Macroinvertebrates Associated with Macroalgae within Integrated Multi-Trophic Aquaculture (IMTA) in Earthen Ponds: Potential for Accessory Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Sampling and Sorting of Macrofauna
3. Results
3.1. Environmental Parameters
3.2. Macroalgae
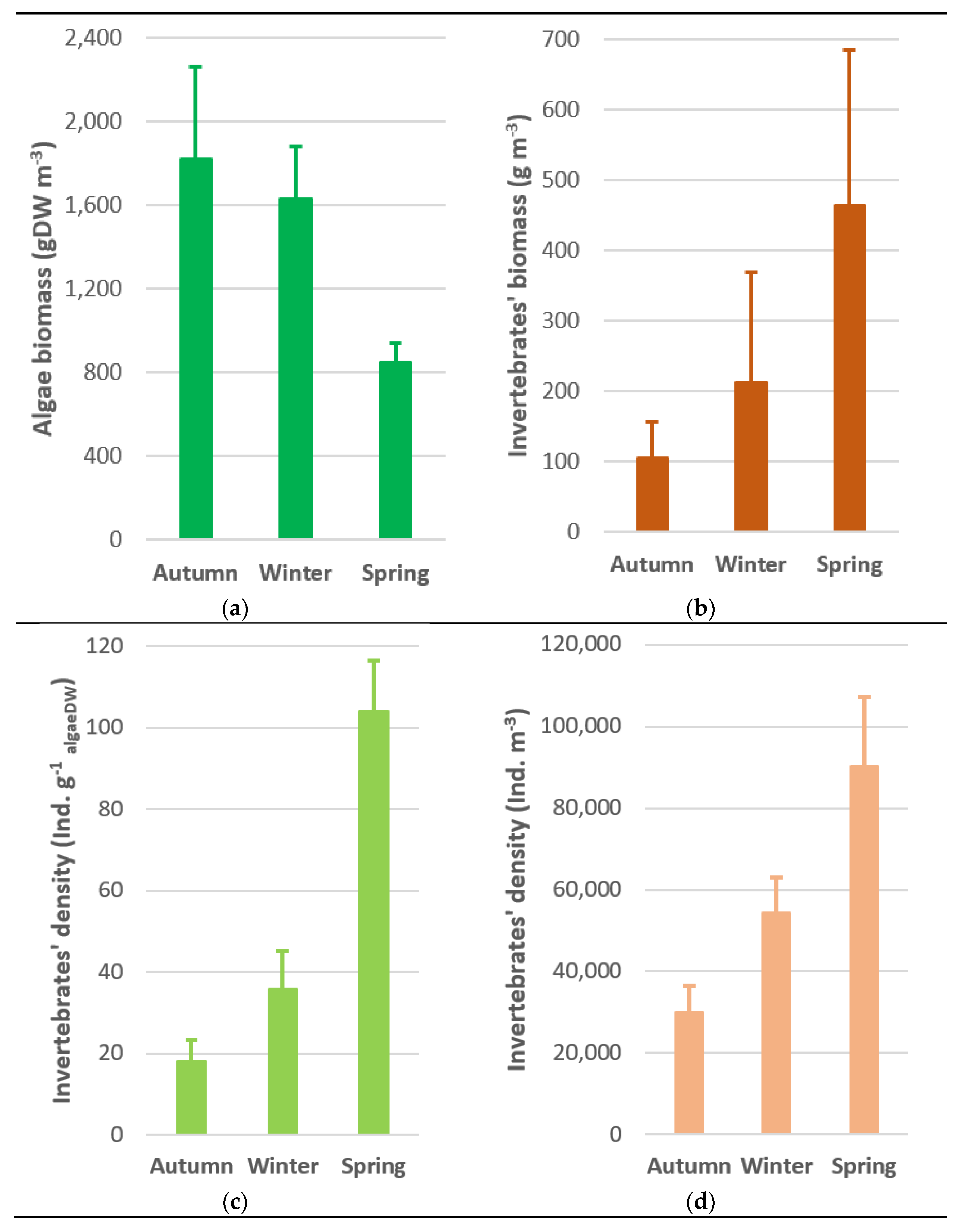
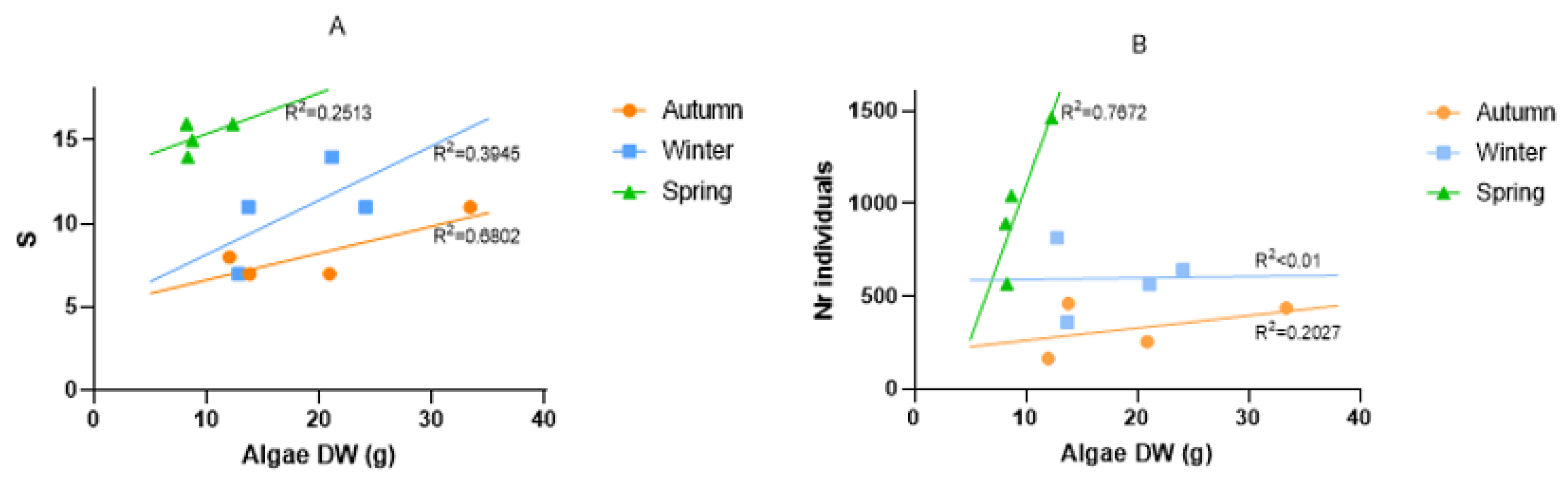
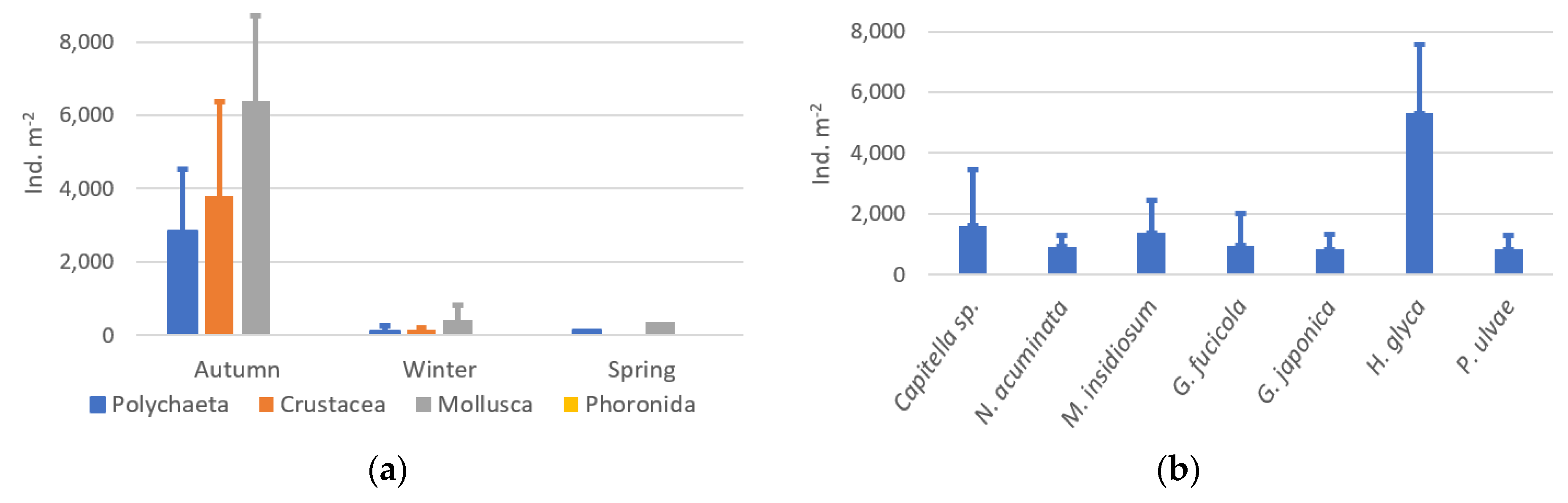
3.3. Macroinvertebrates Associated with Macroalgae
3.4. Benthic Macroinvertebrates
4. Discussion
4.1. Sample Characterization and Species Composition
4.2. Macroalgae as a Substrate and Habitat for Macroinvertebrate Epifauna
4.3. Invertebrates as Feed for Aquaculture
4.4. Macroinvertebrate Role in Nutrient Recycling and in the Trophic Web
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chopin, T. Aquaculture, Integrated Multi-trophic (IMTA). In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 184–205. [Google Scholar] [CrossRef]
- Biswas, G.; Kumar, P.; Ghoshal, T.K.; Kailasam, M.; De, D.; Bera, A.; Mandal, B.; Sukumaran, K.; Vijayan, K.K. Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity, and economic return in brackishwater ponds. Aquaculture 2020, 516, 734626. [Google Scholar] [CrossRef]
- Hossain, A.; Senff, P.; Glaser, M. Lessons for Coastal Applications of IMTA as a way towards Sustainable Development: A Review. Appl. Sci. 2022, 12, 11920. [Google Scholar] [CrossRef]
- Manan, H.; Jalilah, M.; Fauzan, F.; Ikhwanuddin, M.; Amin-Safwan, A.; Abdullah, N.S.; Nur-Syahirah, M.; Kasan, N.A. Recent developments in aquaculture—A review. Ann. Anim. Sci. 2023, 23, 663–680. [Google Scholar] [CrossRef]
- Gamito, S. Three main stressors acting on the Ria Formosa lagoonal system (Southern Portugal): Physical stress, organic matter pollution and the land-ocean gradient. Estuar. Coast. Shelf Sci. 2008, 77, 710–720. [Google Scholar] [CrossRef]
- Gamito, S.; Quental-Ferreira, H.; Parejo, A.; Aubin, J.; Christensen, V.; Cunha, M.E. Integrated multi-trophic aquaculture systems: Energy transfers and food web organization in coastal earthen ponds. Aquac. Environ. Interact. 2020, 12, 457–470. [Google Scholar] [CrossRef]
- Cunha, M.E.; Quental-Ferreira, H.; Parejo, A.; Gamito, S.; Ribeiro, L.; Moreira, M.; Monteiro, I.; Soares, F.; Pousão-Ferreira, P. Understanding the individual role of fish, oysters, phytoplankton and macroalgae in the ecology of integrated production in earthen ponds. Aquaculture 2019, 512, 734297. [Google Scholar] [CrossRef]
- Dudley, T.L.; Cooper, S.D.; Hemphill, N. Effects of Macroalgae on a Stream Invertebrate Community. J. N. Am. Benthol. Soc. 1986, 5, 93–106. [Google Scholar] [CrossRef]
- Brönmark, C. Interactions between epiphytes, macrophytes and freshwater snails: A review. J. Molluscan Stud. 1989, 55, 299–311. [Google Scholar] [CrossRef]
- Downes, B.J.; Lake, P.S.; Schreiber, E.S.G.; Glaister, A. Habitat structure, resources and diversity: The separate effects of surface roughness and macroalgae on stream invertebrates. Oecologia 2000, 123, 569–581. [Google Scholar] [CrossRef]
- Umanzor, S.; Ladah, L.; Calderon-Aguilera, L.E.; Zertuche-González, J.A. Intertidal macroalgae influence macroinvertebrate distribution across stress scenarios. Mar. Ecol. Prog. Ser. 2017, 584, 67–77. [Google Scholar] [CrossRef]
- Guttman, L.; Boxman, S.E.; Barkan, R.; Neori, A.; Shpigel, M. Combinations of Ulva and periphyton as biofilters for both ammonia and nitrate in mariculture fishpond effluents. Algal Res. 2018, 34, 235–243. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Miroshnikova, N.V. Laboratory experiment to determine the potential of two macroalgae from the Russian Far-East as biofilters for integrated multi-trophic aquaculture (IMTA). Bioresour. Technol. 2011, 102, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G. Productivity, fisheries and aquaculture in temperate estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 953–967. [Google Scholar] [CrossRef]
- Dhont, J.; Dierckens, K.; Støttrup, J.; Van Stappen, G.; Wille, M.; Sorgeloos, P. Rotifers, Artemia and copepods as live feeds for fish larvae in aquaculture. In Advances in Aquaculture Hatchery Technology; Allen, G., Ed.; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- van der Meeren, T.; Karlsen, Ø.; Liebig, P.L.; Mangor-Jensen, A. Copepod production in a saltwater pond system: A reliable method for achievement of natural prey in start-feeding of marine fish larvae. Aquac. Eng. 2014, 62, 17–27. [Google Scholar] [CrossRef]
- Hamre, K. Nutrient profiles of rotifers (Brachionus sp.) and rotifer diets from four different marine fish hatcheries. Aquaculture 2016, 450, 136–142. [Google Scholar] [CrossRef]
- Moren, M.; Suontama, J.; Hemre, G.I.; Karlsen, Ø.; Olsen, R.E.; Mundheim, H.; Julshamn, K. Element concentrations in meals from krill and amphipods, Possible alternative protein sources in complete diets for farmed fish. Aquaculture 2006, 261, 174–181. [Google Scholar] [CrossRef]
- Baeza-Rojano, E.; García, S.; Garrido, D.; Guerra-García, J.M.; Domingues, P. Use of Amphipods as alternative prey to culture cuttlefish (Sepia officinalis) hatchlings. Aquaculture 2010, 300, 243–246. [Google Scholar] [CrossRef]
- Vargas-Abúndez, J.A.; Simões, N.; Mascaró, M. Feeding the lined seahorse Hippocampus erectus with frozen amphipods. Aquaculture 2018, 491, 82–85. [Google Scholar] [CrossRef]
- Carvalho, S.; Barata, M.; Pereira, F.; Gaspar, M.B.; da Fonseca, L.C.; Pousão-Ferreira, P. Distribution patterns of macrobenthic species in relation to organic enrichment within aquaculture earthen ponds. Mar. Pollut. Bull. 2006, 52, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.E.; Quental-Ferreira, H.; Parejo, A.; Gamito, S.; Ribeiro, L.; Moreira, M.; Monteiro, I.; Soares, F.; Pousão-Ferreira, P. Methodology for assessing the individual role of fish, oyster, phytoplankton and macroalgae in the ecology of integrated production in earthen ponds. MethodsX 2019, 6, 2570–2576. [Google Scholar] [CrossRef]
- Gamito, S. Benthic ecology of semi-natural coastal lagoons, in the Ria Formosa (Southern Portugal), exposed to different water renewal regimes. Hydrobiologia 2006, 555, 75–87. [Google Scholar] [CrossRef]
- Favot, G.; Engelen, A.E.; Cunha, M.E.; Serrão, M.E.A. Identification of Ulva sp. Grown in Multitrophic Aquaculture Systems. J. Aquac. Fish. 2019, 3, 024. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, A.; Salovius-Laurén, S.; Mattila, J. Epifaunal community composition in five macroalgal species—What are the consequences if some algal species are lost? Estuar. Coast. Shelf Sci. 2018, 207, 402–413. [Google Scholar] [CrossRef]
- Carvalho, S.; Falcão, M.; Cúrdia, J.; Moura, A.; Serpa, D.; Gaspar, M.B.; Dinis, M.T.; da Fonseca, L.C. Benthic dynamics within a land-based semi-intensive aquaculture fish farm: The importance of settlement ponds. Aquac. Int. 2009, 17, 571–587. [Google Scholar] [CrossRef]
- Martínez-Laiz, G.; Ros, M.; Guerra-García, J.M. Marine exotic isopods from the Iberian Peninsula and nearby waters. PeerJ 2018, 6, e4408. [Google Scholar] [CrossRef]
- Jourde, J.; Sauriau, P.G.; Guenneteau, S.; Caillot, E. First record of Grandidierella japonica Stephensen, 1938 (Amphipoda: Aoridae) from Mainland Europe. BioInvasions 2013, 2, 51–55. [Google Scholar] [CrossRef]
- Droual, G.; Le Garrec, V.; Cabelguen, J.; Gélinaud, G.; Grall, J. The spread goes on: The non-indigenous species Grandidierella japonica Stephensen, 1938 (Amphipoda: Aoridae) has reached Brittany (Gulf of Morbihan). Aod-Les Cah. Nat. L’observatoire Mar. 2017, V, 21–29. [Google Scholar]
- Munari, C.; Bocchi, N.; Mistri, M. Grandidierella japonica (Amphipoda: Aoridae): A non-indigenous species in a Po delta lagoon of the northern Adriatic (Mediterranean Sea). Mar. Biodivers. Rec. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Marchini, A.; Ferrario, J.; Nasi, E. Arrival of the invasive amphipod Grandidierella japonica to the Mediterranean Sea. Mar. Biodivers. Rec. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Sheader, M. Distribution and reproductive biology of Corophium insidiosum (amphipoda) on the north-east coast of england. J. Mar. Biol. Assoc. UK 1978, 58, 585–596. [Google Scholar] [CrossRef]
- Veiga, P.; Rubal, M.; Sousa-Pinto, I. Structural complexity of macroalgae influences epifaunal assemblages associated with native and invasive species. Mar. Environ. Res. 2014, 101, 115–123. [Google Scholar] [CrossRef]
- Appadoo, C.; Myers, A.A. Observations on the tube-building behaviour of the marine amphipod Cymadusa filosa Savigny (Crustacea: Ampithoidae). J. Nat. Hist. 2003, 37, 2151–2164. [Google Scholar] [CrossRef]
- Legeżyńska, J.; Kędra, M.; Walkusz, W. When season does not matter: Summer and winter trophic ecology of Arctic amphipods. Hydrobiologia 2012, 684, 189–214. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; De Figueroa, J.T.; Navarro-Barranco, C.; Ros, M.; Sánchez-Moyano, J.E.; Moreira, J. Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J. Sea Res. 2014, 85, 508–517. [Google Scholar] [CrossRef]
- Suhaimi, H.; Rahman, M.I.A.; Ashaai, A.; Ikhwanuddin, M.; Rasdi, N.W. Adaptation and potential culture of wild Amphipods and Mysids as potential live feed in aquaculture: A review. PeerJ 2024, 12, e17092. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Rojano, E.; Hachero-Cruzado, I.; Guerra-García, J.M. Nutritional analysis of freshwater and marine amphipods from the Strait of Gibraltar and potential aquaculture applications. J. Sea Res. 2014, 85, 29–36. [Google Scholar] [CrossRef]
- Cabral, H.N. Comparative feeding ecology of sympatric Solea solea and S. senegalensis, within the nursery areas of the Tagus estuary, Portugal. J. Fish. Biol. 2000, 57, 1550–1562. [Google Scholar] [CrossRef]
- Pita, C.; Gamito, S.; Erzini, K. Feeding habits of the gilthead seabream (Sparus aurata) from the Ria Formosa (southern Portugal) as compared to the back seabream (Spondyliosoma cantharus) and the annular seabream (Diplodus annularis). J. Appl. Ichthyol. 2002, 18, 81–86. [Google Scholar] [CrossRef]
- Gamito, S.; Pires, A.; Pita, C.; Erzini, K. Food availability and the feeding ecology of ichthyofauna of a Ria Formosa (South Portugal) water reservoir. Estuaries 2003, 26, 938–948. [Google Scholar] [CrossRef]
- Pinczon Du Sel, G.; Blanc, A.; Daguzan, J. The diet of the cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) during its life cycle in the Northern Bay of Biscay (France). Aquat. Sci. 2000, 62, 167–178. [Google Scholar] [CrossRef]
- Jimenez-Prada, P.; Hachero-Cruzado, I.; Guerra-García, J.M. Importancia de los anfípodos en la dieta de especies de interés acuícola del litoral andaluz. Zool. Baetica 2015, 26, 3–29. [Google Scholar]
- Jimenez-Prada, P.; Hachero-Cruzado, I.; Guerra-García, J.M. Aquaculture waste as food for amphipods: The case of Gammarus insensibilis in marsh ponds from southern Spain. Aquac. Int. 2021, 29, 139–153. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, V.; Toledo-Guedes, K.; Valero-Rodriguez, J.M.; Agraso, M.; Sanchez-Jerez, P. Harvesting amphipods applying the integrated multitrophic aquaculture (IMTA) concept in off-shore areas. Aquaculture 2018, 489, 62–69. [Google Scholar] [CrossRef]


| Autumn | Winter | Spring | |
|---|---|---|---|
| Temperature (°C) | 17.4 | 16.4 | 24.3 |
| Salinity | 35.8 | 35.7 | 37.1 |
| Dissolved Oxygen (%) | 108.9 | 100.2 | 101.0 |
| pH | 8.1 | 8.0 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, R.; Mateus, M.Â.; Afonso, C.M.L.; Soares, F.; Pousão-Ferreira, P.; Gamito, S. Macroinvertebrates Associated with Macroalgae within Integrated Multi-Trophic Aquaculture (IMTA) in Earthen Ponds: Potential for Accessory Production. J. Mar. Sci. Eng. 2024, 12, 1369. https://doi.org/10.3390/jmse12081369
Vieira R, Mateus MÂ, Afonso CML, Soares F, Pousão-Ferreira P, Gamito S. Macroinvertebrates Associated with Macroalgae within Integrated Multi-Trophic Aquaculture (IMTA) in Earthen Ponds: Potential for Accessory Production. Journal of Marine Science and Engineering. 2024; 12(8):1369. https://doi.org/10.3390/jmse12081369
Chicago/Turabian StyleVieira, Rafael, Miguel Ângelo Mateus, Carlos Manuel Lourenço Afonso, Florbela Soares, Pedro Pousão-Ferreira, and Sofia Gamito. 2024. "Macroinvertebrates Associated with Macroalgae within Integrated Multi-Trophic Aquaculture (IMTA) in Earthen Ponds: Potential for Accessory Production" Journal of Marine Science and Engineering 12, no. 8: 1369. https://doi.org/10.3390/jmse12081369








