Enhanced Continental Weathering and Intense Upwelling Drove the Deposition of Organic-Rich Shales in the Late Permian Dalong Formation, South China
Abstract
:1. Introduction
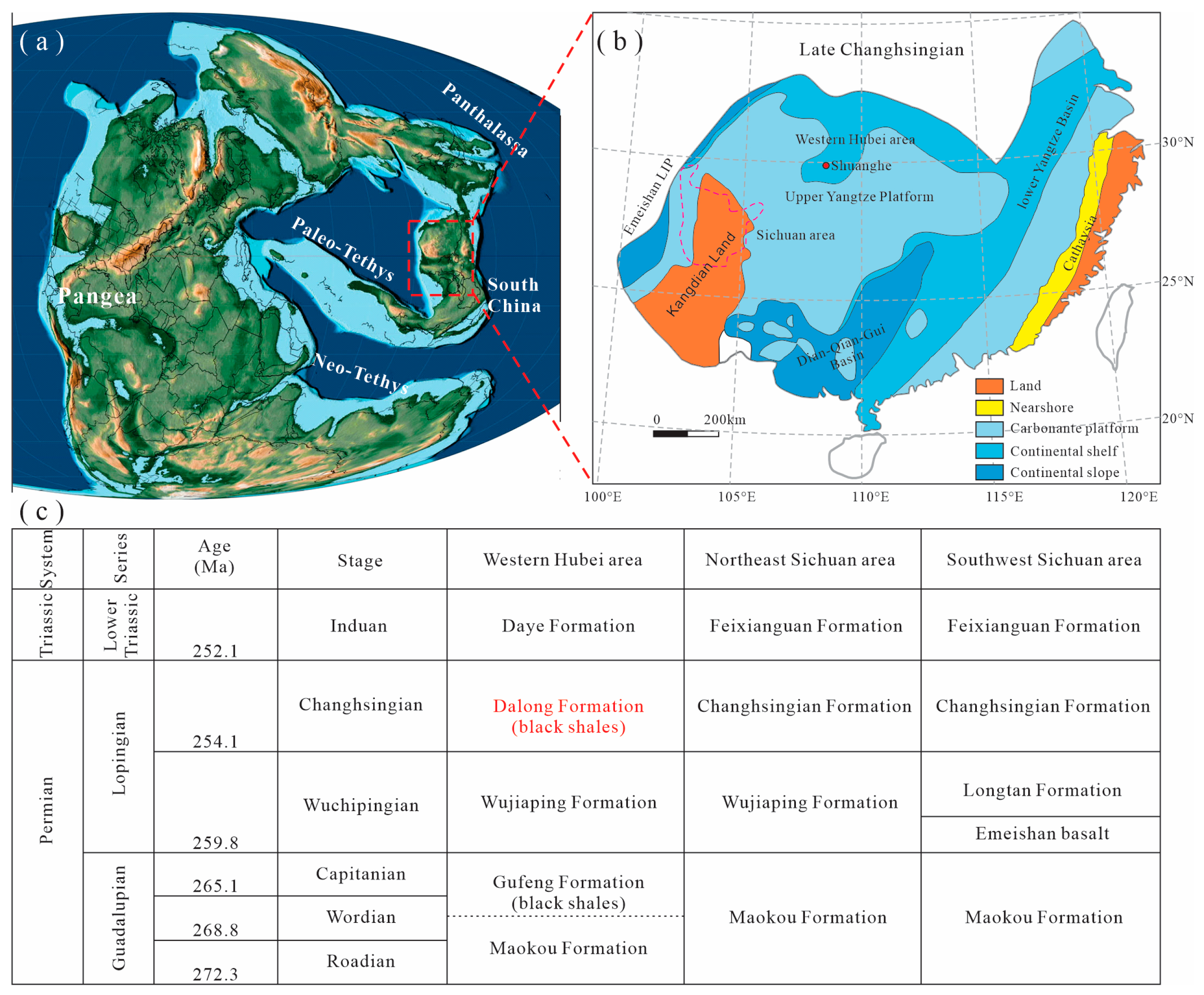
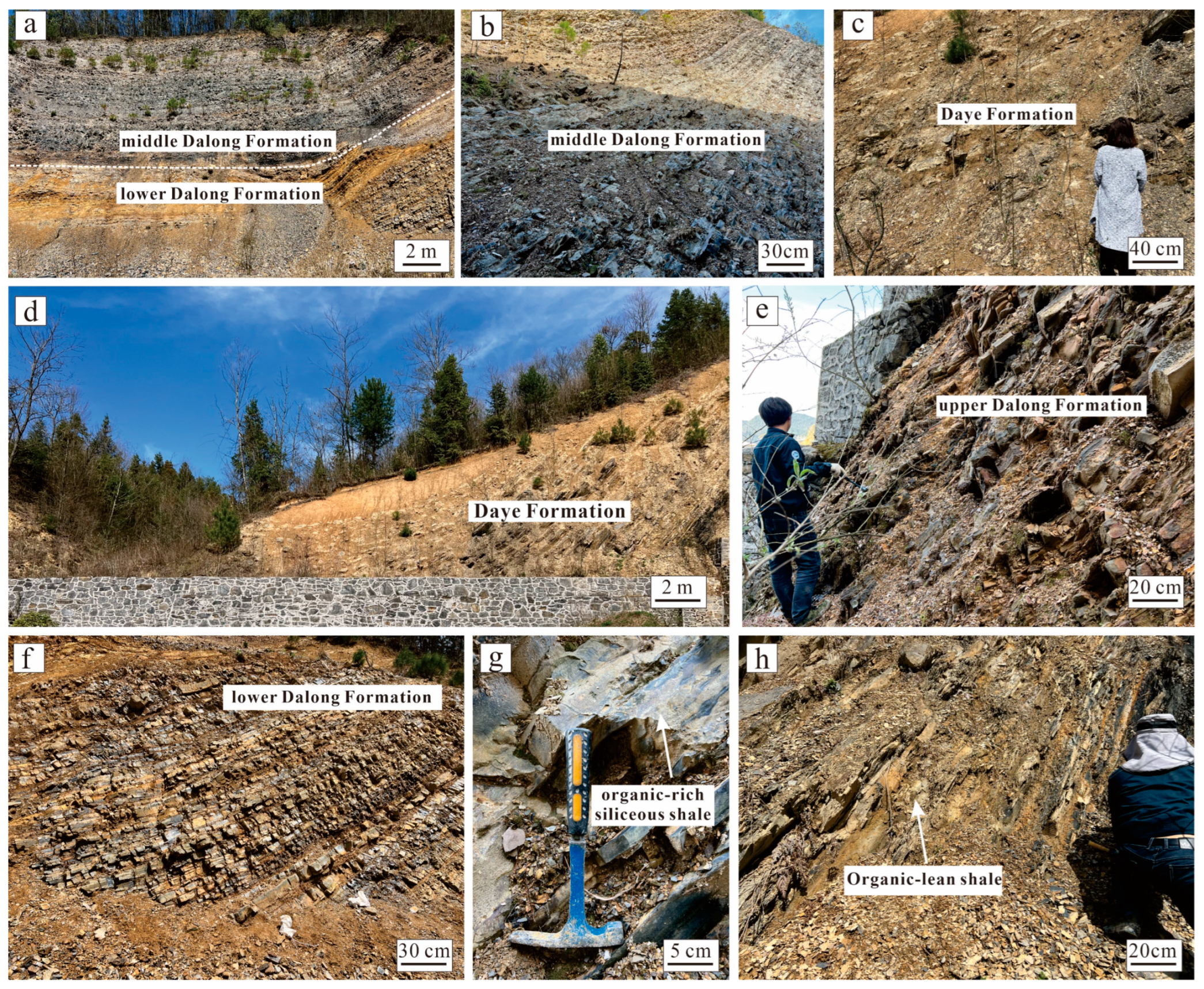
2. Geological Setting
3. Materials and Methods
3.1. TOC and δ13Corg
3.2. Major and Trace Elements
3.3. Mg Isotopes in Silicate
3.4. The Mineralogical Compositions
4. Results
4.1. TOC and δ13Corg Values
4.2. Major Elements
4.3. Trace Elements
4.4. The Mineralogical Compositions
5. Discussion
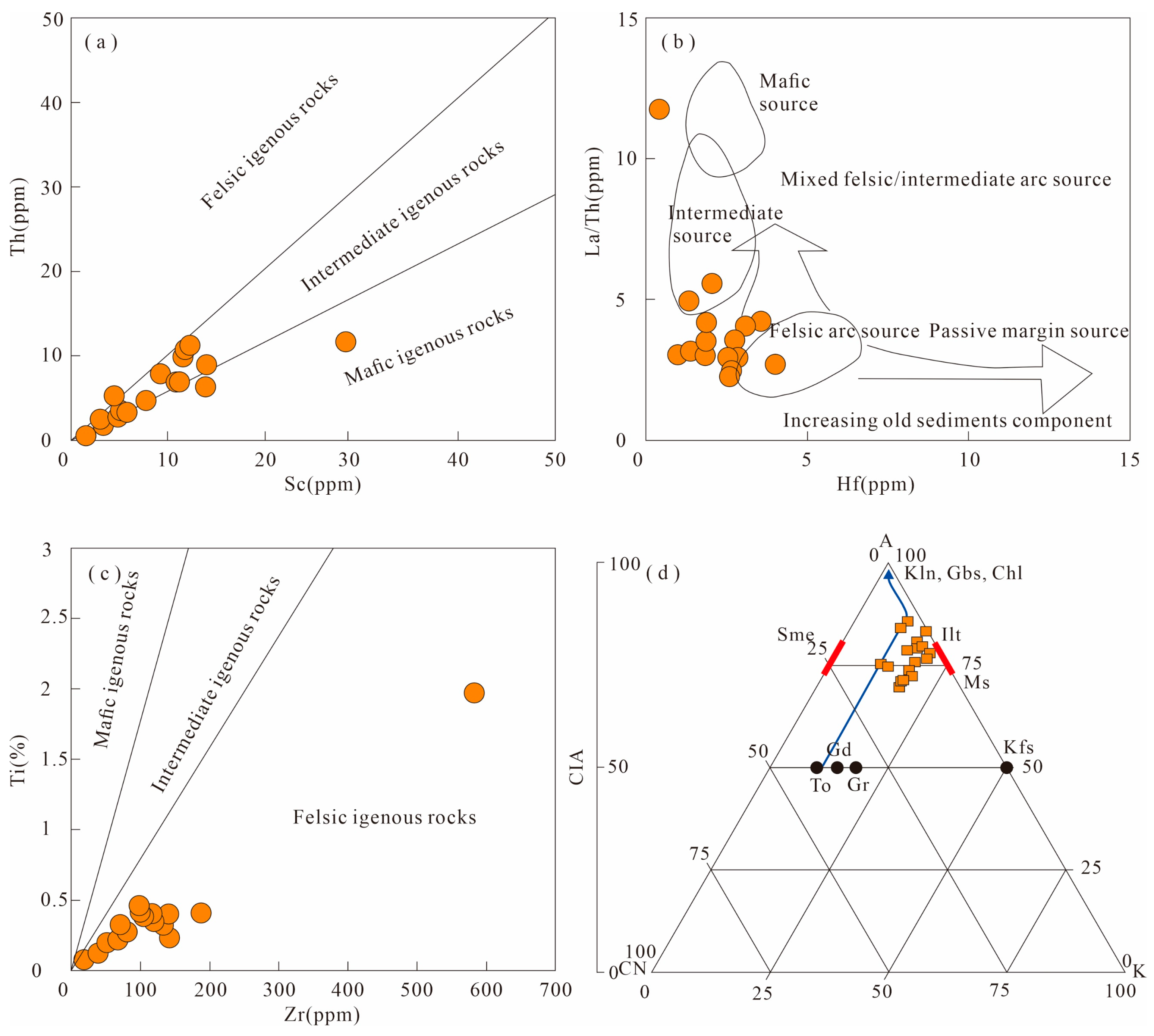
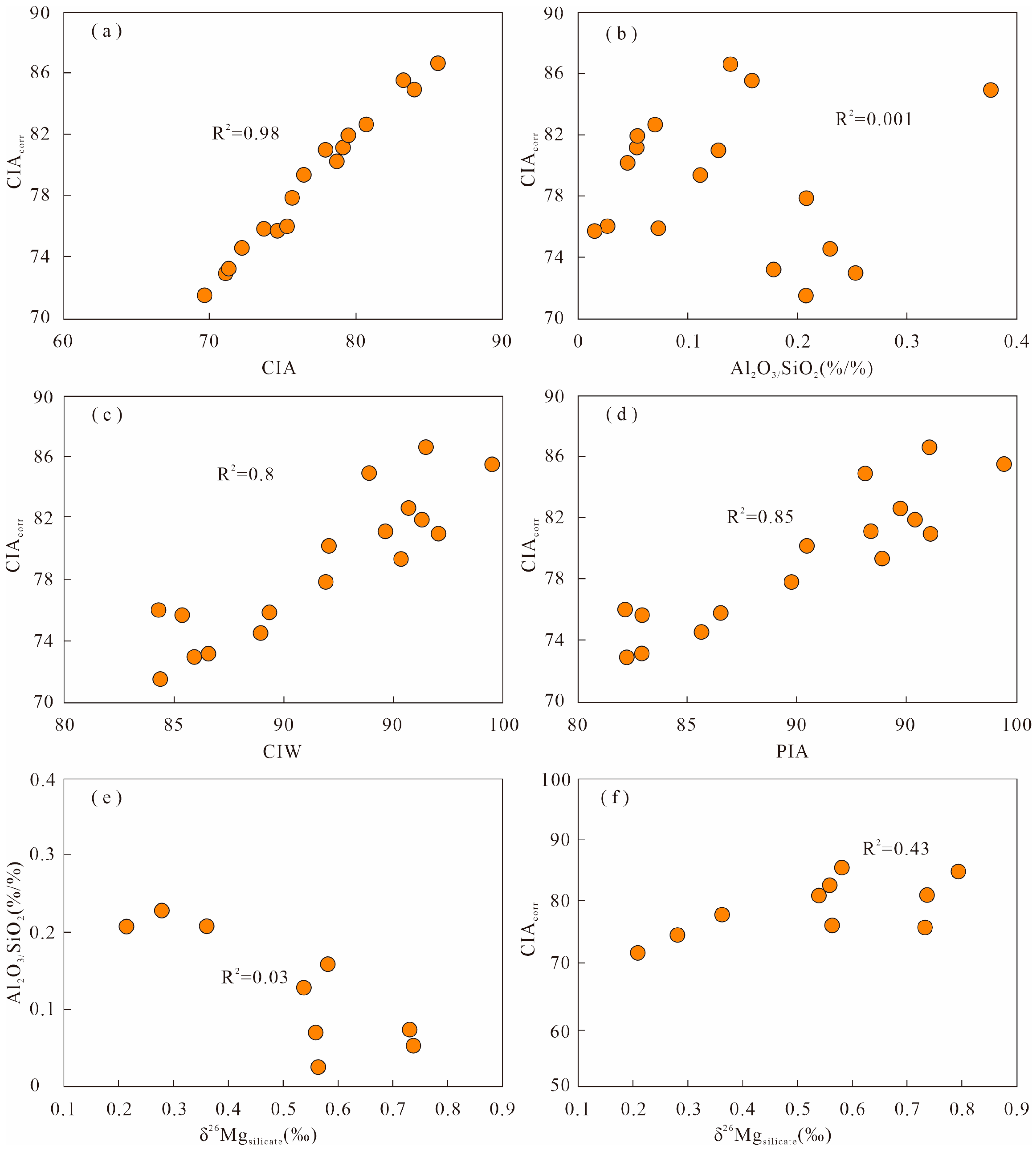
5.1. Evaluation of the Chemical Weathering Index
5.2. Evaluation of the δ26Mg Values
5.2.1. Influence of Authigenic Clay Minerals
5.2.2. Influences of Mineralogy, Granularity, and Diagenesis
5.3. Continental Weathering Trend in the Late Permian
5.3.1. Fluctuations in CIAcorr Values During the Late Permian
5.3.2. Fluctuations in δ26Mgsilicate Values During the Late Permian
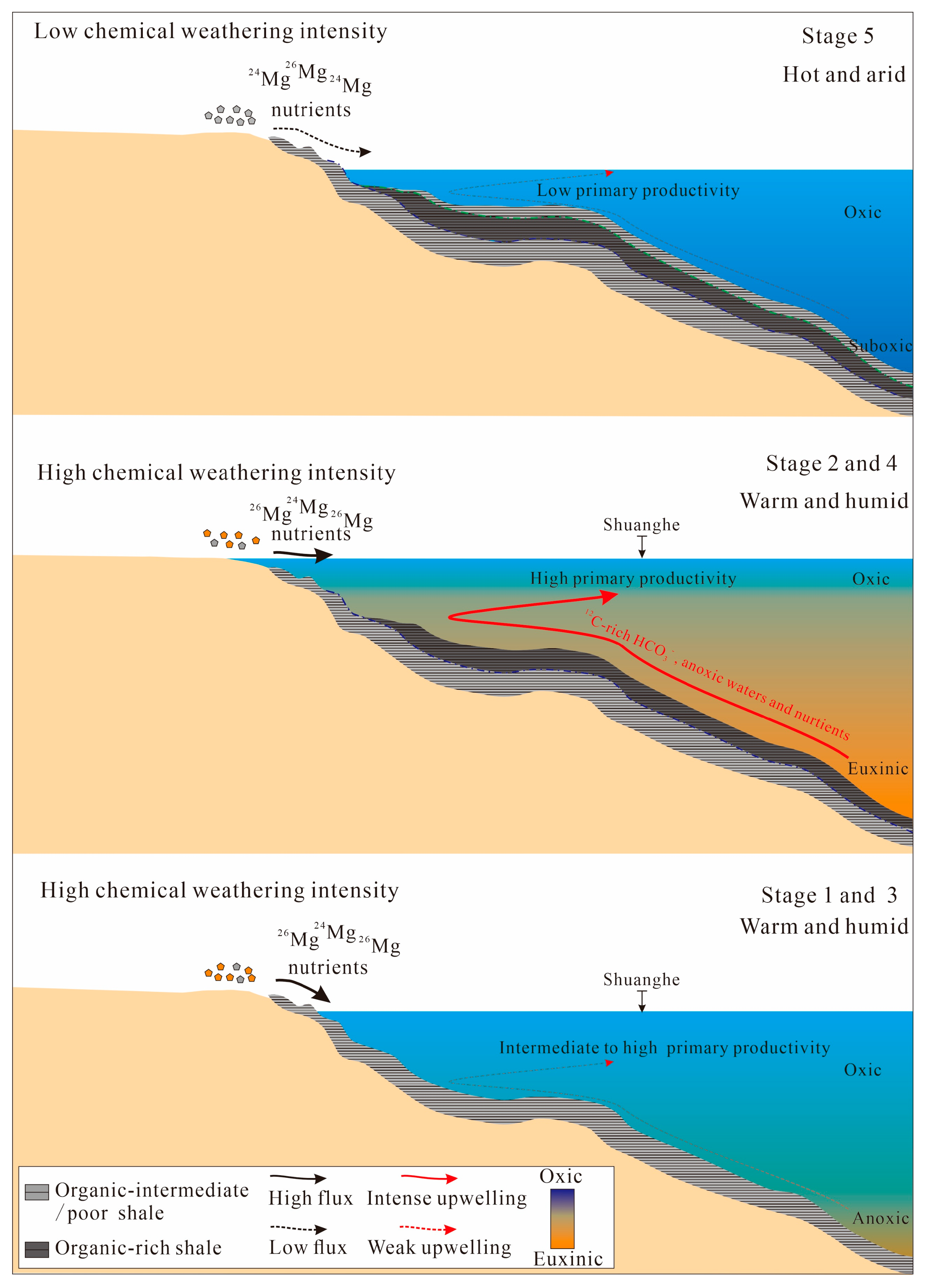
5.4. The Hydrographic Conditions
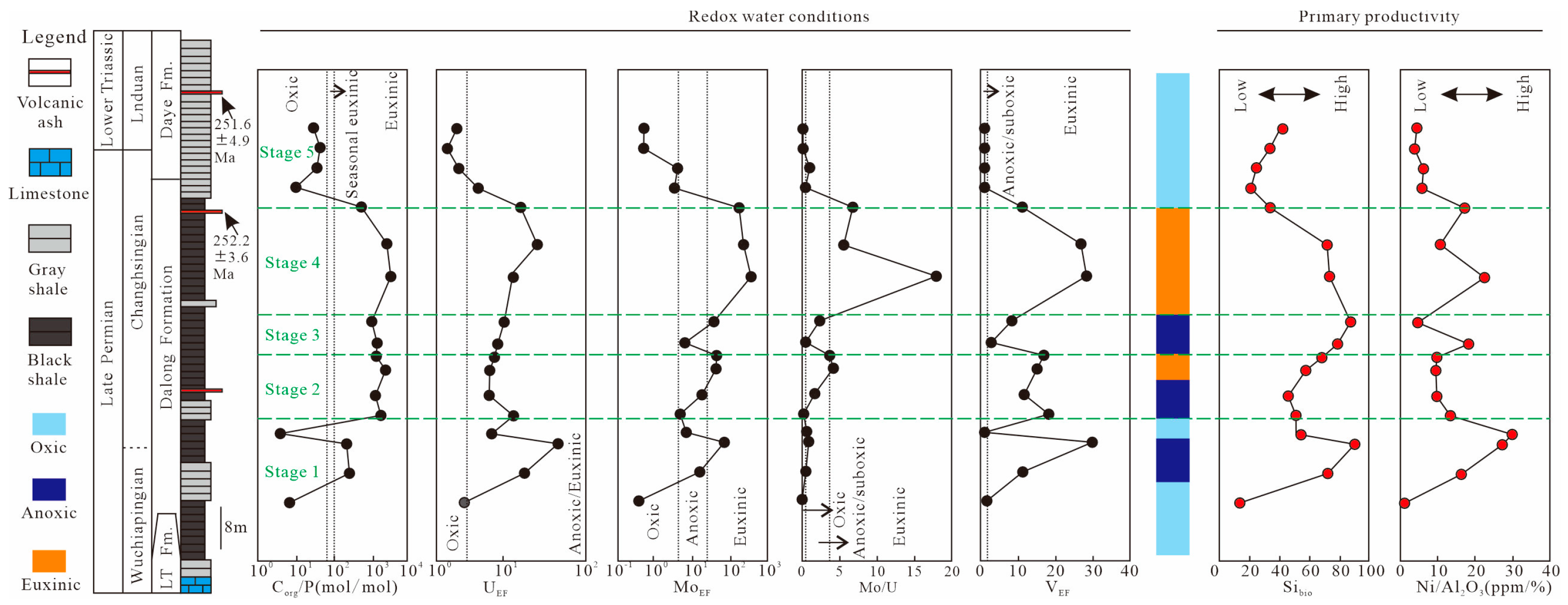
5.5. Variations in Primary Productivity and Redox Water Conditions
5.5.1. Evaluation of Primary Productivity
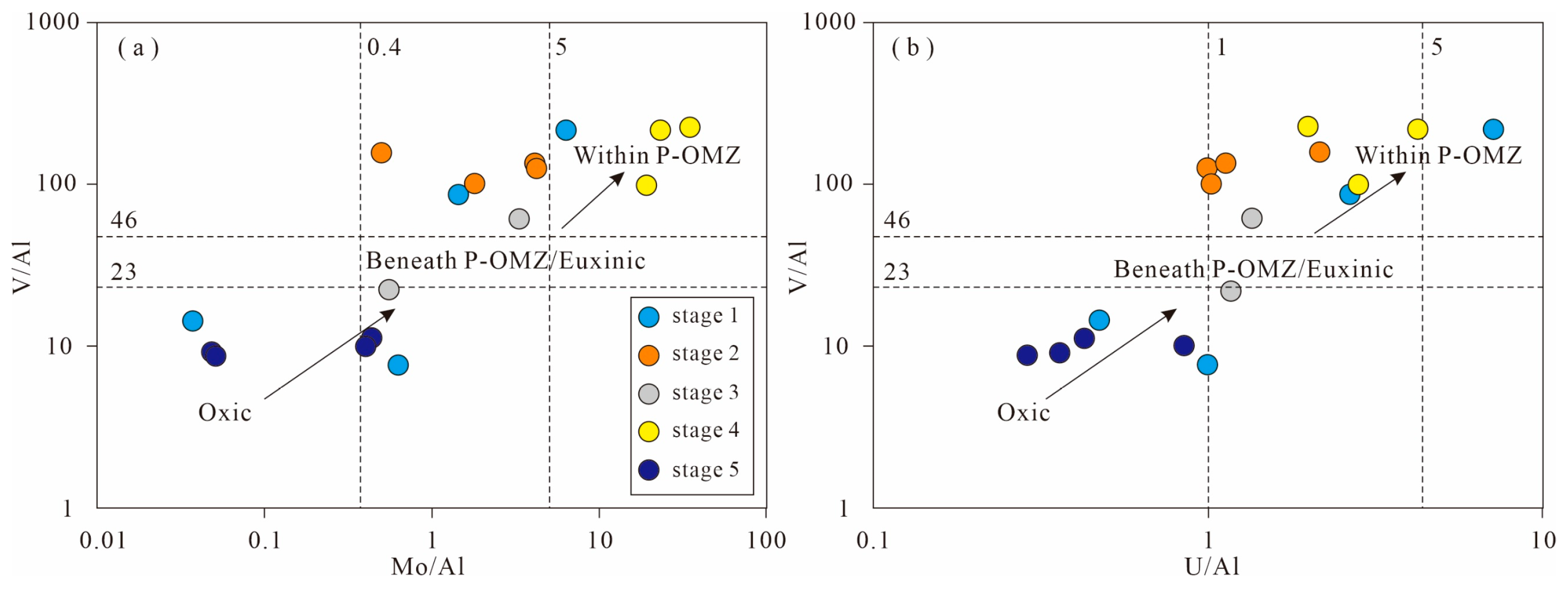
5.5.2. Evaluation of Redox States
5.6. Factors Influencing the Accumulation of Organic Matter in the Dalong Formation
5.7. Implications for the End-Permian Mass Extinction
6. Conclusions
- (1)
- Five stages can be identified on the basis of TOC and environmental variations, with the highest TOC values (>2%) being observed in stages 2 and 4, intermediate TOC (~1% to 2%) values being observed in stages 1 and 3, and the lowest TOC values (<1%) being observed in stage 5.
- (2)
- The deposition of organic-rich shales (stages 2 and 4) in the late Permian Dalong Formation is related to the enhanced continental weathering and intense upwelling. In stages 2 and 4, the enhanced continental weathering (high CIAcorr and δ26Mgsilicate values) and intense upwelling (high Mo/TOC ratios, low δ13Corg and CoEF × MnEF values) contributed to the high primary productivity (high Sibio and Ni/Al2O3) and anoxic water conditions (high Corg/P and Mo/U ratios and high MoEF, UEF, and VEF values), which is in accordance with the high TOC values in shales (>2%).
- (3)
- Both the enhanced continental weathering and upwelling contributed to high-frequency redox fluctuations in the late Permian and early Triassic, in which case the development of anoxia/euxinia of seawater further triggered the EPME.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zou, C.; Qiu, Z.; Poulton, S.W.; Dong, D.; Wang, H.; Chen, D.; Lu, B.; Shi, Z.; Tao, H. Ocean euxinia and climate change “double whammy” drove the Late Ordovician mass extinction. Geology 2018, 46, 535–538. [Google Scholar] [CrossRef]
- Yang, X.R.; Yan, D.T.; Liu, M.; Liu, X.Y.; Gong, Y.; Zhang, L.W.; Zhang, B.; Chen, D.Z. Zinc isotopic evidence for enhanced continental weathering and organic carbon burial in the Early Silurian. Chem. Geol. 2024, 662, 122209. [Google Scholar] [CrossRef]
- Piper, D.Z.; Calvert, S.E. A marine biogeochemical perspective on black shale deposition. Earth-Sci. Rev. 2009, 95, 63–96. [Google Scholar] [CrossRef]
- Zhang, F.F.; Romaniello, S.J.; Algeo, T.J.; Lau, K.V.; Clapham, M.E.; Richoz, S.; Herrmann, A.D.; Smith, H.; Horacek, M.; Anbar, A.D. Multiple episodes of extensive marine anoxia linked to global warming and continental weathering following the latest Permian mass extinction. Sci. Adv. 2018, 4, e1602921. [Google Scholar] [CrossRef]
- Yang, X.R.; Yan, D.T.; Wei, X.S.; Zhang, L.W.; Zhang, B.; Xu, H.W.; Gong, Y.; He, J. Different formation mechanism of quartz in siliceous and argillaceous shales: A case study of Lungmachi Formation in South China. Mar. Pet. Geol. 2018, 94, 80–94. [Google Scholar]
- Yang, X.R.; Yan, D.T.; Zhang, B.; Zhang, L.W.; Wei, X.S.; Li, T.; Zhang, J.F.; She, X.H. The impact of volcanic activity on the deposition of organic-rich shales: Evidence from carbon isotope and geochemical compositions. Mar. Pet. Geol. 2021, 128, 105010. [Google Scholar] [CrossRef]
- Fang, C.G.; Zhang, C.C.; Meng, G.X.; Xu, J.L.; Xu, N.C.; Li, H.L.; Liu, M.; Liu, B. Organic Matter Accumulation in the Upper Permian Dalong Formation from the Lower Yangtze Region, South China. Acta Geol. Sin. (Engl. Ed.) 2024, 98, 150–167. [Google Scholar] [CrossRef]
- Liao, Z.W.; Hu, W.X.; Cao, J.; Wang, X.L.; Hu, Z.Y. Petrologic and geochemical evidence for the formation of organic-rich siliceous rocks of the Late Permian Dalong Formation, Lower Yangtze region, southern China. Mar. Pet. Geol. 2019, 103, 41–54. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, X.X.; Qiao, Y.; Xu, Y.; Mou, C.L.; Wu, W.; Yao, J.X. Climate-driven paleoceanography change controls on petrology and organic matter accumulation in the upper Permian Dalong Formation, western Hubei Province, southern China. Sediment. Geol. 2022, 440, 106259. [Google Scholar] [CrossRef]
- Sageman, B.B.; Murphy, A.E.; Werne, J.P.; Straeten, C.A.V.; Hollander, D.J.; Lyons, T.W. A tale of shales: The relative roles of production, decomposition, and dilution in the accumulation of organic-rich strata, Middle-Upper Devonian, Appalachian basin. Chem. Geol. 2003, 195, 229–273. [Google Scholar] [CrossRef]
- Wei, Z.F.; Wang, Y.L.; Wang, G.; Sun, Z.P.; Zhang, T.; Xu, L.; Ma, X.Y.; He, W. Paleoenvironmental conditions of organic-rich Upper Permian Dalong Formation shale in the Sichuan Basin, southwestern China. Mar. Pet. Geol. 2018, 91, 152–162. [Google Scholar] [CrossRef]
- Demaison, G.J.; Moore, G.T. Anoxic environments and oil source bed genesis. AAPG Bull. 1980, 64, 1179–1209. [Google Scholar] [CrossRef]
- Arthur, M.A.; Sageman, B.B. Marine black shales e depositional mechanisms and environments of ancient-deposits. Annu. Rev. Earth Planet. Sci. 1994, 22, 499–551. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Wang, Y.; Xie, T.; Shi, X.; Chen, W. Evaluation of shale gas resource potential of Late Permian Dalong Formation in western Hubei province. Lithol. Reserv. 2018, 30, 84–90, (In Chinese with English Abstract). [Google Scholar]
- Wu, L.L.; Horsfield, B. Initial insights into releasing bound biomarkers from kerogen matricesusing microscale sealed vessel catalytic hydrogenation (MSSV-HY). Org. Geochem. 2019, 130, 22–32. [Google Scholar] [CrossRef]
- Qiu, Z.; Zou, C.N. Controlling factors on the formation and distribution of “sweet-spot areas” of marine gas shales in South China and a preliminary discussion on unconventional petroleum sedimentology. J. Asian Earth Sci. 2020, 194, 103989. [Google Scholar] [CrossRef]
- Liu, W.Q.; Yao, J.X.; Tong, J.N.; Qiao, Y.; Chen, Y. Organic matter accumulation on the Dalong Formation (Upper Permian) in western Hubei, South China: Constraints from multiple geochemical proxies and pyrite morphology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 514, 677–689. [Google Scholar] [CrossRef]
- Wu, W.; Liu, W.Q.; Mou, C.L.; Liu, H.; Qiao, Y.; Pan, J.N.; Ning, S.Y.; Zhang, X.X.; Yao, J.X.; Liu, J.D. Organic-rich siliceous rocks in the upper Permian Dalong Formation (NW middle Yangtze): Provenance, paleoclimate and paleoenvironment. Mar. Pet. Geol. 2021, 123, 104728. [Google Scholar] [CrossRef]
- Yu, Y.M.; Li, P.P.; Guo, R.X.; Zhao, Y.Z.; Li, S.; Zou, H.Y. Upwelling-induced organic matter enrichment of the Upper Permian Dalong Formation in the Sichuan Basin, SW China and its paleoenvironmental implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 576, 110510. [Google Scholar] [CrossRef]
- Zheng, B.S.; Mou, C.L.; Wang, Y.C. Organic matter accumulation in response to tectonism: New data from the Upper Permian Dalong Formation black shales in the Western Hubei Basin, South China and its implications for the end-Permian mass extinction. Sediment. Geol. 2024, 464, 106621. [Google Scholar] [CrossRef]
- Yang, X.R.; Yan, D.T.; Zhang, B.; Zhang, L. Anomalous weathering trends during the Early Silurian warming: Implications for the biotic crisis and recovery. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 632, 111859. [Google Scholar] [CrossRef]
- West, A.J.; Galy, A.; Bickle, M. Tectonic and climatic controls on silicate weathering. Earth Planet. Sci. Lett. 2005, 235, 211–228. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Jenkyns, H.C.; Woodfine, R.G. Lithium isotope evidence for enhanced weathering during Oceanic Anoxic Event 2. Nat. Geosci. 2013, 6, 668–672. [Google Scholar] [CrossRef]
- Zhang, B.; Wignall, P.B.; Yao, S.P.; Hu, W.X.; Liu, B. Collapsed upwelling and intensified euxinia in response to climate warming during the Capitanian (Middle Permian) mass extinction. Gondwana Res. 2021, 89, 31–46. [Google Scholar] [CrossRef]
- Harnois, L. The CIW index: A new chemical index of weathering. Sediment. Geol. 1988, 55, 319–322. [Google Scholar] [CrossRef]
- Price, J.R.; Velbel, M.A. Chemical weathering indices applied to weathering profiles developed on heterogeneous felsic metamorphic parent rocks. Chem. Geol. 2003, 202, 397–416. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.S.; Yu, W.C.; Algeo, T.J.; Zhou, Q.; Xu, Y.; Qi, L.; Yuan, L.J.; Pan, W. The chemical index of alteration (CIA) as a proxy for climate change during glacial-interglacial transitions in Earth history. Earth-Sci. Rev. 2020, 201, 103032. [Google Scholar] [CrossRef]
- Cao, C.; Bataille, C.P.; Song, H.J.; Saltzman, M.R.; Cramer, K.T.; Wu, H.C.; Korte, C.; Zhang, Z.F.; Liu, X.M. Persistent late permian to early triassic warmth linked to enhanced reverse weathering. Nat. Geosci. 2022, 15, 1071. [Google Scholar] [CrossRef]
- Huang, K.J.; Teng, F.Z.; Shen, B.; Xiao, S.H.; Lang, X.G.; Ma, H.R.; Fu, Y.; Peng, Y.B. Episode of intense chemical weathering during the termination of the 635 Ma Marinoan glaciation. Proc. Natl. Acad. Sci. USA 2016, 113, 14904–14909. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Z.; Li, W.Y.; Rudnick, R.L.; Gardner, L.R. Contrasting lithium and magnesium isotope fractionation during continental weathering. Earth Planet. Sci. Lett. 2010, 300, 63–71. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zhang, X.L.; Shen, Y.N. Quantitative constraints on carbon cycling and temporal changes in episodic euxinia during the end-Permian mass extinction in South China. Chem. Geol. 2021, 562, 120036. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, L.; Deng, X.; Wang, Y.; Li, Y.; Guo, Y. Geochemical features of the Middle–Upper Permian cherts and implications for origin, depositional environment in the Sichuan Basin, SW China. Geol. J. 2019, 55, 1493–1506. [Google Scholar] [CrossRef]
- Zheng, B.; Mou, C.; Zhou, R.; Wang, X.; Xiao, Z.; Chen, Y. Nature and origin of the volcanic ash beds near the Permian–Triassic boundary in South China: New data and their geological implications. Geol. Mag. 2020, 157, 677–689. [Google Scholar] [CrossRef]
- Yu, H.; Chen, D.Z.; Wei, H.Y.; Wang, J.G. Origin of bedded chert and organic matter accumulation in the Dalong Formation of Upper Permian in western Hubei Province. Acta Petrol. Sin. 2012, 28, 1017–1027. [Google Scholar]
- Huang, B.C.; Yan, Y.G.; Piper, J.D.A.; Zhang, D.H.; Yi, Z.Y.; You, S.; Zhou, T.H. Paleomagnetic constraints on the paleogeography of the East Asian blocks during Late Paleozoic and Early Mesozoic times. Earth-Sci. Rev. 2018, 186, 8–36. [Google Scholar] [CrossRef]
- Zhao, G.C.; Zhang, G.W.; Wang, Y.J.; Huang, B.C.; Dong, Y.P.; Li, S.Z.; Yu, S. Geological reconstructions of the East Asian blocks: From the breakup of Rodinia to the assembly of Pangea. Earth-Sci. Rev. 2018, 186, 262–286. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- McLennan, S.M. Weathering and global denudation. J. Geol. 1993, 101, 295–303. [Google Scholar] [CrossRef]
- Fedo, C.M.; Nesbitt, H.W.; Young, G.M. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Schroeder, J.O.; Murray, R.W.; Leinen, M.; Pflaum, R.C.; Janecek, T.R. Barium in equatorial pacific carbonate sediment: Terrigenous, oxide, and biogenic associations. Paleoceanography 1997, 12, 125–146. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Environmental influences on the chemical composition of shales and clays. Phys. Chem. Earth 1971, 8, 307–333. [Google Scholar] [CrossRef]
- Taylor, S.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Malden, MA, USA, 1985. [Google Scholar]
- Hu, Z.Y.; Yang, S.Y.; Yang, C.F.; Guo, Y.L.; Xu, J.; Zhang, C. Mg isotopes of siliciclastic sediments on continental marginal sea: Insights for the potential to trace silicate weathering. Glob. Planet. Change 2023, 231, 104307. [Google Scholar] [CrossRef]
- Bhatia, M.R.; Crook, K.A. Trace element characteristics of graywackes andtectonic setting discrimination of sedimentary basins. Contrib. Mineral. Petrol. 1986, 92, 181–193. [Google Scholar] [CrossRef]
- Isson, T.T.; Planavsky, N.J. Reverse weathering as a long-term stabilizer of marine pH and planetary climate. Nature 2018, 560, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Z.; Shen, B.; Wang, X.L.; Ma, H.R.; Li, C.; Zhou, C.M. Extremely 26Mg-enriched authigenic clays from the Ediacaran Doushantuo Formation (South China) indicating the coupled carbonate-silicate diagenesis. Glob. Planet. Change 2024, 239, 104500. [Google Scholar] [CrossRef]
- Dunlea, A.G.; Murray, R.W.; Ramos, D.P.S.; Higgins, J.A. Cenozoic global cooling and increased seawater Mg/Ca via reduced reverse weathering. Nat. Commun. 2017, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, Z.; Jin, Z.; Wen, X.; Geng, Y. Origin of authigenic quartz in organic- rich shales of the Wufeng and Longmaxi formations in the Sichuan Basin, south China: Implications for pore evolution. J. Nat. Gas Sci. Eng. 2017, 38, 21–38. [Google Scholar] [CrossRef]
- Yan, D.T.; Chen, D.Z.; Wang, Q.C.; Wang, J.G. Large-scale climatic fluctuations in the latest Ordovician on the Yangtze block, South China. Geology 2010, 38, 599–602. [Google Scholar] [CrossRef]
- Chen, J.B.; Guo, Y.; Wei, H.B.; Liu, H.Y.; Ma, R.Y.; Xiao, Z.; Feng, Z. Evaluation of chemical weathering proxies by comparing drilled cores versus outcrops and weathering history during the Permian–Triassic transition. Glob. Planet. Change 2022, 214, 103855. [Google Scholar] [CrossRef]
- Cao, Y.; Song, H.; Algeo, T.J.; Chu, D.; Du, Y.; Tian, L.; Wang, Y.H.; Tong, J.N. Intensified chemical weathering during the Permian-Triassic transition recorded in terrestrial and marine successions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 519, 166–177. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, P.X.; Yang, M.F.; Shao, L.Y.; Hilton, J. Continental records of organic carbon isotopic composition (δ13Corg), weathering, paleoclimate and wildfire linked to the End-Permian Mass Extinction. Chem. Geol. 2020, 558, 119764. [Google Scholar] [CrossRef]
- Fielding, C.R.; Frank, T.D.; McLoughlin, S.; Vajda, V.; Mays, C.; Tevyaw, A.P.; Winguth, A.; Winguth, C.; Nicoll, R.S.; Bocking, M.; et al. Age and pattern of the southern high-latitude continental end-Permian extinction constrained by multiproxy analysis. Nat. Commun. 2019, 10, 385. [Google Scholar] [CrossRef]
- Chen, B.; Joachimski, M.M.; Shen, S.Z.; Lambert, L.L.; Lai, X.L.; Wang, X.D.; Chen, J.; Yuan, D.X. Permian ice volume and paleoclimate history: Oxygen isotope proxies revisited. Gondwana Res. 2013, 24, 77–89. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.Z.; Li, X.H.; Xu, Y.G.; Joachimski, M.M.; Bowring, S.A.; Erwin, D.H.; Yuan, D.X.; Chen, B.; Zhang, H.; et al. High-resolution SIMS oxygen isotope analysis on conodont apatite from South China and implications for the end-Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 448, 26–38. [Google Scholar] [CrossRef]
- Sun, Y.D.; Joachimski, M.M.; Wignall, P.B.; Yan, C.B.; Chen, Y.L.; Jiang, H.S.; Wang, L.N.; Lai, X.L. Lethally Hot Temperatures during the Early Triassic Greenhouse. Science 2012, 338, 366–370. [Google Scholar] [CrossRef]
- Yang, J.H.; Cawood, P.A.; Condon, D.J.; Liu, J.Z.; Deng, X.S.; Wang, J.F.; Du, Y.S.; Yuan, D.X. Anomalous weathering trends indicate accelerated erosion of tropical basaltic landscapes during the Permo-Triassic warming. Earth Planet. Sci. Lett. 2022, 577, 117256. [Google Scholar] [CrossRef]
- Algeo, T.J.; Twitchett, R.J. Anomalous Early Triassic sediment fluxes due to elevated weathering rates and their biological consequences. Geology 2010, 38, 1023–1026. [Google Scholar] [CrossRef]
- Young, E.D.; Galy, A. The isotope geochemistry and cosmochemistry of magnesium. Rev. Mineral. Geochem. 2004, 55, 197–230. [Google Scholar] [CrossRef]
- Wang, S.J.; Teng, F.Z.; Rudnick, R.L.; Li, S.G. The behavior of magnesium isotopes in low-grade metamorphosed mudrocks. Geochim. Cosmochim. Acta 2015, 165, 435–448. [Google Scholar] [CrossRef]
- Teng, F.Z. Magnesium isotope geochemistry. Rev. Mineral. Geochem. 2017, 82, 219–287. [Google Scholar] [CrossRef]
- Huang, K.J.; Teng, F.Z.; Wei, G.J.; Ma, J.L.; Bao, Z.Y. Adsorption- and desorption-controlled magnesium isotope fractionation during extreme weathering of basalt in Hainan Island, China. Earth Planet. Sci. Lett. 2012, 359–360, 73–83. [Google Scholar] [CrossRef]
- Zhang, G.J.; Chen, D.Z.; Huang, K.J.; Liu, M.; Huang, T.Y.; Yeasmin, R.; Fu, Y. Dramatic attenuation of continental weathering during the Ediacaran-Cambrian transition: Implications for the climatic-oceanic-biological co-evolution. Glob. Planet. Change 2021, 203, 103518. [Google Scholar] [CrossRef]
- Wimpenny, J.; Gíslason, S.R.; James, R.H.; Gannoun, A.; Pogge Von Strandmann, P.A.E. The behaviour of Li and Mg isotopes during primary phase dissolution and secondary mineral formation in basalt. Geochim. Cosmochim. Acta 2010, 74, 5259–5279. [Google Scholar] [CrossRef]
- Algeo, T.J.; Rowe, H. Paleoceanographic applications of trace-metal concentration data. Chem. Geol. 2012, 324, 6–18. [Google Scholar] [CrossRef]
- Sweere, T.; van den Boorn, S.; Dickson, A.J.; Reichart, G.J. Definition of new trace metal proxies for the controls on organic matter enrichment in marine sediments based on Mn, Co, Mo and Cd concentrations. Chem. Geol. 2016, 441, 235–245. [Google Scholar] [CrossRef]
- Addison, J.A.; Barron, J.; Finney, B.; Kusler, J.; Bukry, D.; Heusser, L.E.; Alexander, C.R. A Holocene record of ocean productivity and upwelling from the northern California continental slope. Quat. Int. 2018, 469, 96–108. [Google Scholar] [CrossRef]
- Gao, P.; Xiao, X.M.; Meng, G.M.; Lash, G.G.; Li, S.J.; Han, Y.Q. Quartz types and origins of the Upper Permian Dalong Formation shale of the Sichuan Basin: Implications for pore preservation in deep shale reservoirs. Mar. Pet. Geol. 2023, 156, 106461. [Google Scholar] [CrossRef]
- Lu, Y.B.; Jiang, S.; Lu, Y.C.; Xu, S.; Shu, Y.; Wang, Y.X. Productivity or preservation? The factors controlling the organic matter accumulation in the late Katian through Hirnantian Wufeng organic-rich shale, South China. Mar. Pet. Geol. 2019, 109, 22–35. [Google Scholar] [CrossRef]
- Challands, T.J.; Armstrong, H.; Maloney, D.; Davies, J.; Wilson, D.; Owen, A. Organic-carbon deposition and coastal upwelling at mid-latitude during the Upper Ordovician (late Katian): A case study from the Welsh Basin, UK. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 273, 395–410. [Google Scholar] [CrossRef]
- Kaufman, A.J.; Knoll, A.H.; Narbonne, G.M. Isotopes, Ice Ages, and Terminal Proterozoic Earth History. Proc. Natl. Acad. Sci. USA 1997, 94, 6600–6660. [Google Scholar] [CrossRef] [PubMed]
- Werne, J.P.; Hollander, D.J. Balancing Supply and Demand: Controls on Carbon Isotope Fractionation in the Cariaco Basin (Venezuela) Younger Dryas to Present. Mar. Chem. 2004, 92, 275–293. [Google Scholar] [CrossRef]
- Yu, H.; Wei, H.Y. A Kungurian Oceanic Upwelling on Yangtze Platform: Evidenced by δ13Corg and Authigenic Silica in the Lower Chihsia Formation of Enshi Section in South China. J. Earth Sci. 2015, 26, 211–218. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredoxand paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Schoepfer, S.D.; Shen, J.; Wei, H.Y.; Tyson, R.V.; Ingall, E.; Algeo, T.J. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth-Sci. Rev. 2015, 149, 23–52. [Google Scholar] [CrossRef]
- Vink, S.; Chambers, R.M.; Smith, S.V. Distribution of phosphorus in sediments from Tomales Bay, California. Mar. Geol. 1997, 139, 157–179. [Google Scholar] [CrossRef]
- Ingall, E.; Jahnke, R. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 1994, 58, 2571–2575. [Google Scholar] [CrossRef]
- Algeo, T.J.; Li, C. Redox classification and calibration of redox thresholds in sedimentary systems. Geochim. Cosmochim. Acta 2020, 287, 8–26. [Google Scholar] [CrossRef]
- Li, S.; Wignall, P.B.; Poulton, S.W. Co-application of rhenium, vanadium, uranium and molybdenum as paleo-redox proxies: Insight from modern and ancient environments. Chem. Geol. 2025, 674, 122565. [Google Scholar] [CrossRef]
- Wei, H.Y.; Algeo, T.J.; Yu, H.; Wang, J.G.; Guo, C.; Shi, G. Episodic euxinia in the Changhsingian (late Permian) of South China: Evidence from framboidal pyrite and geochemical data. Sediment. Geol. 2015, 319, 78–97. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, B.L.; Cao, J.; Yao, S.P.; Hu, W.X.; Lang, X.G.; Xing, F.C.; Liao, Z.W.; Li, Y.P.; Yang, J. Paleoenvironmental evolution preceding the end-Permian mass extinction in the Lower Yangtze region (South China) and its controls on extreme enrichment of organic matter. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 636, 111967. [Google Scholar] [CrossRef]
- Liao, Z.W.; Hu, W.X.; Cao, J.; Wang, X.L.; Fu, X.G. Oceanic anoxia through the late Permian Changhsingian Stage in the lower Yangtze region, South China: Evidence from sulfur isotopes and trace elements. Chem. Geol. 2020, 532, 119371. [Google Scholar] [CrossRef]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Baudin, F.; Riboulleau, A. Analysis of marine environmental conditions based on molybdenum–uranium covariation—Applications to Mesozoic paleoceanography. Chem. Geol. 2012, 324, 46–58. [Google Scholar] [CrossRef]
- Wanty, R.B.; Goldhaber, M.B. Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochim. Cosmochim. Acta 1992, 56, 1471–1483. [Google Scholar] [CrossRef]
- Bennett, W.W.; Canfield, D.E. Redox-sensitive trace metals as paleoredox proxies: A review and analysis of data from modern sediments. Earth-Sci. Rev. 2020, 204, 103175. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, M.C.; Li, J.; Huang, C.M. Late Permian ~6 My cooling induced by basaltic weathering of the Emeishan large igneous province: Evidence from interbasaltic paleosols. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 609, 111305. [Google Scholar] [CrossRef]
- Fan, J.; Shen, S.; Erwin, D.H.; Sadler, P.M.; MacLeod, N.; Cheng, Q.; Hou, X.; Yang, J.; Wang, X.; Wang, Y.; et al. A high-resolution summary of Cambrian to Early Triassic marine invertebrate biodiversity. Science 2020, 367, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Present, T.M.; Li, M.; Shen, Y.; Adkins, J.F. Carbonate associated sulfate (CAS) δ34S heterogeneity across the end-Permian mass extinction in South China. Earth Planet. Sci. Lett. 2021, 574, 117172. [Google Scholar] [CrossRef]
- Song, H.; Hu, S.; Benton, M.; Jiang, D. Editorial preface to special issue: Recovery of marine ecosystem after the Permian-Triassic mass extinction: New progress from South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 633, 111899. [Google Scholar] [CrossRef]
- Harper, D.A.T. Late Ordovician Mass Extinction: Earth, fire and ice. Natl. Sci. Rev. 2024, 11, nwad319. [Google Scholar] [CrossRef]

| Samples | Height (m) | Formation | TOC | δ13Corg | δ26Mgsilicate | δ25Mgsilicate | SiO2 | Al2O3 | MgO | Na2O | K2O | P2O5 | TiO2 | CaO | TFe2O3 | MnO | CIA | CIAcorr | CIW | PIA | Sibio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH-06 | 3.7 | Dalong | 1.52 | −24.4 | 1.51 | 0.79 | 56.57 | 21.27 | 0.95 | 0.13 | 2.45 | 2.00 | 1.98 | 0.64 | 2.17 | 0.00 | 84.0 | 84.9 | 93.9 | 93.1 | 13.6 |
| SH-12 | 8.1 | Dalong | 1.77 | −25.8 | 1.08 | 0.56 | 84.48 | 5.95 | 0.48 | 0.12 | 1.06 | 0.06 | 0.24 | 0.04 | 2.31 | 0.00 | 80.7 | 82.7 | 95.7 | 94.7 | 72.5 |
| SH-16 | 12.6 | Dalong | 1.03 | −25.9 | / | / | 93.51 | 1.41 | 0.13 | 0.09 | 0.22 | 0.04 | 0.07 | 0.05 | 0.91 | 0.00 | 74.6 | 75.7 | 85.4 | 82.9 | 90.7 |
| SH-18 | 14.1 | Dalong | 0.11 | −25.4 | / | / | 75.39 | 10.48 | 0.75 | 0.13 | 1.28 | 0.25 | 0.32 | 0.09 | 6.21 | 0.05 | 85.6 | 86.6 | 96.5 | 96.0 | 54.2 |
| SH-21 | 16.9 | Dalong | 12.47 | −27.3 | 1.03 | 0.54 | 69.04 | 8.83 | 0.88 | 0.13 | 2.05 | 0.06 | 0.41 | 0.03 | 0.70 | 0.00 | 78.0 | 81.0 | 97.0 | 96.1 | 51.2 |
| SH-25 | 19.9 | Dalong | 9.87 | −27.2 | 1.13 | 0.58 | 67.36 | 10.71 | 0.75 | 0.03 | 1.94 | 0.07 | 0.41 | 0.00 | 2.41 | 0.01 | 83.3 | 85.5 | 99.5 | 99.4 | 45.7 |
| SH-29 | 23.8 | Dalong | 8.58 | −27.7 | / | / | 74.87 | 8.37 | 0.70 | 0.24 | 2.00 | 0.03 | 0.35 | 0.01 | 0.79 | 0.00 | 76.5 | 79.4 | 95.4 | 93.9 | 58.0 |
| SH-31 | 25.8 | Dalong | 7.23 | −27.4 | 1.40 | 0.73 | 80.15 | 5.87 | 0.45 | 0.40 | 1.28 | 0.05 | 0.23 | 0.02 | 0.83 | 0.00 | 73.7 | 75.9 | 89.4 | 86.5 | 68.3 |
| SH-34 | 27.8 | Dalong | 2.96 | −26.7 | / | / | 87.06 | 4.00 | 0.27 | 0.20 | 0.68 | 0.02 | 0.19 | 0.01 | 1.59 | 0.01 | 78.6 | 80.2 | 92.0 | 90.4 | 79.0 |
| SH-36 | 31.1 | Dalong | 1.42 | −26.6 | 1.09 | 0.56 | 92.68 | 2.50 | 0.15 | 0.27 | 0.33 | 0.01 | 0.12 | 0.01 | 0.24 | 0.00 | 75.3 | 76.0 | 84.3 | 82.2 | 87.6 |
| SH-40 | 37.9 | Dalong | 6.50 | −27.5 | 1.42 | 0.74 | 82.41 | 4.39 | 0.38 | 0.14 | 0.84 | 0.02 | 0.20 | 0.01 | 1.71 | 0.01 | 79.1 | 81.2 | 94.6 | 93.3 | 73.5 |
| SH-43 | 42.8 | Dalong | 9.06 | −27.4 | / | / | 80.86 | 4.37 | 0.43 | 0.08 | 0.88 | 0.03 | 0.28 | 0.02 | 0.42 | 0.01 | 79.5 | 81.9 | 96.3 | 95.3 | 72.0 |
| SH-46 | 48.4 | Dalong | 10.64 | −26.8 | 0.70 | 0.36 | 58.53 | 12.20 | 0.79 | 0.62 | 2.63 | 0.17 | 0.40 | 0.03 | 6.27 | 0.01 | 75.7 | 77.9 | 91.9 | 89.7 | 33.9 |
| SH-47 | 51.4 | Daye | 0.26 | −26.2 | 0.53 | 0.28 | 39.05 | 8.98 | 1.26 | 0.34 | 2.15 | 0.23 | 0.33 | 23.80 | 2.55 | 0.18 | 72.2 | 74.6 | 88.9 | 85.6 | 20.9 |
| SH-48 | 54.4 | Daye | 0.65 | −26.9 | / | / | 50.06 | 12.67 | 1.47 | 0.63 | 2.83 | 0.16 | 0.46 | 13.10 | 4.00 | 0.11 | 71.1 | 73.0 | 85.9 | 82.2 | 24.5 |
| SH-49 | 57.4 | Daye | 0.52 | −26.5 | 0.41 | 0.21 | 57.50 | 11.97 | 2.10 | 0.67 | 2.77 | 0.11 | 0.42 | 9.33 | 3.46 | 0.09 | 69.6 | 71.5 | 84.4 | 80.2 | 33.3 |
| SH-50 | 60.4 | Daye | 0.48 | −25.8 | / | / | 66.26 | 11.82 | 1.45 | 0.56 | 2.70 | 0.14 | 0.39 | 6.07 | 2.74 | 0.04 | 71.3 | 73.2 | 86.6 | 82.9 | 42.4 |
| Samples | Height (m) | Formation | V/Al | Mo/Al | U/Al | UEF | MoEF | VEF | Corg/P | Mo/U | Mo/TOC | CoEF × MnEF | Ni/Al2O3 | Sc | V | Ni | Zr | Mo | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH-06 | 3.7 | Dalong | 14.4 | 0.0 | 0.5 | 2.4 | 0.4 | 1.7 | 4.5 | 0.1 | 0.5 | 0.0 | 1.1 | 28.5 | 284.8 | 24.2 | 582.5 | 0.8 | 11.6 | 9.4 |
| SH-12 | 8.1 | Dalong | 86.4 | 1.4 | 2.6 | 15.0 | 15.2 | 11.3 | 177.5 | 0.5 | 5.1 | 0.1 | 16.3 | 5.8 | 535.0 | 97.1 | 140.6 | 8.9 | 3.5 | 16.4 |
| SH-16 | 12.6 | Dalong | 218.1 | 6.4 | 7.1 | 41.7 | 70.8 | 29.8 | 148.4 | 0.9 | 9.6 | 0.4 | 27.2 | 1.4 | 333.8 | 38.4 | 17.3 | 9.9 | 0.5 | 10.9 |
| SH-18 | 14.1 | Dalong | 7.7 | 0.6 | 1.0 | 5.5 | 6.5 | 1.0 | 2.6 | 0.6 | 62.3 | 1.7 | 30.0 | 13.8 | 81.2 | 314.3 | 133.0 | 6.7 | 6.4 | 10.6 |
| SH-21 | 16.9 | Dalong | 159.0 | 0.5 | 2.2 | 10.6 | 4.6 | 18.2 | 1211.6 | 0.2 | 0.3 | 0.0 | 13.5 | 11.2 | 1278.3 | 118.8 | 188.0 | 4.0 | 7.0 | 17.3 |
| SH-25 | 19.9 | Dalong | 102.0 | 1.8 | 1.0 | 5.1 | 16.8 | 11.7 | 849.2 | 1.8 | 1.8 | 0.0 | 9.8 | 11.0 | 996.5 | 104.5 | 115.6 | 17.8 | 7.1 | 10.0 |
| SH-29 | 23.8 | Dalong | 125.9 | 4.2 | 1.0 | 5.2 | 40.9 | 15.2 | 1616.9 | 4.2 | 3.9 | 0.0 | 9.6 | 7.7 | 1009.5 | 80.4 | 118.0 | 33.9 | 4.8 | 8.0 |
| SH-31 | 25.8 | Dalong | 136.6 | 4.2 | 1.1 | 6.0 | 41.5 | 16.9 | 887.3 | 3.7 | 3.3 | 0.0 | 9.9 | 5.2 | 788.8 | 57.9 | 68.4 | 24.0 | 3.6 | 6.5 |
| SH-34 | 27.8 | Dalong | 22.3 | 0.6 | 1.2 | 6.6 | 5.9 | 2.9 | 953.1 | 0.5 | 0.8 | 0.2 | 18.3 | 3.0 | 93.1 | 73.4 | 49.3 | 2.3 | 2.6 | 4.9 |
| SH-36 | 31.1 | Dalong | 61.5 | 3.3 | 1.4 | 8.0 | 36.7 | 8.4 | 694.0 | 2.5 | 6.4 | 0.0 | 4.8 | 3.2 | 167.3 | 12.1 | 38.8 | 9.1 | 2.0 | 3.7 |
| SH-40 | 37.9 | Dalong | 226.7 | 35.7 | 2.0 | 10.7 | 359.3 | 28.4 | 2275.8 | 18.0 | 23.9 | 0.2 | 22.4 | 4.8 | 989.2 | 98.4 | 51.1 | 155.6 | 2.9 | 8.7 |
| SH-43 | 42.8 | Dalong | 218.9 | 23.4 | 4.2 | 22.3 | 230.5 | 26.8 | 1730.1 | 5.5 | 11.0 | 0.0 | 10.9 | 4.4 | 931.8 | 47.7 | 79.0 | 99.6 | 5.3 | 18.0 |
| SH-46 | 48.4 | Dalong | 100.7 | 19.2 | 2.8 | 13.4 | 170.0 | 11.1 | 369.9 | 6.8 | 19.3 | 0.1 | 17.3 | 14.0 | 1074.9 | 211.1 | 139.4 | 204.9 | 9.0 | 30.1 |
| SH-47 | 51.4 | Daye | 10.1 | 0.4 | 0.8 | 3.7 | 3.2 | 1.0 | 6.7 | 0.5 | 11.0 | 3.8 | 5.9 | 9.3 | 71.8 | 53.4 | 68.7 | 2.9 | 8.0 | 6.0 |
| SH-48 | 54.4 | Daye | 11.3 | 0.4 | 0.4 | 2.0 | 3.8 | 1.2 | 24.5 | 1.0 | 7.4 | 1.8 | 6.3 | 12.3 | 123.4 | 79.9 | 98.3 | 4.8 | 11.4 | 4.6 |
| SH-49 | 57.4 | Daye | 8.8 | 0.1 | 0.3 | 1.4 | 0.5 | 1.0 | 28.8 | 0.2 | 1.1 | 1.2 | 3.9 | 11.5 | 95.1 | 46.6 | 98.4 | 0.6 | 10.2 | 3.1 |
| SH-50 | 60.4 | Daye | 9.1 | 0.0 | 0.4 | 1.9 | 0.5 | 1.1 | 19.9 | 0.1 | 1.2 | 0.3 | 4.4 | 11.7 | 103.4 | 52.2 | 101.8 | 0.6 | 10.9 | 4.1 |
| Samples | Height (m) | Quartz (%) | Illite (%) | Albite (%) | Pyrite (%) | Calcite (%) | Anatase (%) | Siderite (%) |
|---|---|---|---|---|---|---|---|---|
| SH-06 | 3.7 | 48.6 | 45 | 0 | 0.8 | 0 | 5 | 0.6 |
| SH-18 | 14.1 | 88.9 | 11.1 | 0 | 0 | 0 | 0 | 0 |
| SH-21 | 16.9 | 92.1 | 7.2 | 0.8 | 0 | 0 | 0 | 0 |
| SH-47 | 51.4 | 41.8 | 10.2 | 3.5 | 2 | 42.5 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Li, Y.; Yang, P.; Xiang, M.; Zhou, Z.; Zhang, Z.; Niu, X.; Yang, X. Enhanced Continental Weathering and Intense Upwelling Drove the Deposition of Organic-Rich Shales in the Late Permian Dalong Formation, South China. J. Mar. Sci. Eng. 2025, 13, 357. https://doi.org/10.3390/jmse13020357
Gong Y, Li Y, Yang P, Xiang M, Zhou Z, Zhang Z, Niu X, Yang X. Enhanced Continental Weathering and Intense Upwelling Drove the Deposition of Organic-Rich Shales in the Late Permian Dalong Formation, South China. Journal of Marine Science and Engineering. 2025; 13(2):357. https://doi.org/10.3390/jmse13020357
Chicago/Turabian StyleGong, Yin, Yiming Li, Peng Yang, Meng Xiang, Zhou Zhou, Zhongquan Zhang, Xing Niu, and Xiangrong Yang. 2025. "Enhanced Continental Weathering and Intense Upwelling Drove the Deposition of Organic-Rich Shales in the Late Permian Dalong Formation, South China" Journal of Marine Science and Engineering 13, no. 2: 357. https://doi.org/10.3390/jmse13020357
APA StyleGong, Y., Li, Y., Yang, P., Xiang, M., Zhou, Z., Zhang, Z., Niu, X., & Yang, X. (2025). Enhanced Continental Weathering and Intense Upwelling Drove the Deposition of Organic-Rich Shales in the Late Permian Dalong Formation, South China. Journal of Marine Science and Engineering, 13(2), 357. https://doi.org/10.3390/jmse13020357







