 | 1 | ||||
| B. trigonus | 3 | 0.52 | −0.88 * | 1 | |||
| H. dirampha | 4 | 0.05 | −0.3 | 0.49 | 1 | ||
| B. bairdi | 5 | 0.08 | −0.27 | 0.39 | 0.81 * | 1 | |
| S. plicata | 6 | −0.23 | 0.37 | −0.15 | 0 | 0.37 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Rodríguez, A.; Antón, C.; Climent, M.-Á.; Garcés, P.; Montiel, V.; Arroyo-Martínez, E.; Ramos-Esplá, A.A. Development and Succession of Non-Indigenous and Cryptogenic Species over Two Different Substrates in the Port of Alicante (Western Mediterranean). J. Mar. Sci. Eng. 2024, 12, 1188. https://doi.org/10.3390/jmse12071188
Carmona-Rodríguez A, Antón C, Climent M-Á, Garcés P, Montiel V, Arroyo-Martínez E, Ramos-Esplá AA. Development and Succession of Non-Indigenous and Cryptogenic Species over Two Different Substrates in the Port of Alicante (Western Mediterranean). Journal of Marine Science and Engineering. 2024; 12(7):1188. https://doi.org/10.3390/jmse12071188
Chicago/Turabian StyleCarmona-Rodríguez, Alejandro, Carlos Antón, Miguel-Ángel Climent, Pedro Garcés, Vicente Montiel, Elisa Arroyo-Martínez, and Alfonso A. Ramos-Esplá. 2024. "Development and Succession of Non-Indigenous and Cryptogenic Species over Two Different Substrates in the Port of Alicante (Western Mediterranean)" Journal of Marine Science and Engineering 12, no. 7: 1188. https://doi.org/10.3390/jmse12071188
APA StyleCarmona-Rodríguez, A., Antón, C., Climent, M.-Á., Garcés, P., Montiel, V., Arroyo-Martínez, E., & Ramos-Esplá, A. A. (2024). Development and Succession of Non-Indigenous and Cryptogenic Species over Two Different Substrates in the Port of Alicante (Western Mediterranean). Journal of Marine Science and Engineering, 12(7), 1188. https://doi.org/10.3390/jmse12071188







