The Effect of Cold and Warm Anomalies on Phytoplankton Pigment Composition in Waters off the Northern Baja California Peninsula (México): 2007–2016
Abstract
:1. Introduction
2. Materials and Methods
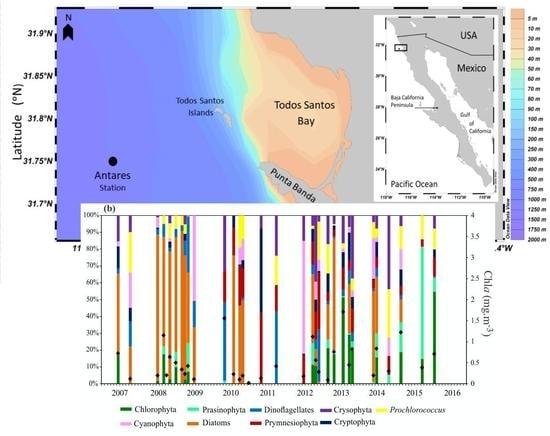
2.1. Sampling Procedure
2.2. HPLC Pigment Analysis
2.3. CHEMTAX Analysis of Pigment Data
2.4. Satellite Data and Climatic Indices
2.5. Statistical Analysis
3. Results
3.1. Satellite Data and Climatic Indices
3.2. Nutrients and Light
3.3. Chlorophyll a Concentration and Phytoplankton Community Composition
3.4. Relationship between Phytoplankton Groups and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plank. Res. 2010, 32, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Miloslavich, P.; Bax, N.J.; Simmons, S.E.; Klein, E.; Appeltans, W.; Aburto-Oropeza, O.; Garcia, M.A.; Batten, S.D.; Benedetti-Cecchi, L.; Checkley, D.M., Jr.; et al. Essential ocean variables for global sustained observations of biodiversity and ecosystem changes. Glob. Chang. Biol. 2018, 24, 2416–2433. [Google Scholar] [CrossRef] [PubMed]
- Polovina, J.J.; Howell, E.A.; Abecassis, M. Ocean’s least productive waters are expanding. Geophys. Res. Lett. 2008, 35, L03618. [Google Scholar] [CrossRef] [Green Version]
- Boyce, D.G.; Lewis, M.L.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellnera, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, X.A.; Lopez-Urrutia, A.; Calvo-Diaz, A.; Li, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Cheung, W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Zeller, D.; Pauly, D. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Chang. Biol. 2010, 16, 24–35. [Google Scholar] [CrossRef]
- Durazo, R. Seasonality of the transitional region of the California Current System off Baja California. J. Geophys. Res. 2015, 120, 1173–1196. [Google Scholar] [CrossRef]
- Castro, R.; Martinez, A. Variabilidad espacial y temporal del campo de viento. In Dinámica del Ecosistema Pelágico Frente a Baja California: 1997–2007, 1st ed.; Gaxiola-Castro, G., Durazo, R., Eds.; Semarnat, Ine, Cicese, Uabc: Ensenada, México, 2010; Volume 1, pp. 129–148. [Google Scholar]
- Linacre, L.P.; Durazo, R.; Hernández–Ayón, M.; Delgadillo-Hinojosa, F.; Cervantes-Díaz, G.; Lara-Lara, J.R.; Camacho-Ibar, V. Temporal variability of the physical and chemical water characteristics at a coastal monitoring observatory: Station ENSENADA. Cont. Shelf Res. 2010, 30, 1730–1742. [Google Scholar] [CrossRef]
- Gaxiola, G.G.; Cepeda-Morales, J.; Nájera-Mártinez, S.; Espinosa-Carreón, T.L.; De la Cruz-Orozco, M.E.; Sosa-Avalos, R.; Aguirre-Hernández, E.; Cantú-Ontiveros, J.P. Biomasa y producción del fitoplancton. In Dinámica del Ecosistema Pelágico Frente a Baja California: 1997–2007, 1st ed.; Gaxiola-Castro, G., Durazo, R., Eds.; Semarnat, Ine, Cicese, Uabc: Ensenada, México, 2010; Volume 1, pp. 59–86. [Google Scholar]
- Lavaniegos, B.E.; Molina-González, O.; Murcia-Riaño, M. Zooplankton functional groups from the California Current and climate variability during 1997–2013. Cicimar Oceán. 2015, 30, 45–62. [Google Scholar]
- Lynn, R.J.; Simpson, J.J. The California Current system: The seasonal variability of its physical characteristics. J. Geophys. Res. 1987, 103, 30825–30853. [Google Scholar] [CrossRef]
- Gómez-Ocampo, E.; Gaxiola-Castro, G.; Durazo, R.; Beier, E. Effects of the 2013–2016 warm anomalies on the California Current phytoplankton. Deep-Sea Res. P. Ii 2017, 151, 64–74. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U.; Bravo–Sierra, E.; Aké-Castillo, J.A. Phytoplankton on the western coast of Baja California in two different seasons in 1998. Sci. Mar. 2007, 71, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Martínez-López, A.; Silverberg, N.; Gaxiola-Castro, G.; Verdugo-Díaz, G.; Nájera-Martínez, N. Fitoplancton silíceo en Cuenca San Lázaro durante las condiciones La Niña 1996 y El Niño 1997–1998. In Dinámica del Ecosistema Pelágico Frente a Baja California: 1997–2007, 1st ed.; Gaxiola-Castro, G., Durazo, R., Eds.; Semarnat, Ine, Cicese, Uabc: Ensenada, México, 2010; Volume 1, pp. 277–290. [Google Scholar]
- Millán-Núñez, E. Variabilidad interanual del nano–microfitoplancton: Inviernos 2001–2007. In Dinámica del Ecosistema Pelágico Frente a Baja California: 1997–2007, 1st ed.; Gaxiola-Castro, G., Durazo, R., Eds.; Semarnat, Ine, Cicese, Uabc: Ensenada, México, 2010; Volume 1, pp. 241–262. [Google Scholar]
- Amaya, D.J.; Miller, A.J.; DeFlorio, M.J. The evolution and known atmospheric forcing mechanisms behind the 2013–2015 North Pacific warm anomalies. US Clivar Var. 2016, 14, 1–6. [Google Scholar]
- Bond, N.A.; Cronin, M.F.; Freeland, H.; Mantua, N. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 2015, 42, 3414–3420. [Google Scholar] [CrossRef]
- Ohman, M.D. Introduction to collection of papers on the response of the southern California Current Ecosystem to the Warm Anomaly and El Niño, 2014–2016. Deep-Sea Res. 2018, 140, 1–3. [Google Scholar] [CrossRef]
- Schiermeier, Q. Hunting the Godzilla El Niño. Nature 2015, 526, 490–491. [Google Scholar] [CrossRef]
- Jacox, M.-G.; Hazen, E.L.; Zaba, K.D.; Rudnick, D.L.; Edwards, C.A.; Moore, A.M.; Bograd, S.J. California Current System: Early assessment and comparison to past events. Geophys. Res. Lett. 2016, 43, 7072–7080. [Google Scholar] [CrossRef]
- Leising, A.W.; Schroeder, I.D.; Bograd, S.J.; Abell, J.; Durazo, R.; Gaxiola-Castro, G.; Bjorkstedt, E.P.; Field, J.; Sakuma, K.; Robertson, R.R.; et al. State of the California Current 2014-15: Impacts of the warm-water “Blob”. Calif. Coop. Ocean. Fish. Investig. Rep. 2015, 56, 31–68. [Google Scholar]
- Durazo, R.; Castro, R.; Miranda, L.E.; Delgadillo-Hinojosa, F.; Mejía-Trejo, A. Anomalous hydrographic conditions off the northwestern coast of the Baja California Peninsula during 2013–2016. Cien. Mar. 2017, 43, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Cavole, L.M.; Demko, A.M.; Diner, R.E.; Giddings, A.; Koester, I.; Pagniello, C.M.L.S.; Paulsen, M.L.; Ramirez-Valdez, A.; Schwenck, S.M.; Yen, N.K.; et al. Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific: Winners, losers, and the future. Oceanography 2016, 29, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Siedlecki, S.; Bjorkstedt, E.; Feely, R.; Sutton, A.; Cross, J.; Newton, J. Impact of the Blob on the Northeast Pacific Ocean biogeochemistry and ecosystems. US Clivar Var. 2016, 14, 7–12. [Google Scholar]
- Sánchez-Velasco, L.; Beier, E.; Godínez, V.M.; Barton, E.D.; Santamaría-del-Angel, E.; Jiménez-Rosemberg, S.P.A.; Marinone, S.G. Hydrographic and Fish Larvae Distribution during the “Godzilla El Niño 2015–2016” in the Northern End of the Shallow Oxygen Minimum Zone of the Eastern Tropical Pacific Ocean. J. Geophys. Res. 2017, 122. Available online: https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/2016JC012622 (accessed on 28 February 2017). [CrossRef]
- Kahru, M.; Jacox, M.G.; Ohman., M.D. Decemberrease in the frequency of oceanic fronts and surface chlorophyll concentration in the California Current System during the 2014–2016 northeast Pacific warm anomalies. Deep Sea-Res. 2018, 140, 4–13. [Google Scholar] [CrossRef]
- Morrow, R.M.; Ohman, M.D.; Goericke, R.; Kelly, T.B.; Stephens, B.M.; Stukel, M.R. Primary production, mesozooplankton grazing, and the biological pump in the California Current Ecosystem: Variability and response to El Niño. Deep Sea-Res. 2018, 140, 52–62. [Google Scholar] [CrossRef]
- Linacre, L.P.; Lara-Lara, J.R.; Mirabal-Gómez, U.; Durazo, R.; Bazán-Guzmán, C. Microzooplankton grazing impact on the phytoplankton community at a coastal upwelling station off northern Baja California, Mexico. Cien. Mar. 2017, 43, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Quiroz, M.C.; Cervantes-Duarte, R.; Funes-Rodríguez, R.; Barón-Campis, S.A.; García-Romero, F.J.; Hernández-Trujillo, S.; Hernández-Becerril, D.U.; González-Armas, R.; Martell-Dubois, R.; Cerdeira-Estrada, S.; et al. Impact of “The Blob” and “El Niño” in the SW Baja California Peninsula: Plankton and Environmental Variability of Bahia Magdalena. Front. Mar. Sci. 2019, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 1st ed.; Cambridge University Press: Cambridge, UK, 1994; p. 509. [Google Scholar]
- Gordon, L.I.; Jennings, J.C., Jr.; Ross, A.A.; Krest, J.M. A Suggested Protocol for Continuous Flow Automated Analysis of Seawater Nutrients (Phosphate, Nitrate, Nitrite and Silicic Acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study; Methods Manual WHPO 91-1; WOCE Hydrographic Program Office: La Jolla, CA, USA, 1993.
- Barlow, R.G.; Cummings, D.G.; Gibb, S.W. Improved resolution of mono– and divinyl chlorophylls a and b and zeaxanthin and lutein in phytoplankton extracts using reverse phase C–8 HPLC. Mar. Ecol. Progr. Ser. 1997, 161, 303–307. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Thomas, C. The HPLC Method. In The Fifth SeaWiFS HPLC Analysis Round–Robin Experiment (SeaHARRE-5); Hooker, S.B., Clementson, L., Thomas, C.S., Schlüter, L., Allerup, M., Ras, J., Claustre, H., Normandeau, C., Cullen, J., Kienast, M., et al., Eds.; GSFC/NASA: Greenbelt, MD, USA, 2012; Chapter 6; pp. 63–72. [Google Scholar]
- Hooker, S.B.; Heukelem, L.V.; Thomas, C.S.; Claustre, H.; Ras, J.; Schlüter, L.; Clementson, L.; Linde, D.; Eker-Develi, E.; Berthon, J.-F.; et al. The Third SeaWiFS HPLC Analysis. Round–Robin Experiment (SeaHARRE–3); NASA/TM-2009-215849; NASA Goddard Space Flight Center: Greenbelt, MA, USA, 2009; p. 97.
- Hooker, S.B.; Clementson, L.; Thomas, C.S.; Schlüter, L.; Allerup, M.; Ras, J.; Schluter, L.; Clementson, L.; van der Linde, D.; Eker-Develi, E.; et al. The Fifth SeaWiFS HPLC Analysis Round–Robin Experiment (SeaHARRE–5); NASA/TM-2012-217503; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2012; p. 95.
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX-a program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Araujo, M.L.V.; Borges-Mendes, C.R.; Tavano, V.M.; Garcia, C.A.E.; Baringer, M.O. Contrasting patterns of phytoplankton pigments and chemotaxonomic groups along 30° S in the subtropical South Atlantic Ocean. Deep-Sea Res. 2017, 120, 112–121. [Google Scholar] [CrossRef]
- Higgins, H.W.; Wright, S.W.; Schlüter, L. Quantitative interpretation of chemotaxonomic pigment data. In Phytoplankon Pigments: Characterization, Chemotaxonomy and Applications in Oceanography, 1st ed.; Roy, S., Llewellyn, C.A., Egeland, S.A., Johnsen, G., Eds.; Cambridge University Press: London, UK, 2011; pp. 257–313. [Google Scholar]
- Jeffrey, S.W.; Writght, S.W.; Zapata, M. Microalgal classes and their signature pigments. In Phytoplankon Pigments: Characterization, Chemotaxonomy and Applications in Oceanography, 1st ed.; Roy, S., Llewellyn, C.A., Egeland, E., Johnsen, G., Eds.; Cambridge University Press: London, UK, 2011; Volume 1, pp. 3–77. [Google Scholar] [CrossRef]
- Goela, P.C.; Danchenko, S.; Icely, J.D.; Lubian, L.M.; Cristina, S.; Newton, A. Using CHEMTAX to evaluate seasonal and interannual dynamics of the phytoplankton community off the South–west coast of Portugal. Est. Coast. Shelf. Sci. 2004, 151, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.W.; Ishikawa, A.; Marchant, H.J.; Davidson, A.T.; van den Enden, R.L.; Nash, G.V. Composition and significance of picophytoplankton in Antarctic waters. Polar Biol. 2009, 32, 797–808. [Google Scholar] [CrossRef]
- Mantua, N.J.; Hare, S.R. The Pacific Decemberadal Oscillation. J. Ocean. 2002, 58, 35–44. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparison by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Wilcoxon, F.; Wilcoxon, R.A. Some Rapid Approximate Statistical Procedures; Pearl River Laboratories, Division of the American Cyanamid Company: New York, NY, USA, 1964; p. 66. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice–Hall Inc.: Upper Saddle River, NJ, USA, 2007; p. 756. [Google Scholar]
- Ohman, M.D.; Barbeau, K.; Franks, P.J.S.; Goericke, R.; Landry, M.R.; Miller, A.J. Ecological transitions in a coastal upwelling ecosystem. Oceanography 2013, 26, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Mejia, E.; Lares, M.L.; Huerta-Diaz, M.A.; Delgadillo-Hinojosa, F. Cadmium and phosphate variability during algal blooms of the dinoflagellate Lingulodinium polyedrum in Todos Santos Bay, Baja California, Mexico. Sci. Total Environ. 2016, 541, 865–876. [Google Scholar] [CrossRef]
- McClatchie, S.; Goericke, R.; Schwing, F.B.; Bograd, S.J.; Peterson, W.T.; Emmett, R.; Hill, K.; Collins, C.; Koslow, J.A.; Gomez-Valdes, J.; et al. The state of the California Current, spring 2008–2009: Cold conditions drive regional differences in coastal production. Calif. Coop. Ocean. Fish. Investig. Rep. 2009, 50, 43–68. [Google Scholar]
- Rodríguez, J.; Tintoré, J.; Allen, J.T.; Blanco, J.M.; Gomis, D.; Reul, A.; Ruiz, J.; Rodriguez, V.; Echevarría, F.; Jiménez-Gómez, F. Mesoscale vertical motion and the size structure of phytoplankton in the ocean. Nature 2001, 410, 360–363. [Google Scholar]
- Latasa, M.; Gutiérrez-Rodríguez, A.; Cabello, A.M.M.; Scharek, R. Influence of light and nutrients on the vertical distribution of marine phytoplankton groups in the deep chlorophyll maximum. Sci. Mar. 2016, 80, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Bjorkstedt, E.P.; Goericke, R.; McClatchie, S.; Weber, E.; William, W.; Lo, N.; Peterson, W.T.; Brodeur, R.D.; Auth, T.; Fisher, J.; et al. State of the California Current 2011–2012: Ecosystems respond to local forcing as La Niña wavers and wanes. Calif. Coop. Ocean. Fish. Investig. Rep. 2012, 53, 41–76. [Google Scholar]
- Venrick, E.L. Phytoplankton species in the California Current System off Southern California: The spatial dimensions. Calif. Coop. Ocean. Fish. Investig. Rep. 2015, 56, 168–184. [Google Scholar]
- Almazán-Becerril, A.; Rivas, D.; García-Mendoza, E. The influence of mesoscale physical structures in the phytoplankton taxonomic composition of the subsurface chlorophyll maximum off western Baja California. Deep-Sea Res. 2012, 70, 91–102. [Google Scholar] [CrossRef]
- L’Heureux, L.M.; Takahashi, K.; Watkins, A.B.; Barnston, A.G.; Becker, E.J.; Di Libert, T.E.; Wittenberg, A.T. Observing and predicting the 2015/16 El Niño. Bull. Am. Meteorol. Soc. 2017, 98, 1363–1382. [Google Scholar] [CrossRef]
- Bouman, H.A.; Ulloa, O.; Barlow, R.; Li, W.K.W.; Platt, T.; Zwirglmaier, K.; Scanlan, D.J.; Sathyendranath, S. Water–column stratification governs the community structure of subtropical marine picophytoplankton. Environ. Microbiol. Rep. 2011, 3, 473–482. [Google Scholar] [CrossRef]
- Linacre, L.P.; Landry, M.R.; Lara-Lara, J.R.; Hernández-Ayón, J.M.; Bazán-Guzmán, C. Picoplankton dynamics during contrasting seasonal oceanographic conditions at a coastal upwelling station off Northern Baja California, México. J. Plank. Res. 2010, 32, 539–557. [Google Scholar] [CrossRef]
- Lopes dos Santos, A.; Pollina, T.; Gourvil, P.; Corre, E.; Marie, D.; Garrido, J.L.; Rodríguez, F.; Noel, M.-H.; Vaulot, D.; Eikrem, W. Chloropicophyceae, a new class of picophytoplanktonic prasinophytes. Sci. Rep. 2017, 7, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Treusch, A.H.; Demir-Hilton, E.; Vergin, K.L.; Worden, A.Z.; Carlson, C.A.; Donatz, M.G.; Burton, R.M.; Giovannoni, S.J. Phytoplankton distribution patterns in the northwestern Sargasso Sea revealed by small subunit rRNA genes from plastids. ISME J. 2012, 6, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Demir-Hilton, E.; Sudek, S.; Cuvelier, M.; Gentemann, C.L.; Zehr, J.; Worden, A.Z. Global distribution patterns of distinct clades of the photosynthetic picoeukaryote Ostreococcus. ISME J. 2011, 5, 1095–1107. [Google Scholar] [CrossRef]
- Checkley, D.M.; Barth, J.A. Patterns and processes in the California Current System. Prog. Oceanogr. 2009, 83, 49–64. [Google Scholar] [CrossRef]
- Miranda-Álvarez, C.; González-Silvera, A.; Santamaria-del-Angel, E.; López-Calderón, J.; Godínez, V.M.; Sánchez-Velasco, L.; Hernández-Walls, R. Phytoplankton pigments and community structure in the northeastern tropical pacific using HPLC–CHEMTAX analysis. J. Oceanogr. 2020, 76, 91–208. [Google Scholar] [CrossRef]





| Year | Date | Year | Date | Year | Date |
|---|---|---|---|---|---|
| 2007 |
| 2010 |
| 2013 |
|
|
|
| |||
|
|
| |||
|
|
| |||
| 2008 |
| 2011 |
|
| |
|
| 2014 |
| ||
|
|
| |||
|
|
| |||
| 2009 |
|
|
| ||
| 2012 |
|
| ||
|
| 2015 |
| ||
|
|
| |||
|
| 2016 |
| ||
|
| Pigment | Abbreviation | Group |
|---|---|---|
| Monovinyl Chlorophyll b | Chlb | Chlorophytes, Prasinophytes |
| Chlorophyll c3 | Chlc3 | Prymnesiophytes, many Bacillariophytes |
| Divinyl Chlorophyll a | DVChla | Prochlorococcus |
| Fucoxanthin | Fuco | Bacillariophytes, Prymnesiophytes |
| Peridinin | Peri | Dinophytes |
| 19′Butanoyloxy fucoxanthin | 19′But | Chrysophytes, Prymnesiophytes |
| 19′Hexanoyloxy fucoxanthin | 19′Hex | Prymnesiophytes |
| Alloxanthin | Allo | Cryptophytes |
| Zeaxanthin | Zea | Cyanobacteria, Prochlorococcus, Chlorophytes |
| Prasinoxanthin | Pras | Prasinophytes |
| Group/Pigment | Chlb | 19′but | 19′hex | Allo | Fuco | Peri | Zea | DVChla | Chlc3 | Pras | Chla |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diatoms | 0 | 0 | 0 | 0 | 0.62 | 0 | 0 | 0 | 0 | 0 | 1 |
| Dinoflagellates | 0 | 0 | 0 | 0 | 0 | 0.56 | 0 | 0 | 0 | 0 | 1 |
| Prymnesiophytes | 0 | 0.05 | 0.42 | 0 | 0.27 | 0 | 0 | 0 | 0.17 | 0 | 1 |
| Chlorophytes | 0.32 | 0 | 0 | 0 | 0 | 0 | 0.03 | 0 | 0 | 0 | 1 |
| Cryptophytes | 0 | 0 | 0 | 0.38 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Prasinophytes | 0.70 | 0 | 0 | 0 | 0 | 0 | 0.06 | 0 | 0 | 0.24 | 1 |
| Cyanobacterias | 0 | 0 | 0 | 0 | 0 | 0 | 0.64 | 0 | 0 | 0 | 1 |
| Prochlorococcus | 0 | 0 | 0 | 0 | 0 | 0 | 0.39 | 1 | 0 | 0 | 0 |
| Chrysophytes | 0 | 0.35 | 0 | 0 | 0.52 | 0 | 0 | 0 | 0 | 0 | 1 |
| Group | nsurf | n40m | Hcalc | Group | nsurf | n40m | Hcalc |
|---|---|---|---|---|---|---|---|
| Diatoms | 26 | 25 | 4.065 | Prasinophytes | 9 | 16 | 71.157 |
| Dinoflagellates | 25 | 27 | 0.037 | Cyanobacteria | 24 | 22 | 4.739 |
| Prymnesiophytes | 25 | 27 | 0.141 | Prochlorococcus | 20 | 22 | 7.536 |
| Chlorophytes | 12 | 20 | 240.06 | Chrysophytes | 23 | 24 | 3.833 |
| Cryptophytes | 17 | 23 | 16.507 |
| 2007–2009 | 2010–2012 | 2013–2016 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Range | n | Average | Range | n | Average | Range | n | |
| Groups | |||||||||
| Diatoms* | 0.21 (46%) | 0.03–0.79 | 21 | 0.051 (12%) | 0–0.47 | 20 | 0.08 (15%) | 0–0.51 | 22 |
| Dinoflagellates | 0.12 (26%) | 0.01–0.92 | 21 | 0.12 (27%) | 0–1.22 | 20 | 0.033 (6.3%) | 0–0.29 | 22 |
| Prymnesiophytes* | 0 (0%) | 0–0.01 | 21 | 0.125 (29%) | 0.01–0.78 | 20 | 0.069 (13%) | 0.01–0.23 | 22 |
| Crysophytes | 0.024 (5.3%) | 0–0.19 | 21 | 0.06 (14%) | 0–0.51 | 20 | 0.07 (13%) | 0–0.33 | 22 |
| Cryptophytes | 0.014 (3.1%) | 0–0.09 | 21 | 0.016 (3.6%) | 0–0.09 | 20 | 0.033 (6.3%) | 0–0.16 | 22 |
| Chlorophytes* | 0.025 (5.5%) | 0–0.21 | 21 | 0.01 (2.3%) | 0–0.13 | 20 | 0.126 (24%) | 0–0.91 | 22 |
| Prasinophytes | 0.008 (1.8%) | 0–0.07 | 21 | 0.01 (2.3%) | 0–0.14 | 20 | 0.039 (7.4%) | 0–0.3 | 22 |
| Cyanobacteria | 0.036 (7.9%) | 0–0.29 | 21 | 0.027 (6.2%) | 0–0.12 | 20 | 0.033 (6.3%) | 0–0.17 | 22 |
| Prochlorococcus | 0.02 (4.4%) | 0–0.08 | 21 | 0.02 (4.6%) | 0–0.09 | 20 | 0.045 (8.5%) | 0–0.19 | 22 |
| Chla (mg m−3) | 0.40 | 0.04–3.25 | 55 | 0.34 | 0.02–2.07 | 63 | 0.44 | 0.04–2.03 | 60 |
| Satellite | |||||||||
| SST (°C)* | 17.28 | 14–21.7 | 36 | 16.94 | 14.4–21.3 | 36 | 18.72 | 14.3–23.6 | 48 |
| ChlaSAT (mg m−3)* | 1.32 | 0.26–6.54 | 36 | 0.63 | 0.17–2.25 | 36 | 0.50 | 0.16–2.05 | 48 |
| Nutrients (μM) and Ze (m) | |||||||||
| [NO3+NO2]* | 5.24 | 0.09–23.8 | 70 | 4.91 | 0.07–21 | 70 | 2.44 | 0–11.65 | 65 |
| H4SiO4 | 6.24 | 0.06–22.8 | 70 | 2.79 | 0.03–18.5 | 70 | 4.78 | 0.4–15.8 | 65 |
| PO4* | 0.87 | 0.14–2.17 | 70 | 2.45 | 0.21–1.45 | 70 | 0.45 | 0.22–1.18 | 65 |
| Ze* | 34 | 24–43 | 12 | 51 | 35–62 | 14 | 63 | 32–89 | 11 |
| Phytoplankton Group | Environmental Variable | rS | n |
|---|---|---|---|
| All data | |||
| Chla | [NO3+NO2] | 0.422 | 46 |
| Diatoms | [NO3+NO2] H4SiO4 PO4 | 0.431 0.490 0.436 | 46 |
| Cyanobacteria | [NO3+NO2] H4SiO4 PO4 | −0.455 −0.412 −0.415 | 46 |
| Surface | |||
| Chla | [NO3+NO2] | 0.421 | 24 |
| 40 m | |||
| Diatoms | [NO3+NO2] PO4 %Ez | 0.421 0.481 −0.609 | 23 |
| Cyanobacteria | [NO3+NO2] H4SiO4 PO4 | −0.658 −0.641 −0.535 | 23 |
| Prochlorococcus | [NO3+NO2] %Ez | −0.419 0.629 | 23 |
| %Ez | [NO3+NO2] PO4 | −0.503 −0.550 | 23 |
| SST | [NO3+NO2] H4SiO4 PO4 | −0.518 −0.494 −0.472 | 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Silvera, A.; Santamaría-del-Ángel, E.; Camacho-Ibar, V.; López-Calderón, J.; Santander-Cruz, J.; Mercado-Santana, A. The Effect of Cold and Warm Anomalies on Phytoplankton Pigment Composition in Waters off the Northern Baja California Peninsula (México): 2007–2016. J. Mar. Sci. Eng. 2020, 8, 533. https://doi.org/10.3390/jmse8070533
González-Silvera A, Santamaría-del-Ángel E, Camacho-Ibar V, López-Calderón J, Santander-Cruz J, Mercado-Santana A. The Effect of Cold and Warm Anomalies on Phytoplankton Pigment Composition in Waters off the Northern Baja California Peninsula (México): 2007–2016. Journal of Marine Science and Engineering. 2020; 8(7):533. https://doi.org/10.3390/jmse8070533
Chicago/Turabian StyleGonzález-Silvera, Adriana, Eduardo Santamaría-del-Ángel, Víctor Camacho-Ibar, Jorge López-Calderón, Jonatan Santander-Cruz, and Alfredo Mercado-Santana. 2020. "The Effect of Cold and Warm Anomalies on Phytoplankton Pigment Composition in Waters off the Northern Baja California Peninsula (México): 2007–2016" Journal of Marine Science and Engineering 8, no. 7: 533. https://doi.org/10.3390/jmse8070533
APA StyleGonzález-Silvera, A., Santamaría-del-Ángel, E., Camacho-Ibar, V., López-Calderón, J., Santander-Cruz, J., & Mercado-Santana, A. (2020). The Effect of Cold and Warm Anomalies on Phytoplankton Pigment Composition in Waters off the Northern Baja California Peninsula (México): 2007–2016. Journal of Marine Science and Engineering, 8(7), 533. https://doi.org/10.3390/jmse8070533