Abstract
In the marine environment, fish parasites are present in most seafood species. The most common are nematodes of the genus Anisakis, which can parasitize human tissues, causing anisakiasis and allergies—in some cases with a strong reaction, such as anaphylactic shock. This happens when people ingest live or dead larvae present in the muscles or viscera of a wide range of fish and cephalopods. Consumer education has been positioned as one of the most effective alternatives for its prevention. This study, carried out in Asturias (northwest Spain), sought to identify the seafood products that present the greatest risk of anisakiasis for consumers, taking into account their consumption, the prevalence of Anisakis, and consumer knowledge about this parasitosis. In the results, hake (Merluccius merluccius) and cod (Gadus morhua), frequently consumed in the region and with high parasite prevalence, do not pose a great risk because they are consumed when well cooked. Instead, sardine (Sardina pilchardus), highly consumed and less parasitized, and anchovy (Engraulis encrasicolus), highly parasitized and less consumed, would exhibit a medium risk. Young participants know more about the risks of anisakiasis from raw seafood. The gaps detected in the knowledge about the ability of temperature treatments to eliminate parasites, especially in allergic people, must be addressed for better prevention. We suggest campaigns adapted to the population sectors.
1. Introduction
Parasites are part of the ecosystems [1] and are normally present in almost all fish species [2], contributing significantly to the food webs. Nematodes are amongst the most prevalent fish parasites, and their type and relative abundance have been considered characteristic of specific regions [3,4]. Some genera can parasitize fish, such as Pseudoterranova, Contracaecum, and, notably, the genus Anisakis, from the Anisakidae family, which can ultimately parasitize humans and cause anisakidosis (anisakiasis when it is caused by Anisakis spp.). Anisakidosis is an emerging zoonosis that causes gastrointestinal disease, with abdominal pain due to erosive or hemorrhagic lesions in the digestive tract [5]. This disease appears when people ingest live larvae from the viscera or muscles of a wide range of fish and cephalopod species. A strong allergic reaction to several nematode proteins is associated with this disease [6], with symptoms that go from urticaria to anaphylactic shocks in sensitive individuals. Allergic responses can also be induced by dead parasites if the allergy-causing proteins or protein domains are preserved [7].
Experts from the FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization) have ranked anisakids fourth among 24 food-borne zoonotic parasites [8]. About 20,000 anisakiasis cases are reported worldwide yearly, mostly in Japan, Spain, the Netherlands, and Germany [9]. Spain has a long list of scientific publications about the Anisakis spp. [10], as it is the country with the highest incidence of anisakiasis in Europe, with an average of over 8000 cases per year [11] and a marked incidence among the population living northwest of the country [12]. The difference in anisakiasis prevalence between countries or regions can be due to several factors, such as seafood consumption per capita; species preferences due to local consumption habits, availability, or price; and other reasons, including the different ways of cooking and eating seafood due to cultural differences [13,14]. The main factor contributing to the high incidence of anisakiasis in Spain is believed to be the high level of consumption of parasite-infested marinated anchovies. There is also evidence of occupational anisakiasis in fishermen and fish industry workers [15].
It appears that a global increase in the consumption of untreated fish products—neither frozen nor cooked—such as sushi, sashimi, gravlax, lomi lomi, and ceviche [16,17], could have led to an increase in the prevalence of anisakiasis in recent years, along with the advancement of new diagnostic techniques. In diagnosed anisakiasis, patients often report consuming raw or undercooked fish [18]. However, we cannot forget that the consumption of seafood is an important part of a balanced diet and a healthy lifestyle [19], and the high life expectancy of the populations with the highest rate of fish consumption per capita showcases these beneficial effects [20]. However, fears about parasitic infections and allergies could lead to a reduction in seafood consumption [21]. So, in order to maintain recommendations for a safe and healthy diet that includes fish, the best way of preventing anisakiasis is to educate consumers to avoid untreated raw or undercooked fish [22]. The present study addressed this issue in northern Spain, where fish consumption is very high [23]. From the perspective of healthy and safe consumption, the research objectives of this work were as follows:
- To assess consumers’ awareness about anisakids and preventive seafood treatments.
- To evaluate the effect of gender and age on the risk of anisakiasis, considering the awareness of each population group.
- To give recommendations for better consumer information regarding anisakiasis prevention, based on the results of objectives 1 and 2.
2. Materials and Methods
To know the population’s fish consumption preferences, a structured short questionnaire was elaborated, following Brace [24]. Two key questions were asked about their consumption patterns, described previously in Blanco-Fernandez et al.’s study [25]. The questionnaires were applied in Asturias, a coastal province in northwestern Spain with just over 1 million inhabitants that has a long fishing tradition and where seafood is an important part of the diet, gastronomy, and economic support of its population [26]. Previously, a pilot test had been carried out (N = 20) to verify that the questionnaire items measured what they were intended to. This survey about fish consumption was administered face-to-face to a heterogeneous group of volunteers. It was possible to collect the responses from 1608 people aged 14 to 63, with an average age of 18.04 years. The anonymity of the respondents was always respected, so the only personal data of the participants collected were their age, gender, and place of residence. Volunteers were invited to provide an email address to share the results obtained and to place them for future research. Here, we will present the results about the frequency of consumption of generic types of fish and invertebrates (hake, salmon, squid, etc.), not identified by their scientific names because the survey was designed for non-experts. Respondents were asked how frequently they ate each type of fish, with the following options: 1, weekly; 2, monthly; 3, occasionally; 4, rarely/never.
In the following research phase, around 10% of the volunteers from the fish consumption survey were contacted by email to participate in a new questionnaire to find out their anisakid awareness, this time, online. They were once again asked to indicate their age, gender, and place of residence. This time, the focus was posed on whether the population is aware of anisakids, where live anisakids’ larvae can be found, and what can be done to kill them (when the parasite is not removed from the seafood) or eliminate them (when the parasite is removed from the seafood). One hundred and forty-nine participants completed the questionnaire, with an age range of 18 to 46 years and an average age of 24.75. In this case, the age range was reduced, and the average age was slightly higher than in the previous one. All survey questions and full raw data are available in the Mendeley dataset [27]. The questions with “Yes/No” answers were the following:
- Are you aware of anisakids?
- 2a/b/c: Do you think that raw/half-cooked/ultrafrozen seafood could have live anisakids?
- 3a/b: Could cooking (>60 °C) or freezing (<−20 °C) eliminate/kill anisakids?
For the statistical analysis, two groups of respondents were created based on their self-declared knowledge about anisakids, differentiated as “aware” and “unaware”. Comparisons between the two groups were made based on their answers to five questions about actual knowledge about anisakids (about the risk of raw, self-cooked, and frozen products, and about the effects of thermal treatment for killing or eliminating anisakids, with values 0—incorrect and 1—correct). The risk ratio (RR), with z tests and their p (H0 being RR = 1), was employed. Then, a multivariate multiple linear regression test was run to evaluate the possible relationships between self-declared knowledge, age, and gender as independent variables, and the five items measuring actual anisakid knowledge as dependent ones.
Data analyses were performed using the free software PAST 4.10 [28]. p-values < 0.05 were considered statistically significant.
3. Results
The results of the awareness survey on anisakids showed that 23.5% of respondents stated that they did not know what anisakids were. The rest of the sample claimed to know about anisakids. Comparing the answers of the two groups of respondents (Figure 1), those declaring to know about anisakids were more aware of the risk of raw and half-cooked products, and of temperature treatments killing anisakids than those declaring not knowing about them (unaware group). The risk ratio (RR) was significant for these three items (Table 1).
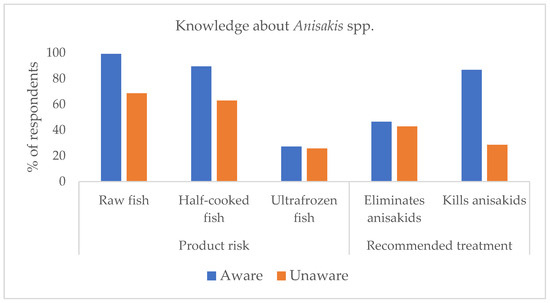
Figure 1.
Knowledge about anisakiasis risks from fish and fish food consumption.

Table 1.
Effect of self-declared knowledge on the real awareness about anisakids. Risk ratio, with 95% confidence interval (CI), for each question about the safety of products and the effect of temperature treatments, comparing the group of respondents self-declaring to know about anisakids and the rest.
In contrast, RR was not significant for the risk of frozen products, which was considered safe by almost 80% of both aware and unaware participants, and for the elimination of anisakids with the recommended treatments, wrongly considered true for about 40% of respondents with no difference between aware and unaware (Table 1, Figure 1).
Multivariate multiple linear regression analysis showed that self-declared awareness predicted three items measuring knowledge: the risk of consuming raw and half-cooked seafood, and the efficiency of temperature treatments for killing Anisakis spp. (Table 2). This was consistent with the results obtained from the risk ratio analysis. Regarding the effects of the demographic variables, age predicted knowledge about raw seafood (R2 = 0.017, p < 0.05), while gender did not predict any knowledge item.

Table 2.
Multiple regression analyses. Product risks and treatment effect as dependent variables, and gender, age, and self-declared knowledge (“Awareness”) of anisakids as independent variables.
From the data provided by the respondents about their consumption of fish and cephalopods, the mean frequency was calculated and is presented in Table 3 (note: as indicated in the Section 2, in the survey, the scale is inverted, and higher values indicate a lower frequency). The range of anisakid prevalence was also given for each type of fish as the percentage of infested individuals per species; these data are taken from published reports [29,30,31].

Table 3.
Average consumption frequency and prevalence range for the most consumed fish and cephalopods.
Regarding the consumption of different types of fish, tuna (Thunnus spp.) was the most consumed, with an average frequency of 2.07 (SD 1.09) (monthly), and eel (Anguilla anguilla) was the least consumed, with an average frequency of 3.84 (SD 0.50) (rarely/almost never). About invertebrates, squid (Loligo vulgaris) had the highest mean frequency 2.27 (SD 0.95) (around monthly), and gooseneck barnacle (Pollicipes pollicipes) had the lowest one 3.64 (SD 0.71) (occasionally/rarely) (Table 3).
The scientific literature we consulted points out that hake, wild salmon, megrim, horse mackerel, and gilt-head seabream are the fish with the highest anisakid prevalence records, while sea bass, eel, and octopus exhibit the lowest ones [29,30,31].
To evaluate the risk of anisakiasis in the population, the maximum prevalence of anisakids in the flesh of each fish and cephalopod type was divided into two groups: over 90% and under 90% average. Within each group, fish were classed by consumption frequency in two groups: high frequency (scoring < 3, that is, at least monthly) and low frequency (scoring > 3) (Table 4).

Table 4.
Classification of fish and cephalopods by the average frequency of consumption and the prevalence of anisakids in them. * Fish that are consumed raw or marinated in the region.
Considering this classification, the seafood products with the highest risk of anisakiasis for Asturian consumers would be hake and cod due to their high levels of consumption and prevalence of anisakids. Although salmon belongs to the same group, reference has been made to the high anisakid prevalence in wild salmon. However, most of the salmon consumed in Asturias and other areas is farmed, corresponding to 80% of the total world salmon supply [32]. Therefore, since farmed Atlantic salmon has not shown the presence of anisakids [33], it will not be considered a species at high risk for anisakiasis in this region.
On the other hand, the species with the lowest risk of anisakiasis in the region were sole, swordfish, and eel, while the rest of the species represented a medium risk if they were not treated adequately.
If gastronomy is considered, taking into account the popular recipes of the region, the risks would remain medium for tuna, anchovy, sardine, and rainbow trout, since they can be consumed as tataki, marinated, and smoked, respectively. The risks for the rest of the fish are almost insignificant, as they are commonly consumed well cooked in this region. Nevertheless, if any of the other high-risk fish (hake and cod) were cooked at low temperatures or consumed raw without prior freezing, the risk of anisakiasis due to their consumption would be high. An example could be ceviche, which, although in the region is generally made with grouper [34], could be prepared with any other white meat fish.
4. Discussion
Considering the results from the anisakid survey, most participants seemed to be aware of anisakids, although more than one-fifth declared to ignore these parasites. Being aware of anisakids is important for the population’s health because, as found in our results, self-declared awareness significantly predicted the actual knowledge about anisakids. Previous studies have confirmed that the improvement in awareness and knowledge about anisakiasis is one of the reasons for the increase in the number of cases reported in a growing number of countries [35]. In the same direction, a current study developed by Ganucci-Cancellieri et al. [36] has shown that habits related to the consumption of raw fish were positively correlated with a higher perceived risk of contracting anisakiasis. Furthermore, prior knowledge of the disease was associated with avoidance of fish consumption, positively correlated with a greater willingness to pay for anisakid-free fish. Moreover, our results showed a significant negative relationship between age and knowledge about anisakids in raw fish and shellfish, with age being a predictor of knowledge concerning raw seafood. It could be explained by the increase in the consumption of raw and half-cooked seafood, such as sushi, ceviche, and sashimi, in different European countries [37,38], mostly by younger people. Previous studies have already shown different food preferences according to gender and age [39,40]. If young people prefer these types of food, they should know more about the safety of raw products. Although it is always advisable to promote more studies on preferences in seafood consumption at different ages, the undeniable fact is that the risk of exposure to this fish-borne disease would be low for conscious populations.
On the other hand, knowledge of the presence of live anisakids in different seafood products and the treatments necessary to kill nematodes are crucial in the prevention and control of anisakiasis, which is based on avoiding the ingestion of live larvae present in raw undercooked seafood. The risk of anisakiasis in the population would be low if everyone knew the temperature treatment, i.e., >60 °C for more than 1 min, or freezing at −20 °C for at least 24 h (the temperature must be reached in the center of the piece), to ensure the death of the anisakids. However, temperature treatment simply kills the parasites but does not eliminate them, so the risk of allergy would still be present. The ingestion of dead parasites in seafood can be potentially dangerous. Some A. simplex allergens are resistant to heat treatments, so cooking or freezing shellfish could kill the parasite but not eliminate its allergenicity [41]. Anisakids’ proteins and DNA have even been found in highly processed seafood products, such as surimi, canned tuna, or croquettes [42,43,44]. In our sample, more than 40% of respondents (whether aware of anisakids or not) mistakenly think that treatments eliminate anisakids, so the risk of allergies does not decrease with knowledge of these parasites. According to the results obtained in this study and the references indicated, part of the population, even being aware of anisakids, is unaware of how prevention methods work. This is an important knowledge gap and an important result of this work that should be addressed in future information campaigns on the safe consumption of seafood.
Regarding preferences of consumption, species with the highest risk of causing anisakiasis or anisakid allergies are expected to be those with a medium–high frequency of consumption, a high prevalence of anisakids, and often consumed raw. According to the aforementioned research by Golden et al. in Portugal, the main risk group for anisakiasis is formed by consumers who prepare raw or half-cooked fish dishes at home. The species most commonly associated with anisakiasis in Europe are herring (Clupea harengus), hake, anchovy, and cod [45]. Anchovies are considered one of the main causes of the high incidence of anisakiasis in Spain, together with raw sardines. In the case of anchovies, the frequency of consumption derived from this study is not one of the highest, but anisakid prevalence has reached 96% (44% in flesh) in recent years [46,47]. From the results obtained, it is observed that for sardines, the frequency of consumption is one of the highest, although anisakid prevalence in flesh is <90%. In any case, marinated anchovies and sardines are also eaten in the studied region, supporting the idea that it is a high-risk product in Spain. According to this, hake could represent a riskier species due to its high average frequency of consumption and high anisakid prevalence in flesh. Hake exhibits anisakid prevalence between 17.7 and 100%, depending on the fishing area, it being higher in the Bay of Biscay and the Atlantic than in the Mediterranean Sea [48]. In fact, hake has already been rejected by consumers who complain about the high infestation of anisakids observed in edible tissues [49,50]. This species would hold a high risk of anisakiasis if consumed raw; however, typical recipes in this region involve cooking at high temperatures for more than one minute, often in cider [51]. Thus, its consumption in the region can be considered safe if well cooked, although not for those with allergies. The rest of the species would have a lower risk due to a lower average frequency of consumption, a lower prevalence of Anisakis spp. larvae, or a combination of both.
The risk caused by this type of parasite is increasing, especially in countries with a high consumption of raw fish [52,53]. Its importance has led to it being addressed within the “Sustainable Development Goals” of the United Nations 2030 Agenda (United Nations 2015), specifically in Goals 2 and 3. Goal 2.1: “Achieve food security and improve healthy nutrition” declares, “By 2030, eliminate hunger and ensure access to safe, nutritious and sufficient food for all people”. To achieve this, it is necessary to provide adequate information aimed at the highest-risk sectors about the possible effects of developing dangerous eating behaviors, such as anisakiasis. Additionally, Goal 3, about health and well-being, focuses on disease prevention (Goal 3d). However, despite the severity of the symptoms caused by these parasites in some cases, knowledge about them and global interest are still low [54]. As already noted, recent studies have shown the importance of identifying the sociological characteristics that help prevent specific diseases, together with biological and medical studies, to favor the development of awareness campaigns about the risks of consuming unsafe fish without adequate treatment. These types of studies are still very scarce and are currently only limited to the two studies presented above in the Iberian Peninsula. Furthermore, we must not forget that fish consumption is part of a healthy and balanced diet, and it is important to avoid unjustified social alarm about its risks. Consequently, the solution is education and the development of specific and targeted campaigns, so that the population continues to consume fish, but healthily and safely.
In Spain, these campaigns started some years ago, for example, the campaign “Come pescado con seguridad” (Eat seafood with safety), developed in Murcia in 2007 [55]; or the Spanish Ministry of Health and Consumer Affairs campaign for anisakiasis prevention launched at a national level in 2006 [56]. In 2021, the Spanish Agency for Food Security and Nutrition (AESAN) published a brochure with the same purpose: (“Comer pescado es seguro y saludable. La anisakiasis es fácil de evitar”) “Eating seafood is safe and healthy. Anisakiasis is easy to prevent” [57]. On a larger scale, in 2021, the European Food Safety Authority launched the communication campaign “EU Choose Safe Food” to inform consumers about safe food consumption [58], although anisakiasis and how to prevent it were not explicitly considered. These campaigns are addressed to the general population. However, following Good Practice of Manufacture (GMP) through the food chain [59,60,61], education campaigns should be adapted to different sectors of the population, including medical doctors [62], and allergic people, as has been shown in this study. The knowledge of and attitudes towards food safety is not the same for all the members of a society. For example, young men are less engaged in safe food handling [63]; safety perception of some foods is different between genders [64], and the prevalence of risky practices increases with socioeconomic status [65].
5. Conclusions
In Asturias, the regional context of our research, anchovy consumption would exhibit a medium risk of anisakiasis, as in the rest of Spain, because of a high prevalence of anisakids but low regional consumption. Sardines, which are highly consumed, marinated, and which exhibit a lower anisakid prevalence, would also have a medium risk of anisakiasis.
In the studied region, the high average frequency of consumption and the high prevalence of anisakids in its flesh could make hake the species with the highest risk of anisakiasis. However, in this region, it can be considered safe for non-allergic consumers because it is traditionally cooked; thus, the parasite is killed.
The conclusions above, grounded on the prevalence of anisakids, are based on a precautionary approach, as not all anisakids are of zoonotic concern. Future investigations to clarify this subject are recommended.
From the data obtained on the population sample studied, a higher awareness of the risk of consuming raw fish in young consumers suggests that sushi consumption habits are associated with a higher perceived risk of contracting anisakiasis.
Our results support the idea that greater knowledge about anisakids implies better knowledge about safe consumption, although the risk of allergies would not be reduced. Therefore, these results reinforce the need for information on how to consume seafood safely, improving public education on the treatments to kill anisakids through campaigns adapted to different sectors of the population. Allergic people would require special information because anisakids’ antigens can trigger allergic reactions even when the parasite is dead.
To contribute to the knowledge of this marine parasite and inform the entire population of its life cycle and ways to reduce its impact on health through ingestion, we filmed a video and posted it on YouTube, to facilitate quick, direct, and free access to this information: https://www.youtube.com/watch?v=Y1-gxvLfocY (accessed on 4 August 2024).
Author Contributions
B.G.-S.: Formal analysis, writing—original draft. P.M.: Survey development. E.G.-V.: Conceptualization, formal analysis, writing—review and editing, supervision, funding acquisition. A.A.: Conceptualization, formal analysis, writing—review and editing, supervision. E.D.: Conceptualization, survey development, methodology, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by the Government of the Principality of Asturias, Grant SV-PA-21-AYUD-2021-50967.
Institutional Review Board Statement
This study adheres to the European Code of Conduct for Research Integrity. This study was approved by the competent Committee of Research Ethics of Asturias Principality, with the reference number 99/16. Prior to the application of the questionnaire, all participants received an information sheet detailing the objectives of the study, that the data were for research purposes only, and that no personal data would be disclosed. Their willingness to participate in the research was guaranteed from informed consent. They were allowed to revise and verify their answers, to have access to the study results, and were informed about their right to withdraw from the process at any time.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data Availability Statements are available in https://data.mendeley.com/datasets/m64np6vg7g/1 (accessed on 4 August 2024).
Acknowledgments
We thank all the people who participated in the different surveys of this study for their kind collaboration. We also express our gratitude to Aida Dopico García for the editing and translation of the video, and for the review and editing of the entire manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lafferty, K.D.; Allesina, S.; Arim, M.; Briggs, C.J.; De Leo, G.; Dobson, A.P.; Johnson, P.T.J.; Kuris, A.M.; Marcogliese, D.J.; Martines, N.D.; et al. Parasites in food webs: The ultimate missing links. Ecol. Lett. 2008, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.M.; Murrell, K.D.; Cross, J.H. Parasites of fish and risks to public health. Rev. Sci. Tech. (Int. Off. Epizoot.) 1997, 16, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.F.; Cabral, H.N.; Busi, M.; D’Amelio, S. Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J. Helminthol. 2006, 80, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Barton, D.P. A critical review of anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Pierce, G.J.; Strachan NJ, C.; Pascual, S.; González-Muñoz, M.; Levsen, A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends Food Sci. Technol. 2019, 86, 298–310. [Google Scholar] [CrossRef]
- Chai, J.Y.; Darwin Murrell, K.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2014. [Google Scholar]
- Pravettoni, V.; Primavesi, L.; Piantanida, M. Anisakis simplex: Current knowledge. Eur. Ann. Allergy Clin. Immunol. 2012, 44, 150–156. [Google Scholar] [PubMed]
- Aydemir, M.E.; Aydemir, S.; Altun, S.K.; Alkan, S. Trends in Anisakis simplex Global Research: A Bibliometric Analysis Study. Turk. Parazitol. Derg 2024, 48, 51–57. [Google Scholar] [CrossRef]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J.C. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef]
- Herrador, Z.; Daschner, Á.; Perteguer, M.J.; Benito, A. Epidemiological scenario of anisakidosis in Spain based on associated hospitalizations: The tip of the iceberg. Clin. Infect. Dis. 2018, 69, 69–76. [Google Scholar] [CrossRef]
- Daschner, A.; Levsen, A.; Cipriani, P.; Cuéllar del Hoyo, C. Anisakis allergy: Unjustified social alarm versus healthy diet. Parasitol. Res. 2021, 120, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Martín Cerdeño, V.J. Consumo de pescados y mariscos. Diferencias sociales y territoriales. Ministerio de Agricultura, Alimentación y Medio Ambiente. Distrib. Y Consumo Madr. 2012, 124, 5–20. [Google Scholar]
- Jerončić, A.; Nonković, D.; Vrbatović, A.; Hrabar, J.; Bušelić, I.; Martínez-Sernández, V.; Lojo Rocamonde, S.A.; Ubeira, F.M.; Jaman, S.; Jeličić, E.C.; et al. Anisakis Sensitization in the Croatian fish processing workers: Behavioral instead of occupational risk factors? PLoS Negl. Trop. Dis. 2020, 14, e0008038. [Google Scholar] [CrossRef]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish—From infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.; Manzabeitia, F.; López Vélex, R.; Onate, J.M. Anisakiasis humana en España por consumo de sardinas crudas. Alimentaria 1992, 28, 57–61. [Google Scholar]
- Quijada, S.G.; Escudero, R.G.; García, L.A.; Martín, A.G.; Serrano, J.V.; Fernández, E.C. Manifestaciones digestivas de la anisakiasis: Descripción de 42 casos. Rev. Clínica Española 2005, 205, 311–315. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The benefits of fish consumption. Nutr. Bull. 2011, 36, 6–19. [Google Scholar] [CrossRef]
- Daschner, A.; Cuéllar, C. Progress in Anisakis allergy research: Milestones and reversals. Curr. Treat. Options Allergy 2020, 7, 457–470. [Google Scholar] [CrossRef]
- Bao, M.; Pierce, G.J.; Strachan NJ, C.; Martínez, C.; Fernández, R.; Theodossiou, I. Consumers’ attitudes and willingness to pay for Anisakis-free fish in Spain. Fish. Res. 2018, 202, 149–160. [Google Scholar] [CrossRef]
- Golden, O.; Caldeira, A.J.; Santos, M. Raw fish consumption in Portugal: A survey on trends in consumption and consumer characteristics. Food Control 2022, 135, 108810. [Google Scholar] [CrossRef]
- Welch, A.; Lund, E.; Amiano, P.; Dorronsoro, M.; Brustad, M.; Kumle, M.; Rodriguez, M.; Lasheras, C.; Janzon, L.; Jansson, J.H.; et al. Variability of fish consumption within the 10 European countries participating in the European Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003, 5, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Brace, I. Questionnaire Design: How to Plan, Structure and Write Survey Material for Effective Market Research; Kogan Page Publishers: London, UK, 2018. [Google Scholar]
- Blanco-Fernandez, C.; Ardura, A.; Masiá, P.; Rodriguez, N.; Voces, L.; Fernandez-Raigoso, M.; Roca, A.; Machado-Schiaffino, G.; Dopico, E.; Garcia-Vazquez, E. Fraud in highly appreciated fish detected from DNA in Europe may undermine the Development Goal of sustainable fishing in Africa. Sci. Rep. 2021, 11, 11423. [Google Scholar] [CrossRef] [PubMed]
- Estrategia Para el Sector Pesquero de Asturias 2021–2030. Available online: https://www.asturias.es/documents/217090/797301/20211230+Estrategia+del+sector+pesquero+en+Asturias+2021+2030.pdf/bf2a70ee-54b8-9cfb-fd8f-3891b5bf5c39?t=1640847918366 (accessed on 6 May 2024).
- García-Sánchez, B.; Dopico, E.; Garcia-Vazquez, E.; Ardura, A. Dataset, Mendeley Data, V1, 2022. Available online: https://data.mendeley.com/datasets/m64np6vg7g/1 (accessed on 3 February 2024).
- Hammer DA, T.; Ryan, P.D.; Hammer, Ø.; Harper DA, T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Debenedetti, Á.L.; Madrid, E.; Trelis, M.; Codes, F.J.; Gil-Gómez, F.; Sáez-Durán, S.; Fuentes, M.V. Prevalence, and risk of anisakid larvae in fresh fish frequently consumed in Spain: An overview. Fishes 2019, 4, 13. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef]
- Audicana, M.T. Anisakis, something is moving inside the fish. Pathogens 2022, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, F.; Hilborn, R. Fished or farmed: Life cycle impacts of salmon consumer decisions and opportunities for reducing impacts. Sci. Total Environ. 2023, 854, 158591. [Google Scholar] [CrossRef]
- González, M.Á.P.; Cavazza, G.; Gustinelli, A.; Caffara, M.; Fioravanti, M. Absence of anisakis nematodes in smoked farmed Atlantic salmon (Salmo salar) products on sale in European countries. Ital. J. Food Saf. 2020, 9, 216–219. [Google Scholar] [CrossRef]
- La Excelencia Gastronómica que Llega del Mar Cantábrico. Available online: https://www.turismoasturias.es/gastronomia/pescados-mariscos (accessed on 24 January 2024).
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. J. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef]
- Ganucci Cancellieri, U.; Amicone, G.; Cicero, L.; Milani, A.; Mosca, O.; Palomba, M.; Mattiucci, S.; Bonaiuto, M. Can Food Safety Practices and Knowledge of Raw Fish Promote Perception of Infection Risk and Safe Consumption Behavior Intentions Related to the Zoonotic Parasite Anisakis? Sustainability 2023, 15, 7383. [Google Scholar] [CrossRef]
- Golden, O.; Araújo, A.C.; Caldeira, A.J.; Santos, M.J. Raw fish consumption in Portugal: Commonly consumed fish species and associated risk factors for anisakiosis. Food Control 2023, 145, 109457. [Google Scholar] [CrossRef]
- Ivanovic, J.; Baltic, M.Z.; Boskovic, M.; Kilibarda, N.; Dokmanovic, M.; Markovic, R.; Janjic, J.; Baltic, B. Anisakis Infection and Allergy in Humans. Procedia Food Sci. 2015, 5, 101–104. [Google Scholar] [CrossRef][Green Version]
- Cooke, L.J.; Wardle, J. Age and gender differences in children’s food preferences. Br. J. Nutr. 2005, 93, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B.; Cheney, M.M.; Chan, N. Exploring comfort food preferences across age and gender. Physiol. Behav. 2003, 79, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, J.; Baltić, M.; Bošković, M.; Kilibarda, N.; Dokmanović, M.; Marković, R.; Janjić, J.; Baltić, B. Anisakis allergy in human. Trends Food Sci. Technol. 2017, 59, 25–29. [Google Scholar] [CrossRef]
- Faeste, C.; Plassen, K.E.; Løvberg, A.; Moen, A.; Egaas, E. Detection of proteins from the fish parasite Anisakis simplex in Norwegian farmed salmon and processed fish products. Food Anal. Methods 2015, 8, 1390–1402. [Google Scholar] [CrossRef]
- Lopez, I.; Pardo, M.A. Evaluation of a real-time polymerase chain (PCR) assay for detection of Anisakis simplex parasite as a food-borne allergen source in seafood products. J. Agric. Food Chem. 2010, 58, 1469–1477. [Google Scholar] [CrossRef]
- Mossali, C.; Palermo, S.; Capra, E.; Piccolo, G.; Botti, S.; Bandi, C.; D´Amelio, S.; Giuffra, E. Sensitive detection, and quantification of anisakid parasite residues in food products. Foodborne Pathog. Dis. 2010, 7, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Attir, B.; Mammeri, A.; Kechroud, N.; Mazouz, K.; Chabira, D.; Chenchouni, H. A survey of nematodes in the European hake (Merluccius merluccius) intended for human consumption. J. Parasit. Dis. 2024, 48, 347–357. [Google Scholar] [CrossRef]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: Dangerous—Dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dessier, A.; Dupuy, C.; Trancart, T.; Audras, A.; Bustamante, P.; Gérard, C. Low diversity of helminth parasites in Sardina pilchardus and Engraulis encrasicolus (Clupeidae) from the Bay of Biscay. Mar. Freshw. Res. 2016, 67, 1583–1588. [Google Scholar] [CrossRef]
- Roca-Geronès, X.; Segovia, M.; Godínez-González, C.; Fisa, R.; Montoliu, I. Anisakis and Hysterothylacium species in Mediterranean and North-East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular, and morphometric discriminant analysis. In J. Food Microbiol. 2020, 325, 108642. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; López-Cuello MD, M.; Benítez, R.; Adroher, F.J. Anisakis spp. in European hake, Merluccius merluccius (L.) from the Atlantic off north-west Africa and the Mediterranean off southern Spain. Acta Parasitol. 2006, 51, 209–212. [Google Scholar] [CrossRef]
- Llarena-Reino, M.; Abollo, E.; Regueira, M.; Rodríguez, H.; Pascual, S. Horizon scanning for management of emerging parasitic infections in fishery products. Food Control 2015, 49, 49–58. [Google Scholar] [CrossRef]
- Recetario del Mar de Asturias. Available online: https://pescadoderula.org/wp-content/uploads/2015/12/Recetario-de-Pescado-de-Rula-con-Artes-Sanos.pdf (accessed on 24 January 2024).
- Mattiucci, S.; Palomba, M.; Nascetti, G. Anisakis. In Encyclopedia of Infection and Immunity, 2nd. ed.; Elsevier: London, UK, 2021; pp. 408–423. [Google Scholar]
- Guardone, L.; Nucera, D.; Lodola, L.B.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int. J. Food Microbiol. 2018, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- Murciasalud.es. Available online: https://www.murciasalud.es/-/sanidad-intensifica-la-prevencion-de-anisakis-con-una-campana-informativa-en-las-pescaderias-de-toda-la-region (accessed on 5 February 2024).
- Lamoncloa.gob.es. Available online: https://www.lamoncloa.gob.es/paginas/archivo/011206-Anisakis.aspx (accessed on 14 April 2024).
- Agencia Española de Seguridad Alimentaria y Nutrición. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/anisakis.htm (accessed on 5 February 2024).
- EFSA. Available online: https://campaigns.efsa.europa.eu/EUChooseSafeFood/#/ (accessed on 14 April 2024).
- AESAN La Alergia por Anisakis y Medidas de Prevención. 2011. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/ALERGIA_ANISAKIS.pdf (accessed on 4 August 2024).
- EU Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 24 January 2024).
- Boletín Oficial del Estado, España. Real Decreto 1420/2006 de 1 de Diciembre, Sobre Prevención de la Parasitosis por Anisakis en Productos de la Pesca Suministrados por Establecimientos que Sirven Comida a Consumidores Finales o a Colectivos. Nº302 de 19.12.2006. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2006-22171 (accessed on 24 January 2024).
- Seal, A.; Harding, C.; Shamsi, S. A preliminary report on the awareness and knowledge of seafood-borne parasitic diseases among medical doctors in Australia. Parasitol. Int. 2020, 74, 101993. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Bredbenner, C.; Maurer, J.; Wheatley, V.; Cottone, E.; Clancy, M. Observed food safety behaviours of young adults. Br. Food J. 2007, 109, 519–530. [Google Scholar] [CrossRef]
- Hamad, M.A.; Ahmed, A.I. Assessing awareness and perception towards food quality and safety among households in Elobeid, North Kordofan-Sudan. MOJ Food Process Technol. 2018, 6, 93–94. [Google Scholar] [CrossRef]
- Wilcock, A.; Pun, M.; Khanona, J.; Aung, M. Consumer attitudes, knowledge, and behaviour: A review of food safety issues. Trends Food Sci. Technol. 2004, 15, 56–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).