Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Area Description
2.2. Sampled Biological Material
2.3. Sample Preparation
2.4. Statistical Analysis
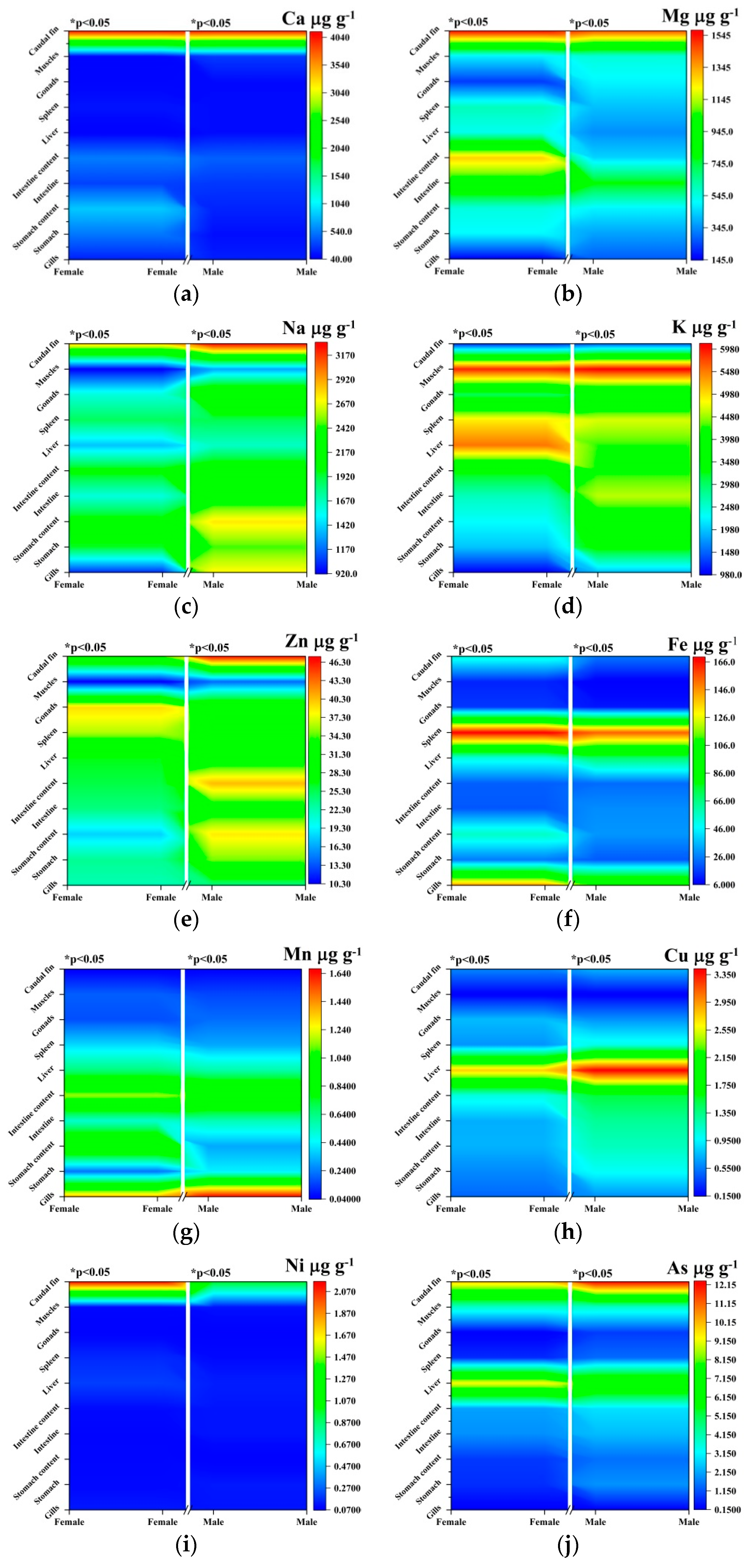
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Song, Y.F.; Luo, Z.; Huang, C.; Liu, X.; Pan, Y.X.; Chen, Q.L. Effects of calcium and copper exposure on lipogenic metabolism, metal element compositions and histology in Synechogobius hasta. Fish Physiol. Biochem. 2013, 39, 1641–1656. [Google Scholar] [CrossRef]
- Santos, I.; Diniz, M.S.; Carvalho, M.L.; Santos, J.P. Assessment of essential elements and heavy metals content on Mytilus galloprovincialis from River Tagus estuary. Biol. Trace Elem. Res. 2014, 159, 233–240. [Google Scholar] [CrossRef]
- Gati, G.; Pop, C.; Brudasca, F.; Gurzau, A.E.; Spinu, M. The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 2016, 25, 688–696. [Google Scholar] [CrossRef]
- Castro, B.B.; Sobral, O.; Guilhermino, L.; Ribeiro, R. An in-situ bioassay integrating individual and biochemical responses using small fish species. Ecotoxicology 2004, 13, 667–681. [Google Scholar] [CrossRef]
- Hartwell, S.I.; Dawson, C.E.; Durell, E.Q.; Alden, R.W.; Adolphson, P.C.; Wright, D.A.; Coelho, G.M.; Magee, J.A. Integrated measures of ambient toxicity and fish community diversity in Chesapeake Bay tributaries. Ecotoxicology 1998, 7, 19–35. [Google Scholar] [CrossRef]
- Lino, A.S.; Galvão, P.M.A.; Longo, R.T.L.; Azevedo-Silva, C.E.; Dorneles, P.R.; Torres, J.P.M.; Malm, O. Metal bioaccumulation in consumed marine bivalves in Southeast Brazilian coast. J. Trace Elem. Med. Biol. 2016, 34, 50–55. [Google Scholar] [CrossRef]
- Capillo, G.; Silvestro, S.; Sanfilippo, M.; Fiorino, E.; Giangrosso, G.; Ferrantelli, V.; Vazzana, I.; Faggio, C. Assessment of electrolytes and metals profile of the Faro Lake (Capo Peloro Lagoon, Sicily, Italy) and its impact on Mytilus galloprovincialis. Chem. Biodivers. 2018. [Google Scholar] [CrossRef]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron profile modulation in Gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel digestive gland as a model for assessing xenobiotics: An overview. Sci. Total Environ. 2018, 613, 220–229. [Google Scholar] [CrossRef]
- Aliko, V.; Hajdaraj, G.; Caci, A.; Faggio, C. Copper, Induced Lysosomal Membrane Destabilisation in Haemolymph Cells of Mediterranean Green Crab (Carcinus aestuarii, Nardo, 1847) from the Narta Lagoon (Albania). Braz. Arch. Biol. Technol. 2015, 58, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Pagano, M.; Porcino, C.; Briglia, M.; Fiorino, E.; Vazzana, M.; Silvestro, S.; Faggio, C. The influence of exposure of cadmium chloride and zinc chloride on haemolymph and digestive gland cells from Mytilus galloprovincialis. Int. J. Environ. Res. 2017, 11, 207–216. [Google Scholar] [CrossRef]
- Vajargah, M.F.; Yalsuyi, A.; Sattari, M.; Prokic, M.; Faggio, C. Effects of Copper Oxide Nanoparticles (CuO-NPs) on Parturition Time, Survival Rate and Reproductive Success of Guppy Fish, Poecilia reticulate. J. Clust. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Savorelli, F.; Manfra, L.; Croppo, M.; Tornambè, A.; Palazzi, D.; Canepa, S.; Trentini, P.L.; Cicero, A.M.; Faggio, C. Fitness evaluation of Ruditapes philippinarum exposed to nickel. Biol. Trace Elem. Res. 2017, 177, 384–393. [Google Scholar] [CrossRef]
- Torre, A.; Trischitta, F.; Faggio, C. Effect of CdCl2 on Regulatory Volume Decrease (RVD) in Mytilus galloprovincialis digestive cells. Toxicol. In Vitro 2013, 27, 1260–1266. [Google Scholar] [CrossRef]
- Kondera, E.; Ługowska, K.; Sarnowski, P. High affinity of cadmium and copper to head kidney of common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2014, 40, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zang, S.; Sun, Q. Health risk assessment of heavy metals in the water environment of Zhalong Wetland, China. Ecotoxicology 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Naimo, T.J. A review of the effects of heavy metals on freshwater mussels. Ecotoxicology 1995, 4, 341–362. [Google Scholar] [CrossRef]
- Lu, G.; Yang, X.; Li, Z.; Zhao, H.; Wang, C. Contamination by metals and pharmaceuticals in northern Taihu Lake (China) and its relation to integrated biomarker response in fish. Ecotoxicology 2013, 22, 50–59. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Yun, X.; Zhang, M.; Li, Q.X.; Wang, J. Distribution and ecological assessment of heavy metals in surface sediments of the East Lake, China. Ecotoxicology 2014, 23, 92–101. [Google Scholar] [CrossRef]
- Harangi, S.; Baranyai, E.; Fehér, M.; Tóth, N.; Herman, P.; Stündl, L.; Fábián, S.; Tóthmérész, B.; Simon, E. Accumulation of metals in juvenile carp (Cyprinus carpio) exposed to sublethal levels of iron and manganese: Survival, body weight and tissue. Biol. Trace Elem. Res. 2017, 177, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Hauser-Davisa, R.A.; Bordonb, I.C.A.C.; Oliveirac, T.F.; Ziolli, R.L. Metal bioaccumulation in edible target tissues of mullet (Mugil liza) from a tropical bay in Southeastern Brazil. J. Trace Elem. Med. Bio. 2016, 36, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenco, H.M.; Afonso, C.; Anacleto, P.; Martins, M.F.; Nunes, M.L.; Nino, A.R. Elemental composition of four farmed fish produced in Portugal. Int. J. Food Sci. Nutr. 2012, 63, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Miranda, J.M.; Nebot, C.; Rodriguez, J.L.; Cepeda, A.; Franco, C.M. Differentiation of farmed and wild turbot (Psetta maxima): Proximate chemical composition, fatty acid profile, trace minerals and antimicrobial resistance of contaminant bacteria. Food Sci. Technol. Int. 2010, 16, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, H.; Palazov, A.; Stancheva, M.; Apostolov, A. Determination of the Black Sea area and coastline length using GIS methods and Landsat 7 satellite images. Geo-Eco-Mar. 2011, 17, 27–31. [Google Scholar]
- Popescu, I. Directorate General for Internal Policies, Policy Department B: Structural and Cohesion Policies, Fisheries in the Black Sea, Note; European Parliament: Brussel, Belgium, 2010.
- Stancheva, M.; Georgieva, S.; Makedonski, L. Polychlorinated biphenyls in fish from Black Sea. Bulgaria, Food Control 2017, 72, 205–210. [Google Scholar] [CrossRef]
- Malakhova, L.; Giragosov, V.; Khanaychenko, A.; Malakhova, T.; Egorov, V.; Smirnov, D. Partitioning and Level of Organochlorine Compounds in the Tissues of the Black Sea Turbot at the South-Western Shelf of Crimea. Turk. J. Fish Aquat. Sci. 2014, 14, 993–1000. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Cai, L.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, J.; Lin, H. Freshness assessment of turbot (Scophthalmus maximus) by Quality Index Method (QIM), biochemical, and proteomic methods. LWT-Food Sci. Technol. 2017, 78, 172–180. [Google Scholar] [CrossRef]
- Lyu, D.; Wang, W.; Luan, S.; Hu, Y.; Kong, J. Estimating genetic parameters for growth traits with molecular relatedness in turbot (Scophthalmus maximus, Linnaeus). Aquaculture 2017, 468, 149–155. [Google Scholar] [CrossRef]
- Li, X.; Chi, L.; Tian, H.; Meng, L.; Zheng, J.; Gao, X.; Liu, Y. Colour preferences of juvenile turbot (Scophthalmus maximus). Physiol. Behav. 2016, 156, 64–70. [Google Scholar] [CrossRef]
- Diaz de Cerio, O.; Bilbao, E.; Ruiz, P.; Pardo, B.G.; Martinez, P.; Cajaraville, M.P.; Cancio, I. Hepatic gene transcription profiles in turbot (Scophthalmus maximus) experimentally exposed to heavy fuel oil nº 6 and to styrene. Mar. Environ. Res. 2017, 123, 14–24. [Google Scholar] [CrossRef]
- Jia, R.; Han, C.; Lei, J.L.; Liu, B.L.; Huang, B.; Huo, H.H.; Yin, S.T. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2015, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, B.L.; Han, C.; Huang, B.; Lei, J.L. The physiological performance and immune response of juvenile turbot (Scophthalmus maximus) to nitrite exposure. Comp. Biochem. Phys. C 2016, 181–182, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Aksungur, N.; Aksungur, M.; Akbulut, B.; Kutlu, I. Effects of Stocking Density on Growth Performance, Survival and Food Conversion Ratio of Turbot (Psetta maxima) in the Net Cages on the Southeastern Coast of the Black Sea. Turk. J. Fish. Aquat. Sci. 2007, 7, 147–152. [Google Scholar]
- Tuzen, M. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphit furnace atomic absorbtion spectrometry. Food Chem. 2003, 80, 119–123. [Google Scholar] [CrossRef]
- Tuzen, M. Toxic and essential trace elemental content in fish species from the Black Sea, Turkey. Food Chem. Toxicol. 2009, 47, 1785–1790. [Google Scholar] [CrossRef]
- Bat, L.; Gundogdu, A.; Yardim, O.; Zoral, T.; Culha, S. Heavy metal amounts in zooplankton and some commercial teleost fish from inner harbor of Sinop, Black Sea. Su Ürünleri Mühendisleri Dern. Derg. 2006, 25, 22–27. [Google Scholar]
- Das, Y.K.; Aksoy, A.; Baskaya, R.H.; Duyar, A.D.; Guvenc, V. Boz, Heavy metal levels of some marine organisms collected in Samsun and Sinop coasts of Black Sea, in Turkey. J. Anim. Vet. Adv. 2009, 8, 496–499. [Google Scholar]
- Nisbet, C.; Terzi, G.; Pilger, O.; Sarac, N. Determination of heavy metal levels in fish samples collected from the Middle Black Sea. Kafkas Univ. Vet. Fakültesi Derg. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Ergönül, M.B.; Altindağ, A. Heavy metal concentrations in the muscle tissues of seven commercial fish species from Sinop coasts of the Black Sea. Annu. Set Environ. Prot. 2014, 16, 34–51. [Google Scholar]
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Schroder, U. Natural chemical composition of commercial fish species: Characterisation of pangasius, wild and farmed turbot and barramundi. Foods 2016, 5, 58. [Google Scholar] [CrossRef]
- Yanchilina, A.G.; Ryan, W.B.F.; McManus, J.F.; Dimitrov, P.; Dimitrov, D.; Slavova, K.M. Filipova-Marinova, Compilation of geophysical, geochronological, and geochemical evidence indicates a rapid Mediterranean-derived submergence of the Black Sea’s shelf and subsequent substantial salinification in the early Holocene. Mar. Geol. 2017, 383, 14–34. [Google Scholar] [CrossRef]
- Black Sea Commission—The Commission of the protection of the Black Sea Against Pollution. Black Sea Transboundary Diagnostic Analysis; Black Sea Commission: Istanbul, Turkey, 2007. [Google Scholar]
- Plavan, G.; Jitar, O.; Teodosiu, C.; Nicoara, M.; Micu, D.; Strungaru, S.A. Toxic metals in tissues of fishes from the Black Sea and associated human health risk exposure. ESPR 2017, 24, 7776–7787. [Google Scholar] [CrossRef]
- Zaitsev, Y.P.; Alexandrov, B.G.; Berlinsky, N.A.; Zenetos, A. Seas around Europe The Black Sea—An Oxygen-Poor Sea. In Europe’s Biodiversity—Biogeographical Regions and Seas; European Environment Agency: Copenhagen, Denmark, 2002. [Google Scholar]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 2014, 32, 369–378. [Google Scholar] [CrossRef]
- Concalves, C.; Martins, M.; Diniz, M.S.; Costa, M.H.; Cairo, S.; Costa, P.M. May sediment contamination be xenoestrogenic to benthic fish? A case study with Solea senegalensis. Mar. Environ. Res. 2014, 99, 170–178. [Google Scholar] [CrossRef]
- Cuevas, N.; Zorita, I.; Costa, P.M.; Larreta, J.; Franco, J. Histopathological baseline levels and confounding factors in common sole (Solea solea) for marine environmental risk assessment. Mar. Environ. Res. 2015, 110, 162–173. [Google Scholar] [CrossRef]
- Samsun, N.; Kalayci, F. Survival rates of black sea turbot (Scophthalmus maeoticus Pallas, 1811) captured by bottom turbot gillnets in different depths and fishing seasons between 1999 and 2004. Turk. J. Fish. Aquat. Sci. 2005, 5, 57–62. [Google Scholar]
- Whitehead, P.J.P.; Bauchot, M.L.; Hureau, J.C.; Nielsen, J.; Tortonese, E. Fishes of the North-eastern Atlantic and The Mediterranean. UNESCO 1986, 3, 1287–1293. [Google Scholar]
- Scophthalmus maximus (Linnaeus, 1758) – Turbot. Available online: https://www.fishbase.se/summary/Scophthalmus-maximus.html (accessed on 3 April 2016).
- Radu, G.; Radu, E. Taxonomically Identifier for Black Sea Fish Species—In Romanian Language (Determinator al Principalelor Specii de Pesti din Marea Neagra); VIROM Publishing Company: Constanța city, Romania, 2008; pp. 504–509. ISBN 978-973-7895-33-2. [Google Scholar]
- Yi, Y.J.; Zhang, S.H. The relationships between fish heavy metal concentrations and fish size in the upper and middle reach of Yangtze River. Procedia Environ. Sci. 2012, 13, 1699–1707. [Google Scholar] [CrossRef] [Green Version]
- Merciai, R.; Guasch, H.; Kumar, A.; Sabater, S. Trace metal concentration and fish size: Variation among fish species in a Mediterranean river. Ecotoxicol. Environ. Saf. 2014, 107, 154–161. [Google Scholar] [CrossRef]
- Monsefrad, F.; Imanpour, N.; Heidary, S. Concentration of heavy and toxic metals Cu, Zn, Cd, Pb and Hg in liver and muscles of Rutilus frisii kutum during spawning season with respect to growth parameters, Iran. J. Fish Sci. 2012, 11, 825–839. [Google Scholar]
- Maximov, V.; Zaharia, T.; Nicolaev, S.; Radu, G. State of the Romanian Black Sea Turbot (Psetta maxima maoetica L.) resources. Rech. Mar. 2013, 43, 296–306. [Google Scholar]
- Eryilmaz, L.; Dalyan, C. Age, growth, and reproductive biology of turbot, Scophthalmus maximus (Actinopterygii: Pleuronectiformes: Scophthalmidae), from the south-western coasts of Black Sea, Turkey. Acta Ichthyol. Piscat. 2015, 45, 181–188. [Google Scholar] [CrossRef]
- Strungaru, S.A.; Nicoara, M.; Jitar, O.; Plavan, G. Influence of urban activity in modifying water parameters, concentration and uptake of heavy metals in Typha latifolia L. into a river that crosses an industrial city. JEHSE 2015, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bulska, E.; Krata, A.; Kalabun, M.; Wojciechowski, M. On the use of certified reference materials for assuring the quality of results for the determination of mercury in environmental samples. Environ. Sci. Pollut. Res. 2017, 24, 7889–7897. [Google Scholar] [CrossRef] [Green Version]
- Bury, N.; Walker, P.A.; Glover, C.N. Nutritive metal uptake in teleost fish. J. Exp. Biol. 2003, 206, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations—United Nations Development Program. ADCP/REP/80/11—Fish Feed Technology; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980; Chapter 7; ISBN 92-5-100901-5. [Google Scholar]
- Wei, Y.; Zhang, J.Y.; Zhang, D.W.; Tu, T.H.; Luo, L.G. Metal concentrations in various fish organs of different fish species from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2014, 104, 182–188. [Google Scholar] [CrossRef]
- Bijvelds, M.J.C.; Velden, V.D.; Kolar, Z.I.; Flik, G. Magnesium transport in freshwater teleosts. J. Exp. Biol. 1998, 201, 1981–1990. [Google Scholar]
- Vijayan, D.K.; Jayarani, R.; Singh, D.K.; Chatterjee, N.S.; Mathew, S.; Mohanty, B.P.; Sankar, T.V.; Anandan, R. Comparative studies on nutrient profiling of two deep sea fish (Neoepinnula orientalis and Chlorophthalmus corniger) and brackish water fish (Scatophagus argus). JOBAZ 2016, 77, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Heath, A. Uptake, Accumulation, Biotransformation, and Excretion of Xenobiotics, Water Pollution and Fish Physiology, Library of Congress Cataloging in Publication data, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 79–86. [Google Scholar]
- Simionov, I.A.; Cristea, V.; Petrea, S.M.; Bocioc Sîrbu, E. Evaluation of heavy metals concentration dynamics in fish from the Black Sea coastal area: An overview. Environ. Eng. Manag. J. 2019, 18, 1097–1110. [Google Scholar]
- Trifan, A.; Breaban, I.G.; Sava, D.; Bucur, L.; Toma, C.C.; Miron, A. Heavy metal content in macroalgae from Roumanian Black Sea. Rev. Roum. Chim. 2015, 60, 915–920. [Google Scholar]
- El-Moselhy, K.M.; Othman, A.I.; El-Azem, H.A.; El-Metwally, M.E.A. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. EJBAS 2014, 1, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.M.; Shu, L.H.; Liu, J.H. Anti-oxidative functions of mt2 and smtB mRNA expression in the gills and brain of zebrafish (Danio rerio) upon cadmium exposure. Fish Physiol. Biochem. 2016, 42, 1709–1720. [Google Scholar] [CrossRef]
- Yuan, S.S.; Lv, Z.M.; Zhu, A.Y.; Zheng, J.L.; Wu, C.W. Negative effect of chronic cadmium exposure on growth, histology, ultrastructure, antioxidant and innate immune responses in the liver of zebrafish: Preventive role of blue light emitting diodes. Ecotoxicol. Environ. Saf. 2017, 139, 18–26. [Google Scholar] [CrossRef]


| Measured Element | Certified Value Mean ± SD (µg∙g−1) | Measured Value Mean ± SD (µg∙g−1) | Analytical Method | Number of Replicates |
|---|---|---|---|---|
| As | 12.7 ± 0.7 | 11.3 ± 0.4 | HR-CS GF-AAS | 6 |
| Ca | 342 | 340 ± 6 | FL-AAS | 6 |
| Cd | 0.0075 ± 0.0018 | 0.0077 ± 0.002 | HR-CS GF-AAS | 6 |
| Cu | 1.67 ± 0.16 | 1.62 ± 0.27 | HR-CS GF-AAS | 6 |
| Fe | 9.4 | 9.5 ± 0.3 | FL-AAS | 6 |
| K | 21400 | 21381 ± 21 | FL-AAS | 6 |
| Mg | 1370 | 1372 ± 7 | FL-AAS | 6 |
| Mn | 0.368 ± 0.028 | 0.361 ± 0.034 | HR-CS GF-AAS | 6 |
| Na | 2800 | 2771 ± 27 | FL-AAS | 6 |
| Zn | 16 ± 1.1 | 15 ± 1.7 | FL-AAS | 6 |
| Biometric Measurement | Gender | |
|---|---|---|
| Female | Male | |
| Total length (cm) | 44.60 ± 1.86 | 48.3 ± 1.55 |
| Maximum width (cm) | 32.6 ± 2.15 | 35.1 ± 0.28 |
| Individual biomass (kg) | 1.83 ± 0.29 | 2.32 ± 0.12 |
| Distance between eyes (cm) | 1.4 ± 0.05 | 1.5 ± 0.02 |
| Caudal fin length (cm) | 8.24 ± 0.05 | 10.18 ± 0.3 |
| Head length (cm) | 8.74 ± 0.67 | 10.21 ± 0.49 |
| Head maximum width (cm) | 8.9 ± 0.02 | 12.56 ± 0.56 |
| Element | Gender | Gills | Stomach | Stomach Content | Intestine | Intestine Content | Liver | Spleen | Gonads | Muscles | Caudal Fin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Female | 180.2 ± 1.1 | 208.9 ± 24.9a | 347.3 ± 50.5a | 307.6 ± 31.2 | 537.9 ± 29.6a | 97.8 ± 3.5a | 143.4 ± 5.6a | 86.2 ± 16.7a | 175.3 ± 13.7a | 4127.1 ± 51 |
| Male | 179 ± 5.9 | 122.6 ± 0.5a | 197.6 ± 19.2a | 274.1 ± 75.9 | 425.9 ± 46.4a | 113.8 ± 6.4a | 113.4 ± 14.4a | 105.7 ± 11.8a | 278.3 ± 98.6a | 4090.9 ± 77.6 | |
| Mg | Female | 149.9 ± 12.4a | 498.6 ± 22.1a | 546.7 ± 8.6a | 977.7 ± 31.6a | 1285.1 ± 170a | 524.3 ± 37.5a | 596.2 ± 54.8a | 228.5 ± 20.3a | 482.5 ± 28.9a | 1574.2 ± 75.3a |
| Male | 273.1 ± 29.9a | 372.1 ± 49.5a | 489.4 ± 45.1a | 793.5 ± 58.5a | 443.1 ± 374.3a | 345.2 ± 6.3a | 415.1 ± 30.9a | 492.8 ± 34.1a | 553.5 ± 32.9a | 1468.4 ± 9.03a | |
| Na | Female | 1029.1 ± 348.8a | 1956.3 ± 381.1a | 2069.2 ± 179.7a | 1592.4 ± 149.5a | 2035.2 ± 19.2a | 1375.5 ± 85.2a | 1868.9 ± 152.1 | 1599.5 ± 89.2a | 926.5 ± 69.1a | 2824.5 ± 168.7a |
| Male | 2713.8 ± 125.4a | 2456.4 ± 52.8a | 2750.7 ± 142.2a | 2269.1 ± 137.7a | 2179.4 ± 147.3a | 1647.5 ± 12.9a | 1888.3 ± 94.4 | 2356.7 ± 19.8a | 1306.5 ± 56.7a | 3295.9 ± 300.8a | |
| K | Female | 986.2 ± 36.5a | 1971.5 ± 575a | 2273.4 ± 85a | 2585.9 ± 76.1a | 3330.5 ± 279.5a | 5516.1 ± 47.7a | 4899.1 ± 162.6a | 3011 ± 154.6a | 5917.3 ± 165.7a | 1162.7 ± 41.4a |
| Male | 1933.7 ± 453a | 3410.3 ± 59.7a | 3883.5 ± 283.9a | 4576.6 ± 286.9a | 4204.1 ± 91.2a | 4261.9 ± 570.2a | 4678.1 ± 353.5a | 3352.6 ± 122.4a | 6085 ± 284.35a | 1415.7 ± 117.9a | |
| Zn | Female | 22.1 ± 1.1a | 23.2 ± 1.9a | 17.9 ± 1.1a | 23.93 ± 0.55a | 25.6 ± 4.7a | 26.5 ± 0.8a | 36 ± 0.56a | 39.1 ± 2.6a | 10.3 ± 2.9a | 34.2 ± 1.3a |
| Male | 24 ± 1.7a | 34.8 ± 2.2a | 38.6 ± 1.06a | 31.4 ± 2.3a | 40.3 ± 7.3a | 30.6 ± 1.7a | 32.3 ± 0.2a | 31.6 ± 0.3a | 14.06 ± 1.43a | 47.15 ± 0.07a | |
| Fe | Female | 144.8 ± 18.2a | 25.5 ± 5.1a | 56.1 ± 23.03a | 20.3 ± 3.7a | 21.05 ± 0.55 | 51.7 ± 5.9a | 169.1 ± 19.3a | 15.1 ± 1.8a | 12.25 ± 2.39 | 52.5 ± 18.4 |
| Male | 109.4 ± 6.5a | 20.3 ± 4.2a | 30.05 ± 3.02a | 28.5 ± 2.1a | 23.25 ± 4.7 | 68.9 ± 6.5a | 154.6 ± 9.8a | 10.4 ± 0.1a | 6.01 ± 1.25 | 25.09 ± 7.68 | |
| Cu | Female | 0.47 ± 0.01a | 0.5 ± 0.03a | 0.72 ± 0.05a | 0.71 ± 0.02a | 1.06 ± 0.14a | 2.7 ± 0.1a | 0.62 ± 0.04a | 0.7 ± 0.07a | 0.15 ± 0.01 | 0.58 ± 0.09a |
| Male | 0.61 ± 0.03a | 0.99 ± 0.03a | 1.17 ± 0.06a | 1.29 ± 0.09a | 1.52 ± 0.11a | 3.4 ± 0.3a | 1.04 ± 0.18a | 0.62 ± 0.01a | 0.15 ± 0.01 | 0.72 ± 0.001a | |
| Ni | Female | 0.13 ± 0.02 | 0.10 ± 0.001a | 0.09 ± 0.00a | 0.08 ± 0.001a | 0.10 ± 0.001a | 0.2 ± 0.001a | 0.16 ± 0.02a | 0.08 ± 0.01 | 0.09 ± 0.02a | 2.15 ± 0.66a |
| Male | 0.12 ± 0.01 | 0.13 ± 0.001a | 0.07 ± 0.001a | 0.12 ± 0.001a | 0.12 ± 0.001a | 0.14 ± 0.001a | 0.10 ± 0.001a | 0.07 ± 0.00 | 0.12 ± 0.02a | 0.99 ± 0.07a | |
| Mn | Female | 1.37 ± 0.06a | 0.22 ± 0.11a | 1.05 ± 0.09a | 0.52 ± 0.04 | 1.1 ± 0.2 | 0.6 ± 0.3a | 0.38 ± 0.03 | 0.16 ± 0.07 | 0.19 ± 0.01 | 0.04 ± 0.001 |
| Male | 1.66 ± 0.01a | 0.42 ± 0.03a | 0.30 ± 0.17a | 0.56 ± 0.07 | 1.05 ± 0.02 | 0.5 ± 0.15a | 0.30 ± 0.01a | 0.21 ± 0.11 | 0.15 ± 0.07a | 0.05 ± 0.01a | |
| As | Female | 0.18 ± 0.03a | 0.73 ± 0.02a | 0.85 ± 0.16a | 1.8 ± 0.4a | 2.06 ± 0.31a | 9.31 ± 4.88 | 0.70 ± 0.68a | 0.18 ± 0.04a | 3.82 ± 0.95a | 9.87 ± 1.34a |
| Male | 0.21 ± 0.01a | 1.90 ± 0.49a | 1.47 ± 0.28a | 2.3 ± 0.5a | 2.85 ± 0.04a | 7.52 ± 2.43 | 1.42 ± 0.7a | 0.88 ± 0.12a | 3.80 ± 1.32 | 12.31 ± 0.89a | |
| Cd | Female | 0.06 ± 0.01a | 0.03 ± 0.001 | 0.04 ± 0.01a | 0.09 ± 0.02 | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.05 ± 0.001a | 0.03 ± 0.0001 | 0.03 ± 0.001 | 0.03 ± 0.001a |
| Male | 0.08 ± 0.01a | 0.03 ± 0.001 | 0.08 ± 0.01a | 0.11 ± 0.03 | 0.11 ± 0.05 | 0.09 ± 0.02 | 0.03 ± 0.001a | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.13 ± 0.05a |
| Element | Male | ||||||||
| Stomach | Intestine Content | Intestine | Liver | Spleen | Gonads | Muscle | Caudal Fin | Gills | |
| Ca | 0.62 | 2.16 | 1.39 | 0.58 | 0.57 | 0.54 | 1.41 | 20.70 | 0.91 |
| Mg | 0.76 | 0.91 | 1.62 | 0.71 | 0.85 | 1.01 | 1.13 | 3.00 | 0.56 |
| Na | 0.89 | 0.79 | 0.82 | 0.60 | 0.69 | 0.86 | 0.47 | 1.20 | 0.99 |
| K | 0.88 | 1.08 | 1.18 | 1.10 | 1.20 | 0.86 | 1.57 | 0.36 | 0.50 |
| Zn | 0.90 | 1.05 | 0.81 | 0.80 | 0.84 | 0.82 | 0.36 | 1.22 | 0.62 |
| Fe | 0.68 | 0.77 | 0.95 | 2.29 | 5.14 | 0.35 | 0.20 | 0.83 | 3.64 |
| Cu | 0.85 | 1.29 | 1.10 | 2.91 | 0.89 | 0.53 | 0.13 | 0.62 | 0.52 |
| Mn | 1.39 | 3.43 | 1.82 | 1.89 | 1.00 | 0.69 | 0.50 | 0.17 | 5.41 |
| Ni | 1.69 | 1.61 | 1.59 | 1.81 | 1.35 | 0.98 | 1.55 | 12.86 | 1.58 |
| Cd | 0.44 | 1.28 | 1.23 | 1.06 | 0.43 | 0.40 | 0.38 | 1.45 | 0.90 |
| As | 1.29 | 1.94 | 1.63 | 5.11 | 0.97 | 0.60 | 2.58 | 8.35 | 0.15 |
| Female | |||||||||
| Ca | 0.59 | 1.54 | 0.88 | 0.28 | 0.41 | 0.24 | 0.5 | 11.89 | 0.42 |
| Mg | 0.91 | 2.35 | 1.79 | 0.96 | 1.09 | 0.42 | 0.88 | 2.88 | 0.27 |
| Na | 0.95 | 0.98 | 0.77 | 0.66 | 0.90 | 0.77 | 0.45 | 1.36 | 0.50 |
| K | 0.87 | 1.46 | 1.14 | 2.43 | 2.15 | 1.32 | 2.60 | 0.51 | 0.43 |
| Zn | 1.30 | 1.43 | 1.33 | 1.48 | 2.01 | 2.18 | 0.57 | 1.91 | 1.24 |
| Fe | 0.46 | 0.38 | 0.36 | 0.92 | 3.01 | 0.27 | 0.22 | 0.94 | 2.58 |
| Cu | 0.82 | 1.47 | 0.98 | 3.84 | 0.86 | 1.04 | 0.22 | 0.80 | 0.66 |
| Mn | 0.21 | 1.09 | 0.50 | 0.62 | 0.37 | 0.16 | 0.18 | 0.04 | 1.30 |
| Ni | 1.07 | 1.14 | 0.96 | 2.16 | 1.74 | 0.89 | 1.04 | 23.15 | 1.40 |
| Cd | 0.87 | 2.46 | 2.16 | 2.05 | 1.25 | 0.71 | 0.71 | 0.86 | 1.48 |
| As | 0.86 | 2.42 | 2.20 | 10.92 | 0.82 | 0.22 | 4.48 | 11.57 | 0.22 |
| References | Macroelements | Microelements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Na | K | Zn | Fe | Cu | Ni | Mn | As | Cd | Cr | Pb | |
| Present study (f.w.) | 176.8 ± 122.5 | 518.1 ± 46.6 | 1116.5 ± 199.7 | 6001.2 ± 241.8 | 12.18 ± 2.95 | 9.13 ± 3.63 | 0.15 ± 0.01 | 0.10 ± 0.02 | 0.17 ± 0.05 | 3.81 ± 1.12 | 0.03 ± 0.001 | < LOD | < LOD |
| [41] (f.w.) | 90 ± 17 | 240 ± 20 | 1084 ± 96 | 2836 ± 183 | 6.1 ± 1.2 | - | - | - | - | 4.6 ± 1.10 | - | - | - |
| [40] (d.w.) | - | - | - | - | 21.4 ± 5.38 | 48.6 ± 9.06 | 0.75 ± 0.25 | - | - | - | 0.021 ± 0.005 | 1.24 ± 0.38 | 0.42 ± 0.10 |
| [22] (f.w.) | 110 ± 40 | 240 ± 30 | 900 ± 100 | 3200 ± 300 | 6.8 ± 0.5 | 2.6 ± 0.4 | 0.17 ± 0.05 | 0.02 ± 0.0 | 0.32 ± 0.11 | - | 0.007 ± 0.005 | 0.28 ± 0.08 | 0.05 ± 0.01 |
| [23] (d.w.) | - | - | - | - | 1.2 ± 0.1 | 0.8 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | <0.1 ± 0.0 | - | < 0.1 ± 0.0 | 0.3 ± 0.1 | < 0.1 ± 0.0 |
| [39] (d.w.) | - | - | - | - | 24.83 ± 1.71 | 21.72 ± 0.83 | 2.13 ± 0.21 | 3.22 ± 0.47 | 3.26 ± 0.32 | - | 0.022 ± 0.007 | - | 0.73 ± 0.21 |
| [38] (d.w.) | - | - | - | - | - | - | - | - | - | 1.56 ± 0.02 | < 0.02 | - | < 0.05 |
| [37] (f.w.) | - | - | - | - | 32.93 | 39.84 | 5.05 | 4.504 | 24.22 | - | 0.053 | - | 0.525 |
| [35] (d.w.) | - | - | - | - | 45.2 ± 2.7 | 36.2 ± 2.4 | 0.75 ± 0.05 | 3.60 ± 0.21 | 3.67 ± 0.22 | 0.15 ± 0.01 | 0.1 ± 0.01 | 1.2 | 0.28 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionov, I.-A.; Cristea, V.; Petrea, S.-M.; Mogodan, A.; Nicoara, M.; Baltag, E.S.; Strungaru, S.-A.; Faggio, C. Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender. J. Mar. Sci. Eng. 2019, 7, 466. https://doi.org/10.3390/jmse7120466
Simionov I-A, Cristea V, Petrea S-M, Mogodan A, Nicoara M, Baltag ES, Strungaru S-A, Faggio C. Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender. Journal of Marine Science and Engineering. 2019; 7(12):466. https://doi.org/10.3390/jmse7120466
Chicago/Turabian StyleSimionov, Ira-Adeline, Victor Cristea, Stefan-Mihai Petrea, Alina Mogodan, Mircea Nicoara, Emanuel Stefan Baltag, Stefan-Adrian Strungaru, and Caterina Faggio. 2019. "Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender" Journal of Marine Science and Engineering 7, no. 12: 466. https://doi.org/10.3390/jmse7120466
APA StyleSimionov, I.-A., Cristea, V., Petrea, S.-M., Mogodan, A., Nicoara, M., Baltag, E. S., Strungaru, S.-A., & Faggio, C. (2019). Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender. Journal of Marine Science and Engineering, 7(12), 466. https://doi.org/10.3390/jmse7120466







