Application of Self-Polishing Copolymer and Tin-Free Nanotechnology Paint for Ships
Abstract
1. Introduction
2. Literature Review
3. Experimental Methods and Records
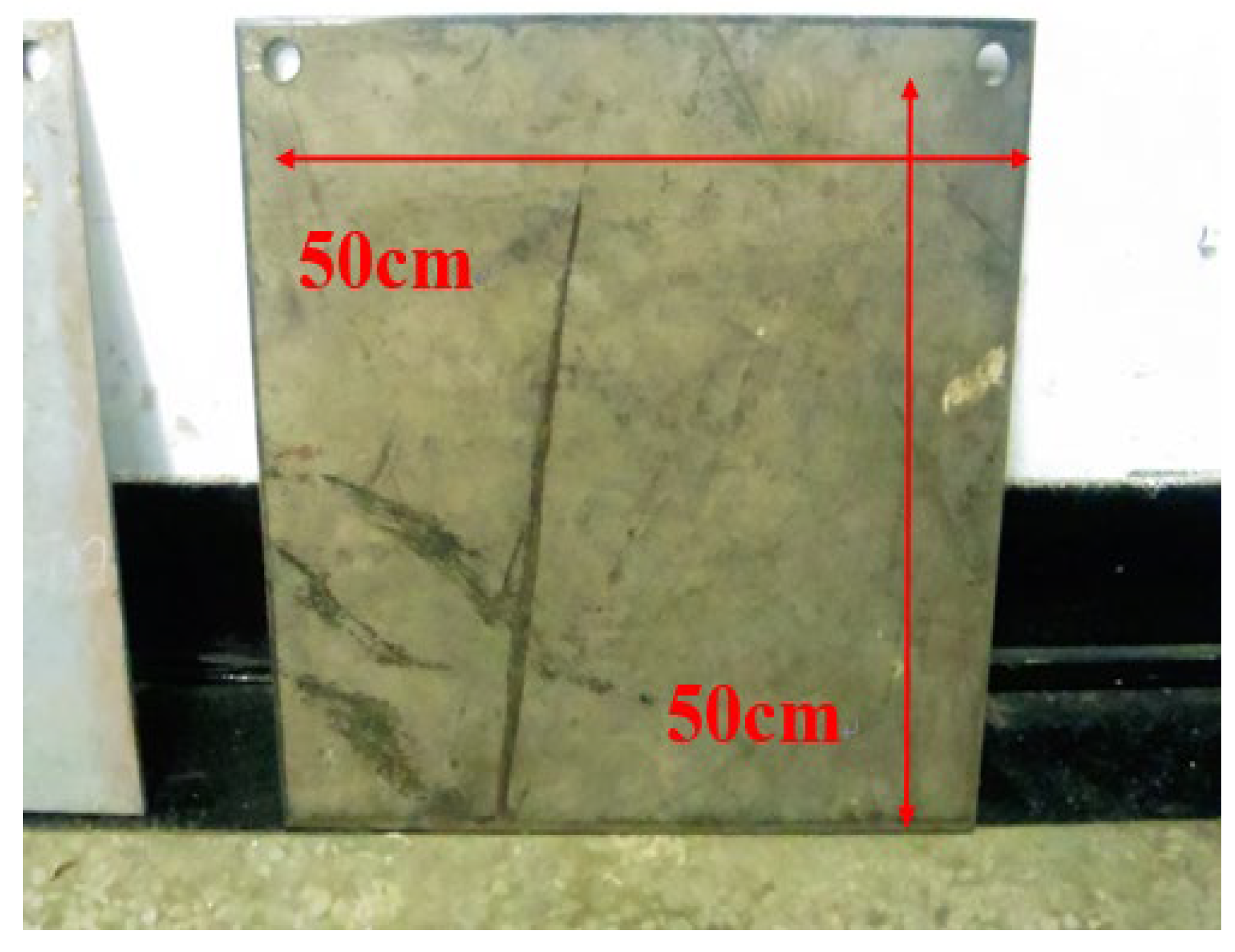
3.1. Paint Film Spraying Experiment
- Type I—Paint systems with topcoats that contain biocide(s), other than copper, which ablate or self-polish.
- Type II—Paint systems with topcoats that contain biocide(s) (copper or other biocide not cited in Type I) that ablate or self-polish.
- Type III—Paint systems with topcoats that are foul-released and contain no biocide.
- Type IV—Paint systems with topcoats that contain biocide(s) (copper or other) that do not ablate or self-polish.
- Class 1—Paint systems for use on rigid, fiberglass, wood, or metallic substrates, other than aluminum.
- Class 2—Paint systems for use on aluminum substrates.
- Class 3—Paint systems for use on elastomeric substrates.
- App1—Paint systems for use on the underwater hull, with a service life of three years.
- App2—Paint systems for use on the underwater hull, with a service life of seven years.
- App3—Paint systems for use on the underwater hull, with a service life of twelve years.
- App4—Paint systems for use on high-speed vessels with a service life of a minimum of two years.
- Models 1 and 2 utilize surface treatment and a self-polishing copolymer spray as a type of antifouling paint.
- Models 3 and 4 involve surface treatment and the spraying of tin-free nanotechnology paint, which is another type of antifouling paint, as shown in Table 2.
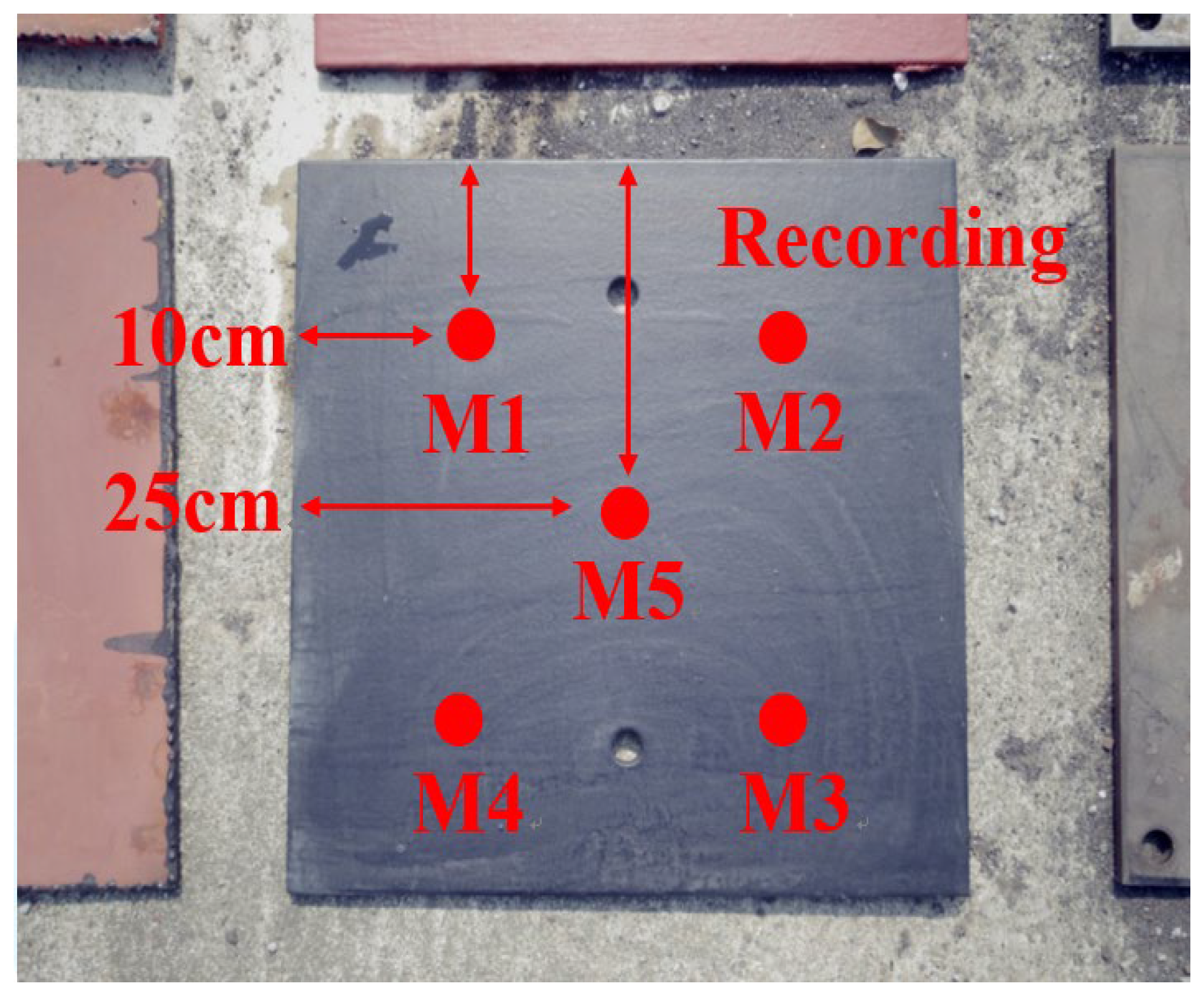
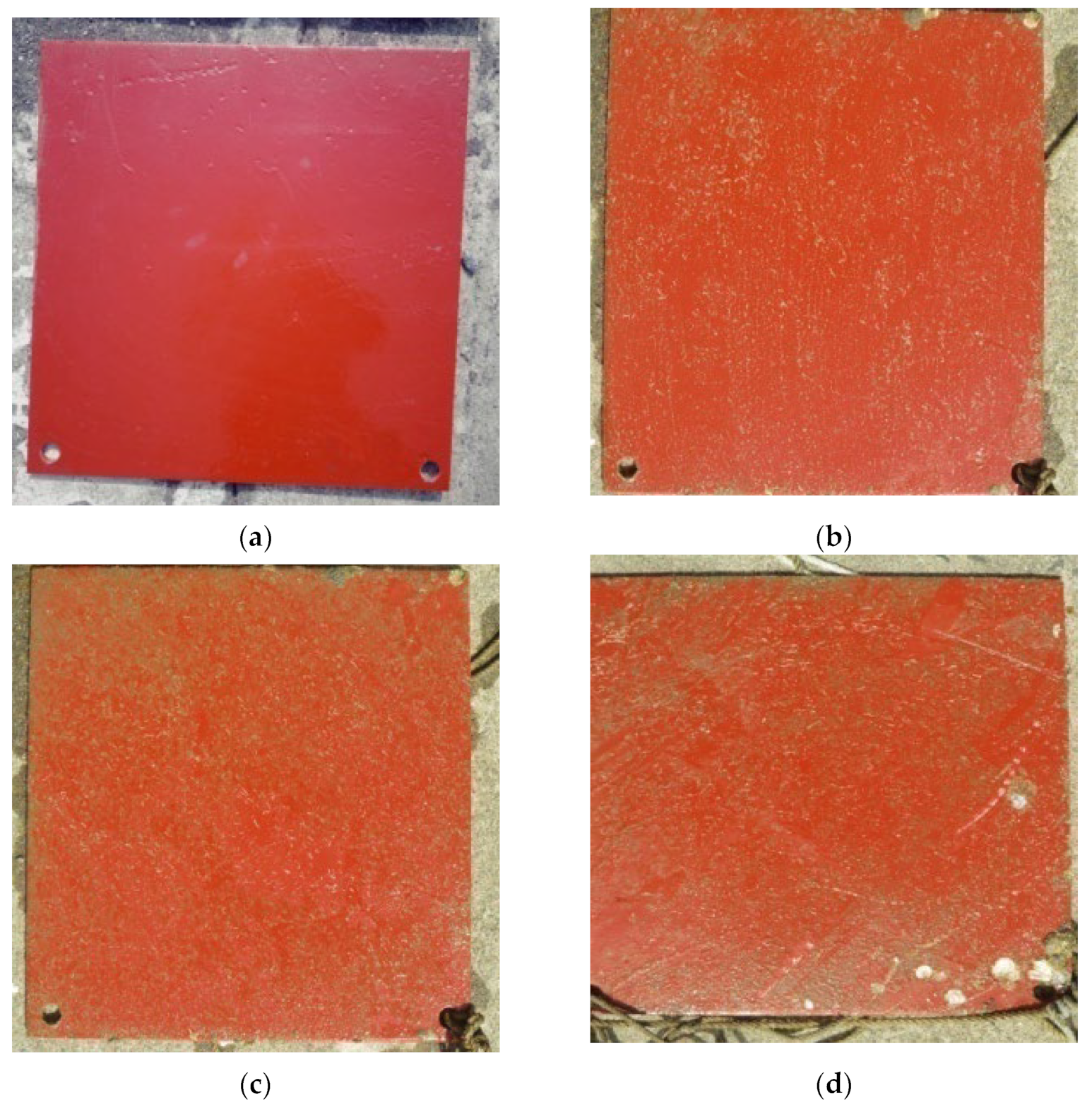
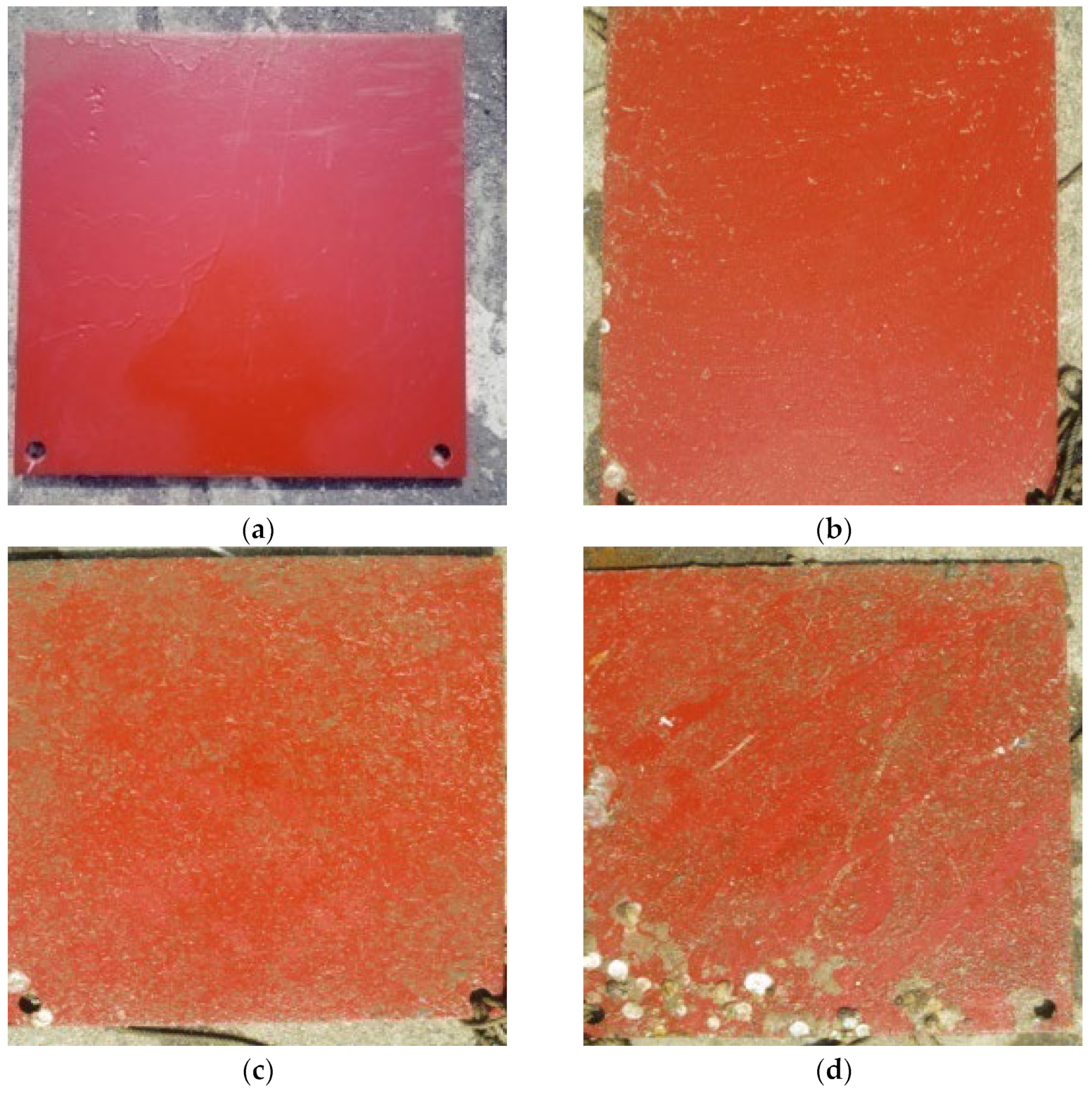
3.2. Hull Spray Paint Film Test
3.3. Ships’ Sea Trial Test
4. Results and Discussion
4.1. Research Model Construction Analysis
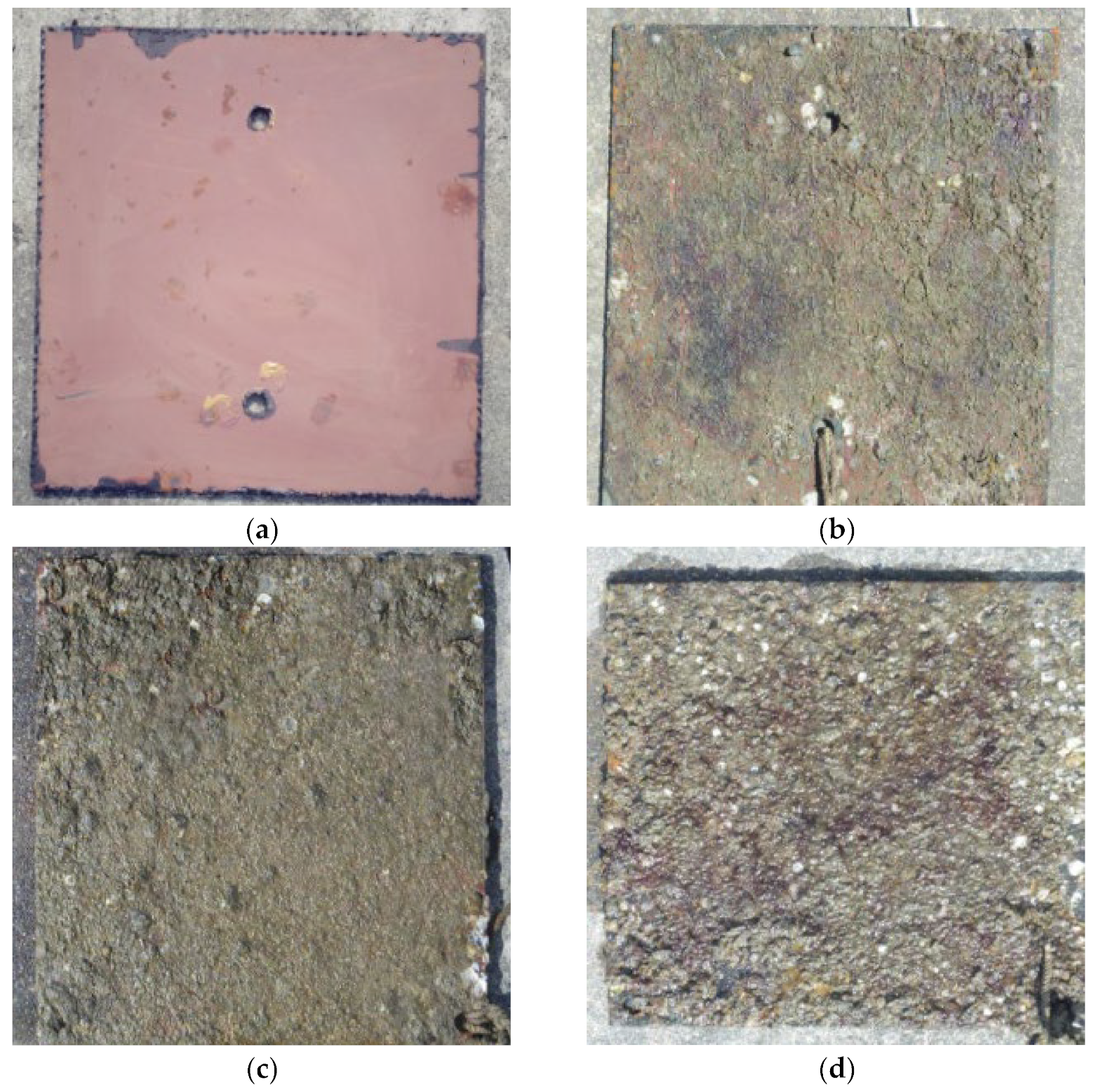
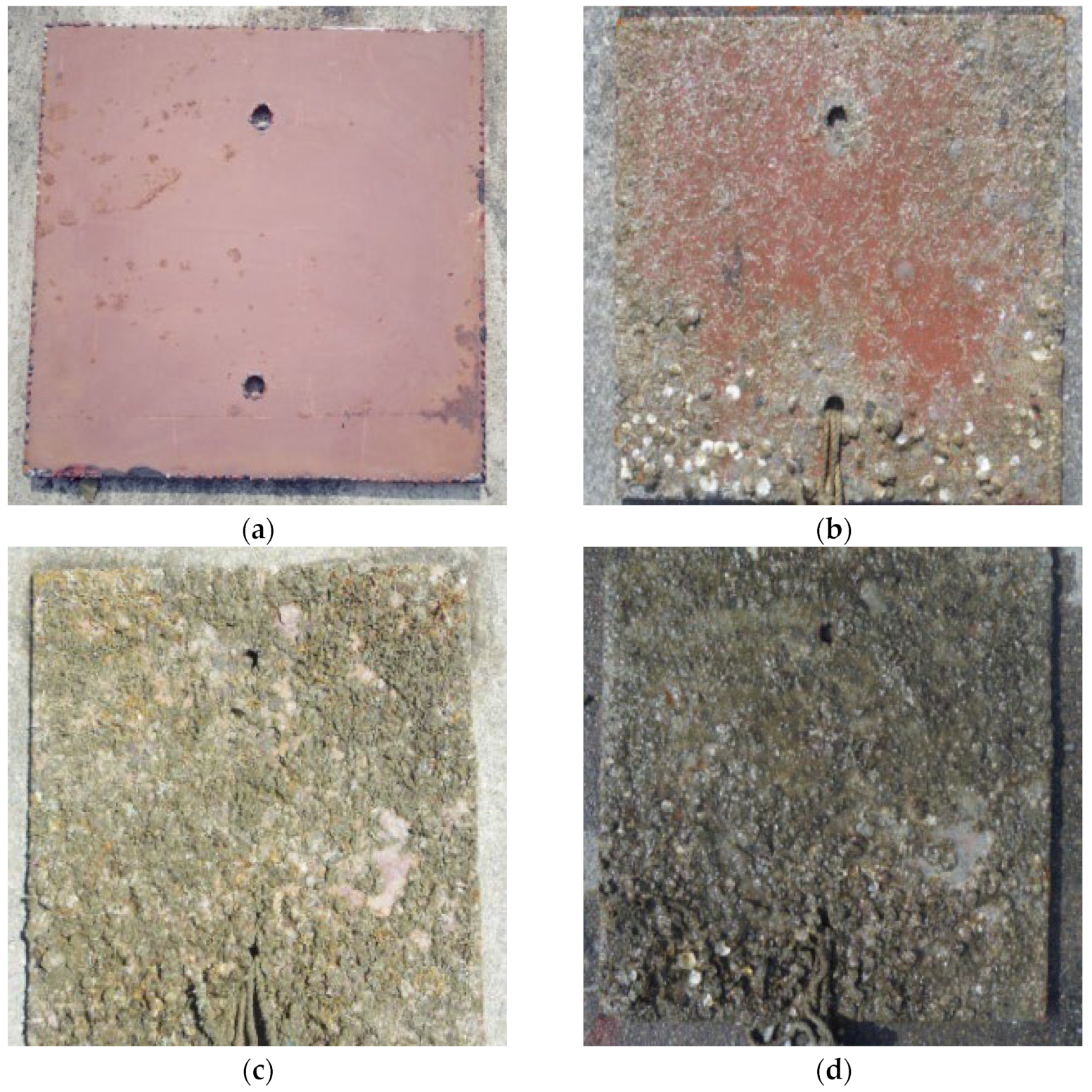
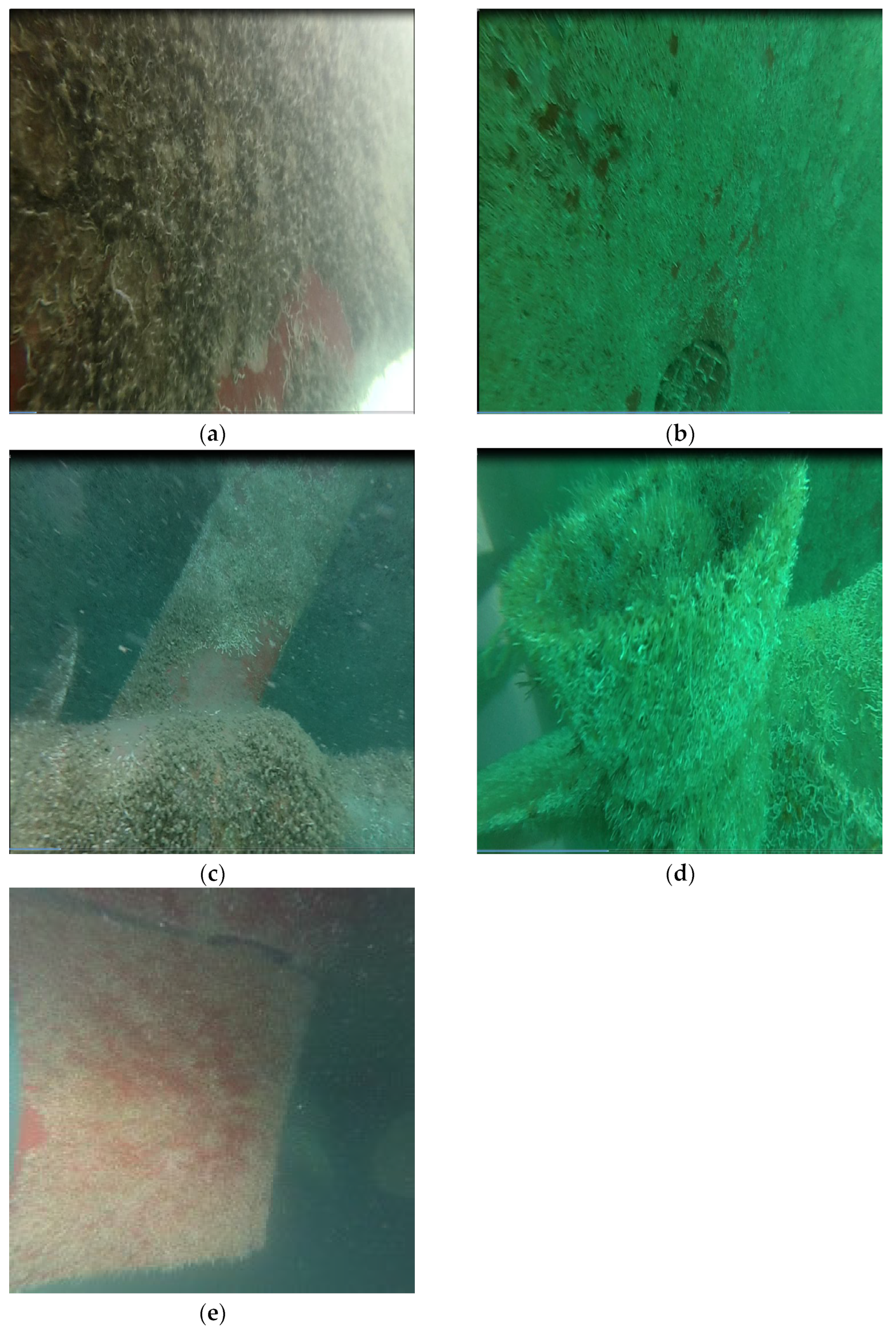
4.2. Perform an Underwater Inspection
4.3. Sea Trial and Fuel Consumption Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrewartha, J.; Perkins, K.; Sargison, J.; Osborn, J.; Walker, G.; Henderson, A. Drag Force and Surface Roughness Measurements on Freshwater Biofouled Surfaces. J. Bioadhesion Biofilm Res. 2010, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Demirel, Y.K.; Atlar, M. An Investigation into the Effect of Biofouling on the Ship Hydrodynamic Characteristics Using CFD. Ocean Eng. 2019, 175, 122–137. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Katranitsas, A.; Castritsi-Catharios, J.; Persoone, G. The Effects of a Copper-Based Antifouling Paint on Mortality and Enzymatic Activity of a Non-Target Marine Organism. Mar. Pollut. Bull. 2003, 46, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Warnken, J.; Dunn, R.J.K.; Teasdale, P.R. Investigation of Recreational Boats as a Source of Copper at Anchorage Sites Using Time-Integrated Diffusive Gradients in Thin Film and Sediment Measurements. Mar. Pollut. Bull. 2004, 49, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Soon, Z.Y.; Jung, J.-H.; Loh, A.; Yoon, C.; Shin, D.; Kim, M. Seawater Contamination Associated with In-Water Cleaning of Ship Hulls and the Potential Risk to the Marine Environment. Mar. Pollut. Bull. 2021, 171, 112694. [Google Scholar] [CrossRef] [PubMed]
- China Corporation Register of Shipping. Technical Circular No. 25. 2006. pp. 6–7. Available online: https://www.crclass.org/en/category/circulars/ (accessed on 10 August 2024). (In Chinese).
- International Maritime Organization. International Convention on the Control of Harmful Anti-Fouling Systems on Ships. 2016. Available online: https://www.imo.org/ (accessed on 10 August 2024).
- Dickon, H.; Brigitte, B. A Review of Surface Roughness in Antifouling Coatings Illustrating the Importance of Cutoff Length. J. Bioadhesion Biofilm Res. 2006, 22, 401–410. [Google Scholar]
- Schultz, M.P. Effects of Coating Roughness and Biofouling on Ship Resistance and Powering. J. Bioadhesion Biofilm Res. 2007, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Y.K.; Mahdi, K.; Osman, T.; Atilla, I.; Schultz, M.P. A CFD Model for the Frictional Resistance Prediction of Antifouling Coatings. Ocean Eng. 2014, 89, 21–23. [Google Scholar] [CrossRef]
- Demirel, Y.K.; Turan, O.; Incecik, A. Predicting the Effect of Biofouling on Ship Resistance Using CFD. Appl. Ocean Res. 2017, 62, 100–118. [Google Scholar] [CrossRef]
- Monty, J.P.; Dogan, E.; Hanson, R.; Scardino, A.J.; Ganapathisubramani, B.; Hutchins, N. An Assessment of the Ship Drag Penalty Arising from Light Calcareous Tubeworm Fouling. Biofouling 2016, 32, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Y.K.; Uzun, D.; Zhang, Y.; Fang, H.C.; Day, A.H.; Osman, T. Effect of Barnacle Fouling on Ship Resistance and Powering. Biofouling 2017, 33, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Y.K.; Soonseok, S.; Osman, T.; Atilla, I. Practical Added Resistance Diagrams to Predict Fouling Impact on Ship Performance. Ocean Eng. 2019, 186, 106–112. [Google Scholar] [CrossRef]
- Naval Sea Systems Command. MIL-PRF-24647D, Performance Specification: Paint System, Anticorrosive and Antifouling, Ship Hull; Naval Sea Systems Command: Washington, DC, USA, 2005. [Google Scholar]
- SSPC-SP 10/NACE No. 2; Joint Surface Preparation Standard, Near-White Metal Blast Cleaning. The Society for Protective Coatings: Pittsburgh, PA, USA; NACE: Houston, TX, USA, 2007.



| Type | Class | App1 | App2 | App3 | App4 |
|---|---|---|---|---|---|
| I | 1 | YES | YES | YES | YES |
| 2 | YES | YES | YES | YES | |
| 3 | YES | YES | YES | NO | |
| II | 1 | YES | YES | YES | YES |
| 2 | NO | NO | NO | NO | |
| 3 | YES | YES | YES | NO | |
| III | 1 | YES | YES | NO | YES |
| 2 | YES | YES | NO | YES | |
| 3 | YES | YES | NO | NO | |
| IV | 1 | YES | NO | NO | NO |
| 2 | YES | NO | NO | NO | |
| 3 | YES | NO | NO | NO |
| Model | Steel Plate Material | Surface Treatment | Types of Antifouling Paint |
|---|---|---|---|
| 1 | EH36 | SSPC-SP 10 | self-polishing copolymer |
| 2 | HY80 | SSPC-SP 10 | self-polishing copolymer |
| 3 | EH36 | SSPC-SP 10 | tin-free nanotechnology paint |
| 4 | HY80 | SSPC-SP 10 | tin-free nanotechnology paint |
| Model | Record Point | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|---|
| 1 | M1 | 2.27 | 4.13 | 6.83 | 8.38 | 10.54 |
| M2 | 2.79 | 3.57 | 5.74 | 8.81 | 12.86 | |
| M3 | 2.16 | 6.86 | 9.13 | 12.02 | 13.57 | |
| M4 | 3.13 | 5.61 | 5.95 | 11.37 | 12.28 | |
| M5 | 2.22 | 4.09 | 8.79 | 10.34 | 12.02 | |
| average value | 2.51 | 4.85 | 7.29 | 10.18 | 12.25 | |
| Record Point | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| M1 | 16.22 | 19.83 | 22.64 | 22.55 | 23.69 | |
| M2 | 14.54 | 15.96 | 17.25 | 20.14 | 22.78 | |
| M3 | 14.89 | 17.68 | 22.48 | 24.76 | 25.36 | |
| M4 | 13.87 | 14.27 | 19.71 | 21.38 | 23.95 | |
| M5 | 14.61 | 17.64 | 18.89 | 22.24 | 25.62 | |
| average value | 14.83 | 17.08 | 20.19 | 22.23 | 24.28 | |
| Record Point | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| M1 | 25.79 | 27.19 | 29.11 | 30.12 | 31.72 | |
| M2 | 23.87 | 25.84 | 26.23 | 27.87 | 29.12 | |
| M3 | 27.95 | 28.47 | 31.48 | 31.94 | 32.29 | |
| M4 | 24.25 | 26.38 | 28.67 | 29.22 | 30.36 | |
| M5 | 26.36 | 28.15 | 29.85 | 30.21 | 30.41 | |
| average value | 25.64 | 27.21 | 29.07 | 29.89 | 30.78 |
| Model | Record Point | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|---|
| 2 | M1 | 2.29 | 3.76 | 5.11 | 7.92 | 11.83 |
| M2 | 2.67 | 6.18 | 9.89 | 11.31 | 12.95 | |
| M3 | 2.21 | 4.74 | 6.27 | 10.68 | 12.16 | |
| M4 | 2.54 | 5.27 | 6.85 | 9.57 | 11.69 | |
| M5 | 2.73 | 4.92 | 8.23 | 11.26 | 13.03 | |
| average value | 2.49 | 4.98 | 7.27 | 10.15 | 12.33 | |
| Record Point | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| M1 | 15.58 | 17.16 | 18.31 | 21.28 | 24.74 | |
| M2 | 16.39 | 19.07 | 22.29 | 23.76 | 25.32 | |
| M3 | 14.37 | 18.92 | 22.38 | 23.04 | 24.12 | |
| M4 | 13.14 | 16.24 | 17.25 | 22.88 | 24.03 | |
| M5 | 15.96 | 16.17 | 18.53 | 19.15 | 20.97 | |
| average value | 15.09 | 17.51 | 19.75 | 22.02 | 23.84 | |
| Record Point | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| M1 | 25.61 | 27.19 | 28.35 | 29.56 | 31.49 | |
| M2 | 26.89 | 25.84 | 28.43 | 29.13 | 30.17 | |
| M3 | 25.25 | 28.47 | 30.98 | 31.26 | 31.86 | |
| M4 | 25.86 | 26.38 | 28.18 | 28.85 | 29.10 | |
| M5 | 21.82 | 28.15 | 29.64 | 30.42 | 31.25 | |
| average value | 25.09 | 27.21 | 29.12 | 29.84 | 30.77 |
| Model | Record Point | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|---|
| 3 | M1 | 0 | 0 | 0.44 | 0.85 | 1.06 |
| M2 | 0 | 0 | 0.51 | 0.91 | 1.20 | |
| M3 | 0 | 0 | 0.32 | 0.65 | 1.18 | |
| M4 | 0 | 0 | 0.49 | 0.88 | 1.13 | |
| M5 | 0 | 0 | 0.48 | 0.93 | 1.16 | |
| average value | 0 | 0 | 0.45 | 0.84 | 1.15 | |
| Record Point | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| M1 | 1.09 | 1.14 | 1.31 | 1.47 | 1.54 | |
| M2 | 1.21 | 1.27 | 1.36 | 1.42 | 1.51 | |
| M3 | 1.57 | 1.65 | 1.69 | 1.82 | 1.94 | |
| M4 | 1.19 | 1.25 | 1.48 | 1.71 | 1.73 | |
| M5 | 1.18 | 1.21 | 1.29 | 1.65 | 1.69 | |
| average value | 1.25 | 1.30 | 1.43 | 1.61 | 1.68 | |
| Record Point | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| M1 | 1.77 | 1.83 | 1.89 | 2.12 | 2.15 | |
| M2 | 1.58 | 1.66 | 1.72 | 1.85 | 1.94 | |
| M3 | 1.98 | 2.02 | 2.11 | 2.23 | 2.25 | |
| M4 | 1.84 | 1.87 | 1.93 | 1.97 | 2.01 | |
| M5 | 1.73 | 1.76 | 1.80 | 1.98 | 2.04 | |
| average value | 1.78 | 1.83 | 1.89 | 2.03 | 2.08 |
| Model | Record Point | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|---|
| 4 | M1 | 0 | 0 | 0.49 | 0.95 | 1.11 |
| M2 | 0 | 0 | 0.56 | 1.18 | 1.21 | |
| M3 | 0 | 0 | 0.37 | 0.69 | 1.07 | |
| M4 | 0 | 0 | 0.31 | 0.78 | 1.19 | |
| M5 | 0 | 0 | 0.48 | 0.92 | 1.02 | |
| average value | 0 | 0 | 0.44 | 0.90 | 1.12 | |
| Record point | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| M1 | 1.22 | 1.27 | 1.34 | 1.42 | 1.42 | |
| M2 | 1.29 | 1.34 | 1.50 | 1.54 | 1.65 | |
| M3 | 1.11 | 1.19 | 1.56 | 1.68 | 1.69 | |
| M4 | 1.24 | 1.56 | 1.58 | 1.64 | 1.77 | |
| M5 | 1.13 | 1.21 | 1.32 | 1.47 | 1.54 | |
| average value | 1.20 | 1.31 | 1.46 | 1.55 | 1.61 | |
| Record point | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| M1 | 1.44 | 1.52 | 1.69 | 1.74 | 1.88 | |
| M2 | 1.82 | 1.89 | 1.99 | 2.28 | 2.33 | |
| M3 | 1.79 | 1.85 | 1.91 | 2.05 | 2.15 | |
| M4 | 1.89 | 1.93 | 2.02 | 2.10 | 2.13 | |
| M5 | 1.56 | 1.84 | 1.96 | 2.01 | 2.08 | |
| average value | 1.70 | 1.81 | 1.91 | 2.04 | 2.11 |
| Speed Mode | Paint Film Material | Engine Design Speed (RPM) | Average Engine Speed per Hour (RPM) | Fuel Consumption Statistics (L) |
|---|---|---|---|---|
| 1 | Self-polishing copolymer | 500 | 500 | 211 |
| Tin-free nanotechnology paint | 499 | 191 | ||
| 2 | Self-polishing copolymer | 1000 | 1001 | 546 |
| Tin-free nanotechnology paint | 1000 | 520 | ||
| 3 | Self-polishing copolymer | 1500 | 1501 | 1493 |
| Tin-free nanotechnology paint | 1497 | 1440 | ||
| 4 | Self-polishing copolymer | 1800 | 1799 | 1902 |
| Tin-free nanotechnology paint | 1799 | 1584 | ||
| 5 | Self-polishing copolymer | 1950 | 1950 | 2381 |
| Tin-free nanotechnology paint | 1947 | 2337 | ||
| 6 | Self-polishing copolymer | 2100 | 2158 | 2473 |
| Tin-free nanotechnology paint | 2096 | 2403 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hsu, C.; Pan, G.; Chen, C. Application of Self-Polishing Copolymer and Tin-Free Nanotechnology Paint for Ships. J. Mar. Sci. Eng. 2024, 12, 1662. https://doi.org/10.3390/jmse12091662
Wang Y, Hsu C, Pan G, Chen C. Application of Self-Polishing Copolymer and Tin-Free Nanotechnology Paint for Ships. Journal of Marine Science and Engineering. 2024; 12(9):1662. https://doi.org/10.3390/jmse12091662
Chicago/Turabian StyleWang, Yushi, Cheunghwa Hsu, Guanhong Pan, and Chenghao Chen. 2024. "Application of Self-Polishing Copolymer and Tin-Free Nanotechnology Paint for Ships" Journal of Marine Science and Engineering 12, no. 9: 1662. https://doi.org/10.3390/jmse12091662
APA StyleWang, Y., Hsu, C., Pan, G., & Chen, C. (2024). Application of Self-Polishing Copolymer and Tin-Free Nanotechnology Paint for Ships. Journal of Marine Science and Engineering, 12(9), 1662. https://doi.org/10.3390/jmse12091662




