Sediment Types with Alternation of Sandy and Rocky Shores Influence the Distribution of Clams in an Area Characterized by High-Energy Hydrodynamic Conditions
Abstract
:1. Introduction
2. Material and Methods
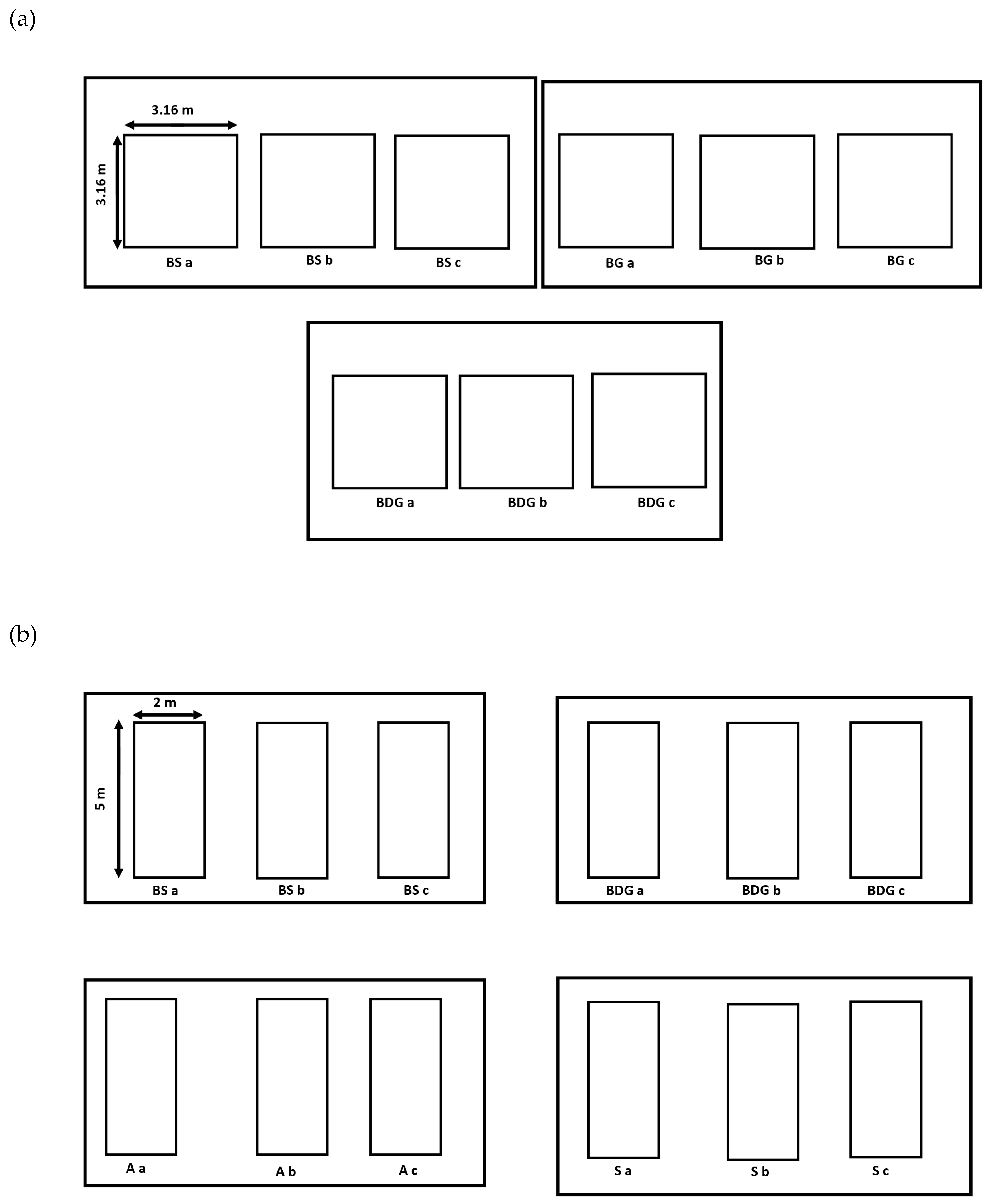
2.1. Sampling Sites
2.2. Clam Sampling Strategies
2.2.1. Quadrat Raking Method
2014 Sampling
2016 Sampling
2018 Sampling
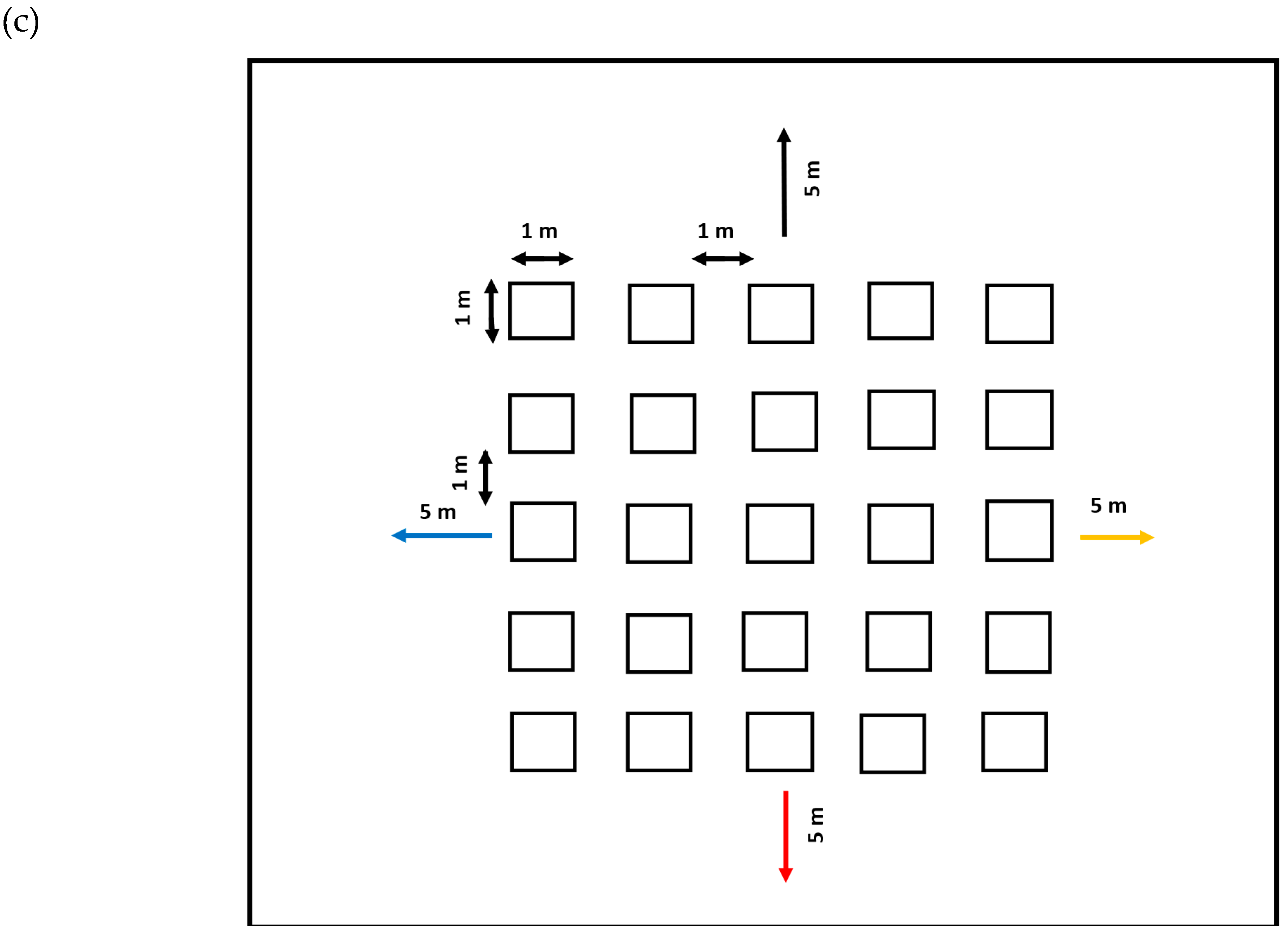
2.2.2. Mark–Recapture Method
2017 Displacement Assessment
2018 Displacement Assessment
2.3. Environmental Data
2.4. Statistical Analyses
3. Results
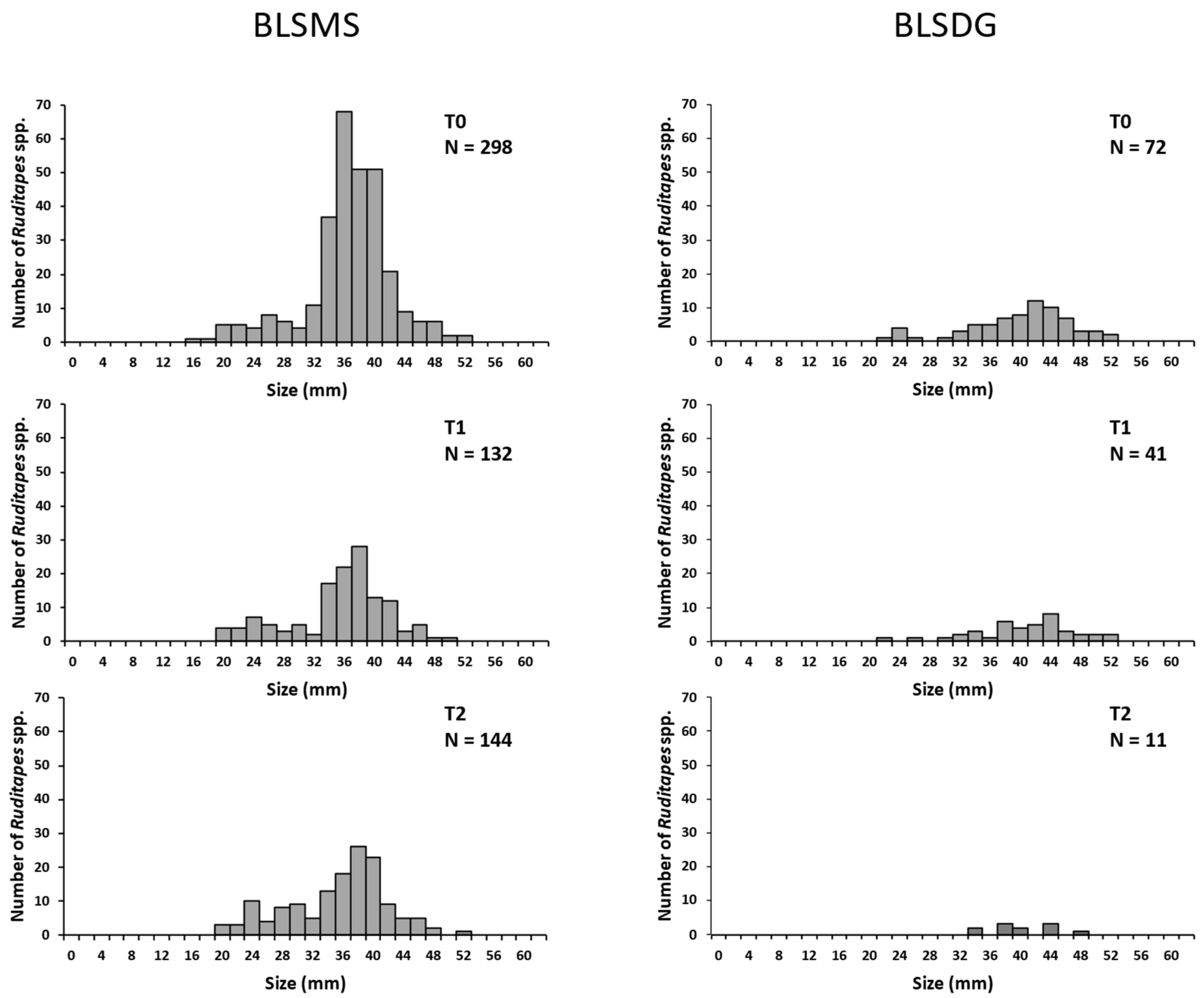
3.1. Quadrat Raking
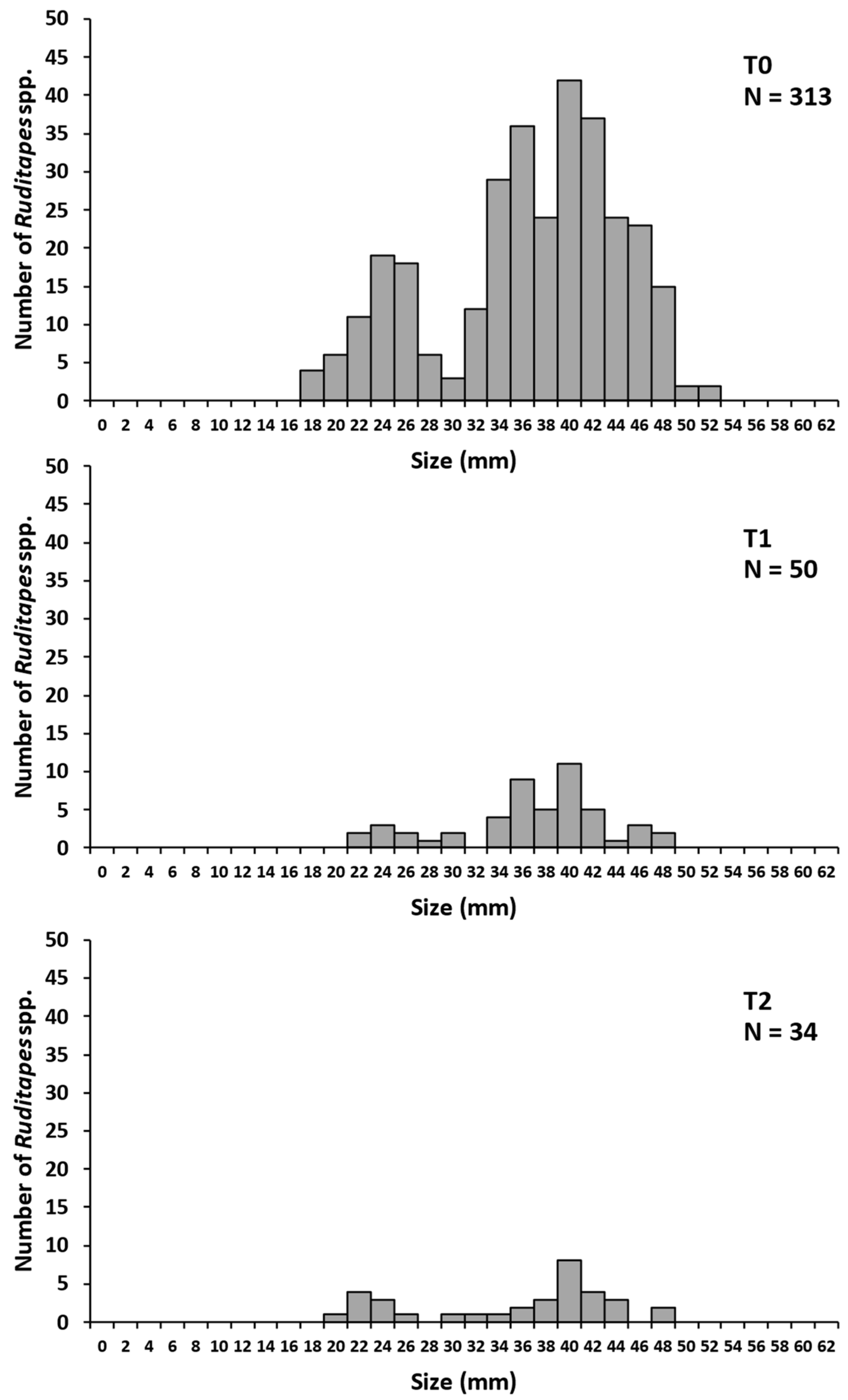
3.1.1. 2014 Sampling
3.1.2. 2016 Sampling
3.1.3. 2018 Sampling
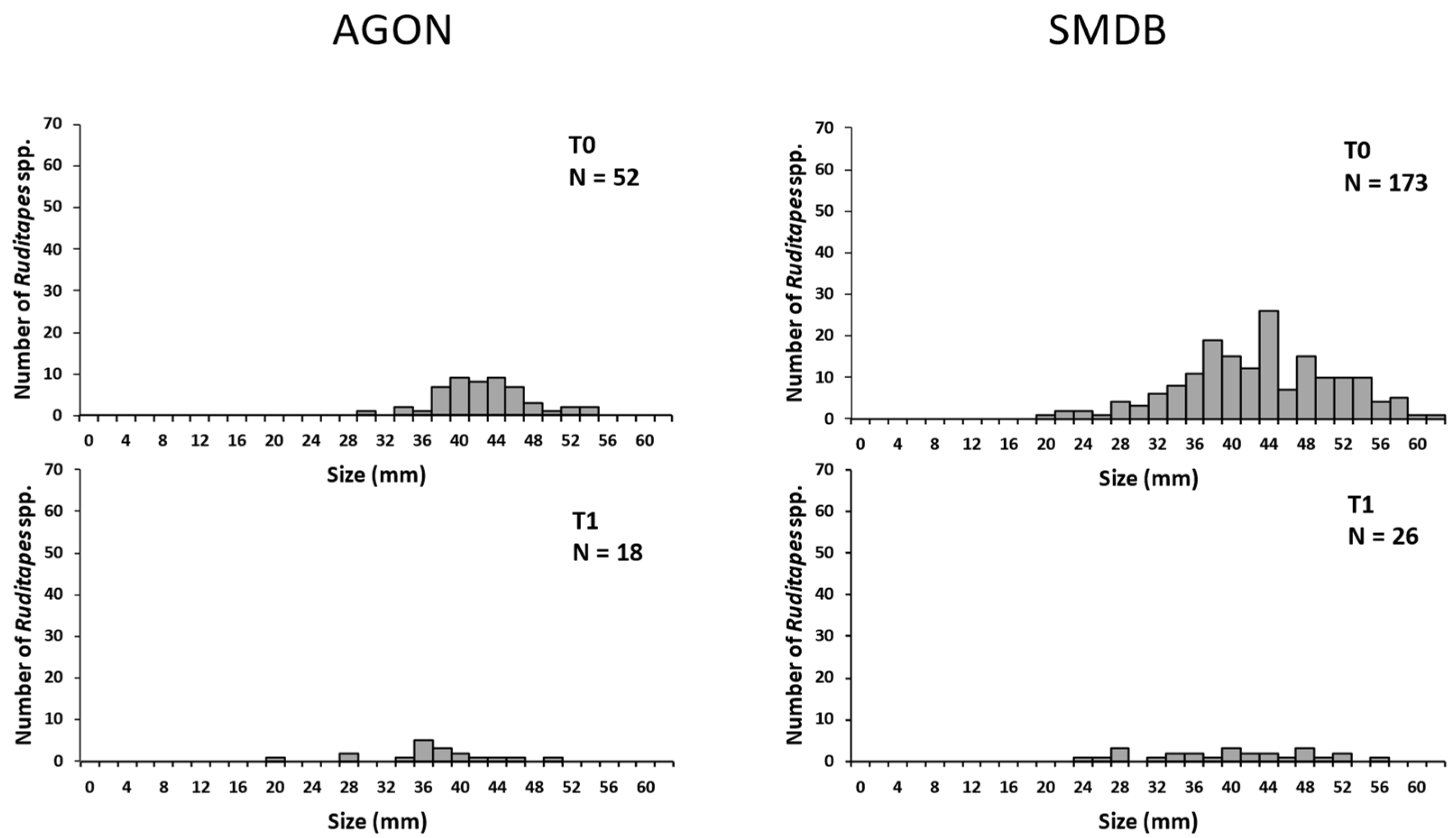
3.2. Mark–Recaptures
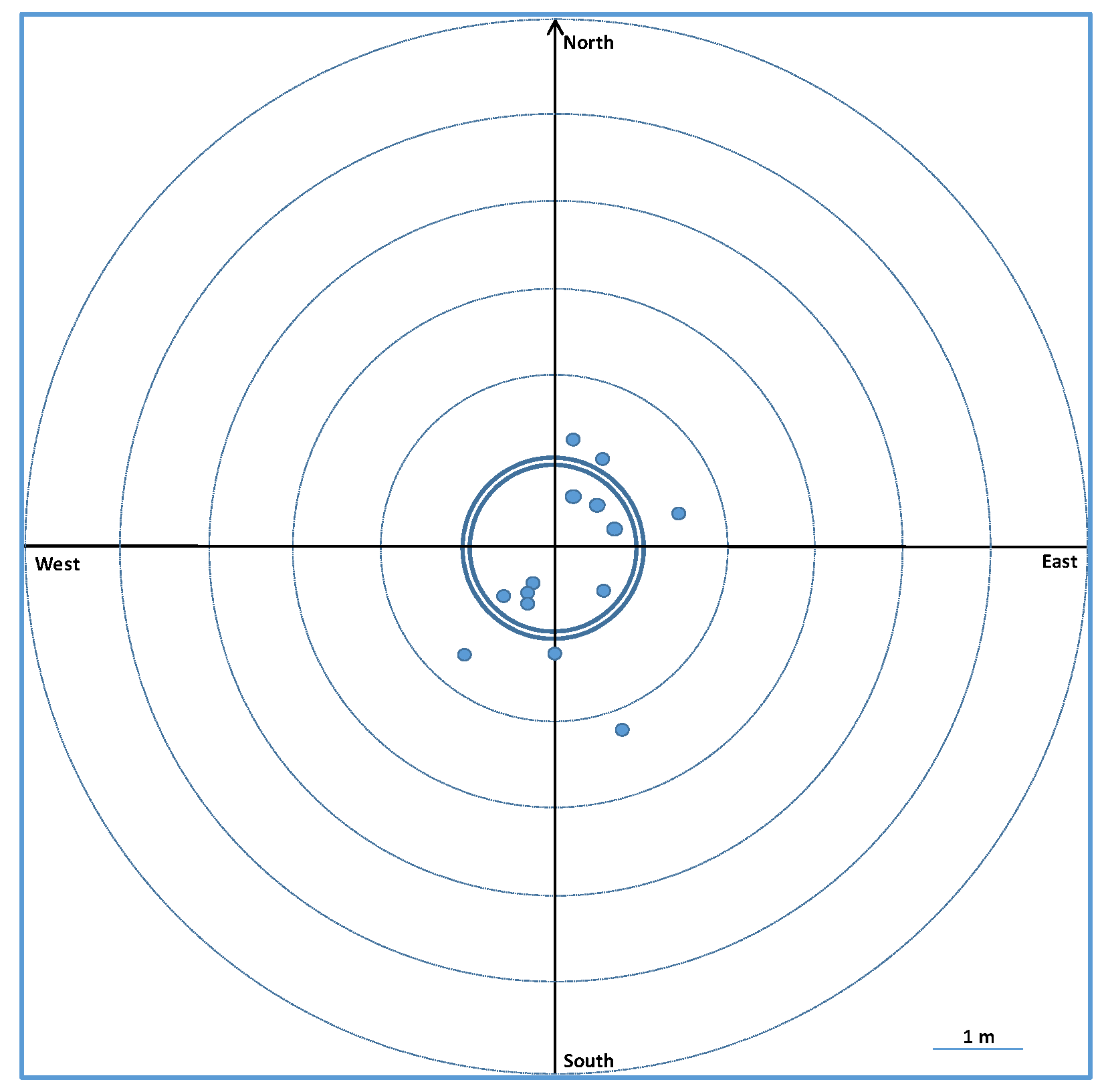
3.2.1. 2017 Recaptures
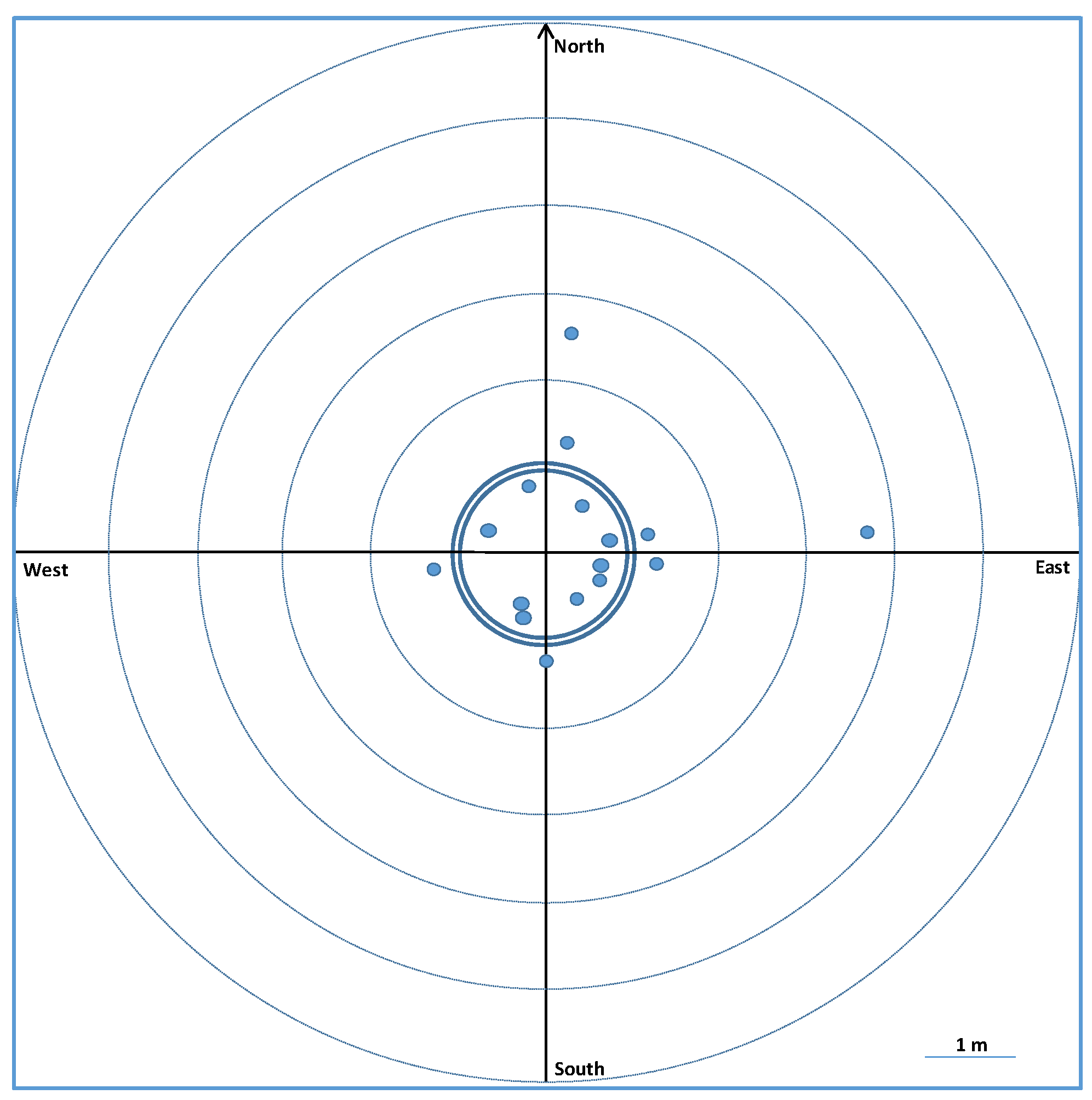
3.2.2. 2018 Recaptures
4. Discussion
4.1. General Patterns of Ruditapes spp. on the Western Coast of Cotentin
4.2. Main Lessons from the Quadrat Raking Method in 2014, 2016 and 2018
4.3. Natural Transport of Clams and Intertidal Sediment Transport
4.4. Remarks on the Sampling and Experimental Design
4.5. Mark–Recapture Method
4.6. Perspectives for Future Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, P.; Mann, R. The post-larval phase of bivalve mollusks: A review of functional ecology and new records of post larval drifting of Chesapeake Bay bivalves. Bull. Mar. Sci. 1997, 61, 409–430. [Google Scholar]
- Beukema, J.J.; de Vlas, D. Tidal-current transport of thread-drifting post larval juveniles of the bivalve Macoma balthica from the Wadden Sea to the North Sea. Mar. Ecol. Prog. Ser. 1989, 52, 193–200. [Google Scholar] [CrossRef]
- Emerson, C.W. A method for the measurement of bedload sediment transport and passive faunal transport on intertidal sandflats. Estuaries 1991, 14, 361–371. [Google Scholar] [CrossRef]
- Emerson, C.W.; Grant, J. The control of soft-shell clam (Mya arenaria) recruitment on intertidal sandflats by bedload sediment transport. Limnol. Oceanogr. 1991, 36, 1288–l300. [Google Scholar] [CrossRef]
- Bouma, H.; Duiker, J.M.C.; de Vries, P.P.; Herman, P.M.J.; Wolf, W.J. Spatial pattern of early recruitment of Macoma balthica (L.) and Cerastoderma edule (L.) in relation to sediment dynamics on a highly dynamic intertidal sandflat. J. Sea Res. 2001, 45, 79–93. [Google Scholar] [CrossRef]
- Hunt, H.L. Transport of juvenile clams: Effects of species and sediment grain size. J. Exp. Mar. Biol. Ecol. 2004, 312, 271–284. [Google Scholar] [CrossRef]
- Hunt, H.L. Effects of sediment source and flow regime on clam and sediment transport. Mar. Ecol. Prog. Ser. 2005, 296, 143–153. [Google Scholar] [CrossRef]
- St-Onge, P.; Miron, G. Effects of current speed, shell length and type of sediment on the erosion and transport of juvenile softshell clams (Mya arenaria). J. Exp. Mar. Biol. Ecol. 2007, 349, 12–26. [Google Scholar] [CrossRef]
- St-Onge, P.; Miron, G.; Moreau, G. Burrowing behaviour of the softshell clams (Mya arenaria) following erosion and transport. J. Exp. Mar. Biol. Ecol. 2007, 340, 103–111. [Google Scholar] [CrossRef]
- Jennings, L.B.; Hunt, H.L. Distances of dispersal of juvenile bivalves (Mya arenaria (Linnaeus), Mercenaria mercenaria (Linnaeus), and Gemma gemma (Totten). J. Exp. Mar. Biol. Ecol. 2009, 376, 76–84. [Google Scholar] [CrossRef]
- Turner, S.J.; Grant, J.; Pridmore, R.D.; Hewitt, J.E.; Wilkinson, M.R.; Hume, T.M.; Morrisey, D.J. Bedload and water-column transport and colonization processes by post-settlement benthic macrofauna: Does infaunal density matter? J. Exp. Mar. Biol. Ecol. 1997, 216, 51–75. [Google Scholar] [CrossRef]
- Norkko, A.; Cummings, V.J.; Thrush, S.F.; Hewitt, J.E.; Hume, T. Local dispersal of juvenile bivalves: Implications for sandflat ecology. Mar. Ecol. Prog. Ser. 2001, 212, 131–144. [Google Scholar] [CrossRef]
- Lundquist, C.J.; Pilditch, C.A.; Cummings, V.J. Behaviour controls post-settlement dispersal by the juvenile bivalves Austrovenus stutchburyi and Macomona liliana. J. Exp. Mar. Biol. Ecol. 2004, 306, 51–74. [Google Scholar] [CrossRef]
- Lundquist, C.J.; Thrush, S.F.; Hewitt, J.E.; Halliday, J.; MacDonald, I.; Cummings, V.J. Spatial variability in recolonisation potential; influence of organism behaviour and hydrodynamics on the distribution of macrofaunal colonists. Mar. Ecol. Prog. Ser. 2006, 324, 67–81. [Google Scholar] [CrossRef]
- Beck, F.; Pezy, J.P.; Baffreau, A.; Dauvin, J.C. Effects of clam rake harvesting on the intertidal Ruditapes habitat of the English Channel. ICES J. Mar. Sci. 2015, 72, 2663–2673. [Google Scholar] [CrossRef]
- Dang, C.; de Montaudouin, X.; Gam, M.; Paroissin, C.; Bru, N.; Caill-Milly, N. The Manila clam population in Arcachon Bay (SW France): Can it be kept sustainable? J. Sea Res. 2010, 63, 108–118. [Google Scholar] [CrossRef]
- Benniger, P.G.; Boldina, I. Fine-scale spatial distribution of the temperate bivalve Tapes (=Ruditapes) philippinarum (Adams and Reeve) on fished and unfished intertidal mudflats. J. Exp. Mar. Biol. Ecol. 2014, 457, 128–134. [Google Scholar] [CrossRef]
- de Montaudouin, X.; Arzul, I.; Caill-Milly, N.; Khayati, A.; Labrousse, J.M.; Lafitte, C.; Paillard, C.; Soudant, P.; Goulletquer, P. Asari clam (Ruditapes philippinarum) in France: History of an exotic species 1972-2015. Bull. Fish. Res. Agency Japan 2016, 42, 35–42. [Google Scholar]
- Moura, P.; Garaulet, L.L.; Vasconcelos, P.; Chainho, P.; Costa, J.L.; Gaspar, M.B. Age and growth of a highly successful invasive species: The Manila clam Ruditapes philippinarum (Adams & Reeve, 1850) in the Tagus Estuary (Portugal). Aquat. Inv. 2017, 12, 133–146. [Google Scholar]
- Basuyaux, O.; Beck, F.; Pezy, J.P.; Baffreau, B.; Joncourt, Y.; Tetard, X.; Dauvin, J.C. Evaluation of Ruditapes spp. clam stock on the western coast of Cotentin (English Channel). J. Mar. Biol. Oceanogr. 2018, 7, 2. [Google Scholar] [CrossRef]
- Grant, J.; Turner, S.J.; Legendre, P.; Hume, T.; Bell, R.G. Patterns of sediment reworking and transport over small spatial scales on an intertidal sandflat, Manukau Harbour, New Zealand. J. Exp. Mar. Biol. Ecol. 1997, 216, 33–50. [Google Scholar] [CrossRef]
- Kakino, J. Dispersal of Japanese littleneck clam Ruditapes philippinarum (Adams and Reeve) in relation to changes of bottom level due to wave action on Banzu tidal flats, Tokyo Bay. Fish. Engineer. 2000, 37, 115–128. [Google Scholar]
- Toba, M.; Ito, M.; Kobayashi, Y. Bedload transport of newly-settled juveniles of the Manila clam Ruditapes philippinarum observed in situ at Banzu Tidal Flat, Tokyo Bay. J. Shellfish Res. 2011, 30, 777–789. [Google Scholar] [CrossRef]
- Günther, C.P. Dispersal of intertidal invertebrates: A strategy to react to disturbances of different scales? Neth. J. Sea Res. 1992, 30, 45–56. [Google Scholar] [CrossRef]
- Hooker, S.H. Preliminary evidence for post-settlement movement of juvenile and adult pipi, Paphies australis (Gmelin, 1790) (bivalvia: Mesodesmatidae). Mar. Fresh. Behav. Physiol. 1995, 27, 37–47. [Google Scholar] [CrossRef]
- Olivier, F.; Vallet, C.; Dauvin, J.C.; Retière, C. Drifting in post-larvae and juveniles in an Abra alba (Wood) community of the eastern part of the Bay of Seine (English Channel). J. Exp. Mar. Biol. Ecol. 1996, 199, 89–109. [Google Scholar] [CrossRef]
- Olivier, F.; Retière, C. The role of physical-biological coupling in the benthic boundary layer under megatidal conditions: The case of the dominant species of the Abra alba community in the eastern Baie de Seine (English Channel). Estuaries 1998, 21, 571–584. [Google Scholar] [CrossRef]
- Montreuil, A.L.; Levoy, F.; Bretel, P.; Anthony, E.J. Morphological diversity and complex sediment recirculation on the ebb delta of a macrotidal inlet (Normandy, France): A multiple LiDAR dataset approach. Geomorphology 2014, 219, 114–125. [Google Scholar] [CrossRef]
- Levoy, F.; Monfort, O.; Larsonneur, C. Transports solides sur les plages macrotidales: Traçage fluorescent et application à la côte ouest du Cotentin (France). Oceanol. Acta 1997, 20, 811–822. [Google Scholar]
- Levoy, F.; Monfort, O.; Larsonneur, C. Hydrodynamic variability on megatidal beaches, Normandy, France. Cont. Shelf Res. 2001, 21, 563–586. [Google Scholar] [CrossRef]
- Levoy, F.; Anthony, E.J.; Monfort, O.; Robin, N.; Bretel, P. Formation and migration of transverse bars along a tidal sandy coast deduced from multi-temporal Lidar datasets. Mar. Geol. 2013, 342, 39–52. [Google Scholar] [CrossRef]
- Dugan, J.E.; McLachlan, A. An assessment of longshore movement in Donax serra Röding (Bivalvia: Donacidae) on an exposed sandy beach. J. Exp. Mar. Biol. Ecol. 1999, 234, 111–124. [Google Scholar] [CrossRef]
- Potvin, C. ANOVA: Experiments in controlled environments. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 1993; pp. 46–68. [Google Scholar]
- Mahé, M.; Delanghe, D.; Grisel, R.; Poggiale, J.C.; Mayot, N. Distribution of Manila clam, Ruditapes philippinarum, into Berre Lagoon according to the environmental condition. Vie Milieu 2020, 70, 269–277. [Google Scholar]
- Bidegain, G.; Juanes, J.A. Does expansion of the introduced Manila clam Ruditapes philippinarum cause competitive displacement of the European native clam Ruditapes decussatus? J. Exp. Mar. Biol. Ecol. 2013, 445, 44–52. [Google Scholar] [CrossRef]
- Commito, J.A.; Thrush, S.F.; Pridmore, R.D.; Hewitt, J.E.; Cummings, V.J. Dispersal dynamics in a wind-driven benthic system. Limnol. Oceanogr. 1995, 40, 1513–1518. [Google Scholar] [CrossRef]
- Gotoh, H.; Sakai, T. Bivalve habitat bases on sediment-transport mechanics. In Proceedings of the Coastal Engineering 1996, Orlando, FL, USA, 2–6 September 1996; pp. 4300–4313. [Google Scholar]
- Cargnelli, L.M.; Griesbach, S.J.; Packer, D.B.; Weissberger, E. Essential Fish Habitat Source Document: Atlantic Surf Clam, Spisula Solidissima, Life History and Habitat Characteristics; NOAA Technical Memorandum NMFS-NE; National Oceanic and Atmospheric Administration: Washington, DC, USA, 1999; Volume 142, 22p. [Google Scholar]
- Bocher, P.; Piersma, T.; Dekinga, A.; Kraan, C.; Yates, M.G.; Guyot, T.; Fomer, E.O.; Radenac, G. Site- and species distribution patterns of molluscs at five intertidal soft-bottom areas in northwest Europe during a single winter. Mar. Biol. 2007, 151, 577–594. [Google Scholar] [CrossRef]
- Evans, O.; Hick, P.; Whittington, R.J. Comparison of two external tagging methods used for the identification of individual adult Pacific oysters Crassotrea gigas. J. Shell. Res. 2016, 35, 837–840. [Google Scholar] [CrossRef]
- Dernie, K.M.; Kaiser, M.J.; Richardson, E.A.; Warwick, R.M. Recovery of soft sediment communities and habitats following physical disturbance. J. Exp. Mar. Biol. Ecol. 2003, 285–286, 415–434. [Google Scholar] [CrossRef]
- Dernie, K.M.; Kaiser, M.J.; Warwick, R.M. Recovery rates of benthic communities following physical disturbance. J. Anim. Ecol. 2003, 72, 1043–1056. [Google Scholar] [CrossRef]
- Dolbeth, M.; Viegas, I.; Martinho, F.; Marques, J.C.; Pardal, M.A. Population structure and species dynamics of Spisula solida, Diogenes pugilator and Branchiostoma lanceolatum along a temporal spatial gradient in the south coast of Portugal. Estuar. Coast. Shelf Sci. 2006, 66, 168–176. [Google Scholar] [CrossRef]
- Berthou, P.; Glémarec, M. Distribution Temporelle et Spatiale des Cohortes de Spisula ovalis (Sowerby) dans le Nord du Golfe de Gascogne: Reflet d’une Compétition Inter-Cohortes? International Council for the Exploration of the Sea. Shellfish Committee C.M. 1988/K:35, 16p. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.ices.dk/sites/pub/CM%2520Doccuments/1988/K/1988_K35.pdf&ved=2ahUKEwjj0Pr-vIWIAxVAs1YBHf1XJoEQFnoECBgQAQ&usg=AOvVaw3OI1u_yv-z45o4BgqjCL62 (accessed on 1 March 2024).
- Ledoux, T.; Clements, J.C.; Gallant, D.; Sonier, R.; Miron, G. Burrowing behaviour of soft-shell clams (Mya arenaria) following human disturbance. J. Exp. Mar. Biol. Ecol. 2023, 565, 151916. [Google Scholar] [CrossRef]




| Sites/Stations | T0 | T1 | Ratio T1/T0 in % |
|---|---|---|---|
| BS a | 4 | 6 | 150.0 |
| BS b | 43 | 33 | 76.7 |
| BS c | 20 | 15 | 75.0 |
| Mean | 22.3 ± 19.6 | 18.0 ± 13.7 | |
| BG a | 133 | 18 | 13.5 |
| BG b | 93 | 8 | 8.6 |
| BG c | 88 | 22 | 25.0 |
| Mean | 105 ± 24.6 | 16 ± 7.2 | |
| BDG a | 92 | 8 | 54.3 |
| BDG b | 61 | 22 | 82.0 |
| BDG c | 46 | 14 | 30.4 |
| Mean | 66.3 ± 23.4 | 14.7 ± 7.0 |
| Variables | Factors | Df | F | p | Tukey Test | |
|---|---|---|---|---|---|---|
| 2014 sampling | Date | 1 | 34.2465 | 0.00007813 *** | T0 > T1 | |
| Sediment type | 2 | 7.9205 | 0.006412 ** | BG > BS | ||
| Date: Sediment type | 2 | 8.7722 | 0.004490 ** | |||
| 2016 rake sampling T0-T1 for BS, S, BDG, A | Clam abundance | Date | 1 | 18.1851 | 0.0005931 *** | T0 > T1 |
| Sediment type | 3 | 13.1754 | 0.0001368 *** | BS > S, BDG, A | ||
| Date: Sediment type | 3 | 2.2058 | 0.1270535 | |||
| 2016 rake sampling T0-T1-T2 for BS and BDG | Date | 2 | 5.1885 | 0.0237838 * | T0 > T1; T2 | |
| Sediment type | 1 | 25.6066 | 0.0002797 *** | BS > BDG | ||
| Date: Sediment type | 2 | 1.3671 | 0.2918395 | |||
| 2016 rake sampling T0-T1 for BS, S, BDG, A | Clam size | Date | 1 | 8.1239 | 0.004482 ** | T0 > T1 |
| Sediment type | 3 | 39.6583 | <0.001 *** | S < BDG; A < BS | ||
| Date: Sediment type | 3 | 3.0810 | 0.026802 * | |||
| 2016 rake sampling T0-T1-T2 for BS and BDG | Date | 2 | 2.1547 | 0.1167 | ||
| Sediment type | 1 | 39.4281 | <0.001 *** | BDG > BS | ||
| Date: Sediment type | 2 | 1.1089 | 0.3305 | |||
| Sites/Stations | T0 | T1 | Ratio T1/T0 in % | T2 | Ratio T2/T1in % |
|---|---|---|---|---|---|
| BS a | 51 | 60 | 117.6 | 65 | 108.3 |
| BS b | 122 | 35 | 28.7 | 49 | 140.0 |
| BS c | 127 | 42 | 33.0 | 32 | 76.2 |
| 100 ± 50.2 | 45.7 ± 12.9 | 48.7 ± 16.5 | |||
| BDG a | 16 | 6 | 37.5 | 3 | 50.0 |
| BDG b | 5 | 11 | 183.3 | 3 | 27.3 |
| BDG c | 52 | 12 | 23.1 | 6 | 50.0 |
| 24.3 ± 24.6 | 9.7 ± 3.2 | 4.0 ± 1.7 | |||
| A a | 23 | 1 | 4.3 | ||
| A b | 19 | 14 | 73.7 | ||
| A c | 10 | 3 | 30.0 | ||
| 17.3 ± 6.6 | 6.0 ± 7.0 | ||||
| S a | 65 | 12 | 18.5 | ||
| S b | 47 | 6 | 12.8 | ||
| S c | 63 | 8 | 12.7 | ||
| 58.3 ± 9.9 | 8.7 ± 3.0 | 3.0 |
| Quadrat | T0 | T1 | Ratio T1/T0 in % | T2 | Ratio T2/T1 in % |
|---|---|---|---|---|---|
| 1-1 | 22 | 3 | 13.7 | 0 | 0 |
| 1-2 | 11 | 2 | 18.2 | 1 | 50.0 |
| 1-3 | 12 | 1 | 8.3 | 0 | 0 |
| 1-4 | 7 | 2 | 28.6 | 1 | 50. |
| 1-5 | 13 | 6 | 46.2 | 4 | 66.7 |
| 2-1 | 16 | 3 | 18.8 | 2 | 66.7 |
| 2-2 | 8 | 2 | 25.0 | 1 | 50.0 |
| 2-3 | 13 | 1 | 7.7 | 2 | 200.0 |
| 2-4 | 12 | 4 | 33.3 | 1 | 25.0 |
| 2-5 | 12 | 6 | 50.0 | 0 | 0 |
| 3-1 | 6 | 2 | 33.3 | 2 | 100.0 |
| 3-2 | 6 | 0 | 0.0 | 3 | - |
| 3-3 | 6 | 0 | 0.0 | 0 | - |
| 3-4 | 8 | 0 | 0.0 | 2 | - |
| 3-5 | 12 | 3 | 25.0 | 1 | 33.3 |
| 4-1 | 12 | 0 | 0.0 | 1 | - |
| 4-2 | 20 | 3 | 15.0 | 1 | 33.3 |
| 4-3 | 12 | 5 | 47.1 | 3 | 60.0 |
| 4-4 | 12 | 0 | 0.0 | 0 | - |
| 4-5 | 20 | 0 | 0.0 | 1 | - |
| 5-1 | 15 | 2 | 13.3 | 2 | 100.0 |
| 5-2 | 21 | 0 | 0.0 | 2 | - |
| 5-3 | 17 | 4 | 23.6 | 2 | 0.5 |
| 5-4 | 9 | 1 | 11.1 | 2 | 200.0 |
| 5-5 | 11 | 0 | 0.0 | 0 | - |
| Total | 313 | 50 | 16.0 | 34 | 68.0 |
| Mean | 12.5 ± 4.8 | 2.1 ± 1.9 | 1.4 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauvin, J.-C.; Basuyaux, O.; Pezy, J.-P. Sediment Types with Alternation of Sandy and Rocky Shores Influence the Distribution of Clams in an Area Characterized by High-Energy Hydrodynamic Conditions. J. Mar. Sci. Eng. 2024, 12, 1488. https://doi.org/10.3390/jmse12091488
Dauvin J-C, Basuyaux O, Pezy J-P. Sediment Types with Alternation of Sandy and Rocky Shores Influence the Distribution of Clams in an Area Characterized by High-Energy Hydrodynamic Conditions. Journal of Marine Science and Engineering. 2024; 12(9):1488. https://doi.org/10.3390/jmse12091488
Chicago/Turabian StyleDauvin, Jean-Claude, Olivier Basuyaux, and Jean-Philippe Pezy. 2024. "Sediment Types with Alternation of Sandy and Rocky Shores Influence the Distribution of Clams in an Area Characterized by High-Energy Hydrodynamic Conditions" Journal of Marine Science and Engineering 12, no. 9: 1488. https://doi.org/10.3390/jmse12091488






