Seagrass of Vasiliko Bay, Eastern Mediterranean: Lost Cause or Priority Conservation Habitat?
Abstract
:1. Introduction
2. Materials and Methods
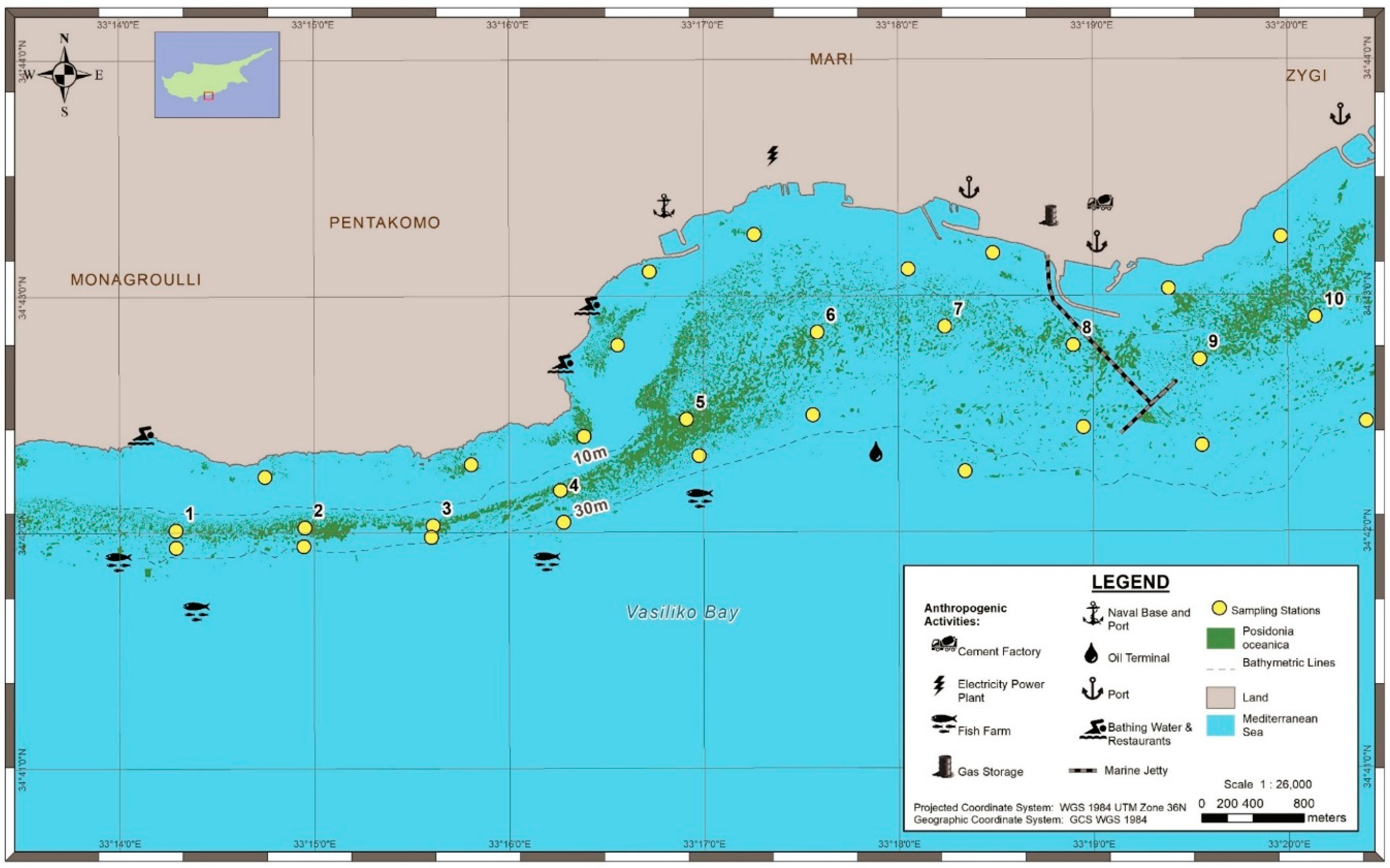
2.1. Study Area
2.2. Seagrass Mapping
2.3. Assessment of P. oceanica Meadows
2.3.1. Shoot Densities
2.3.2. Leaf Morphometrics and Epiphytes
2.3.3. Ecological Status
2.4. Statistical Analyses
3. Results
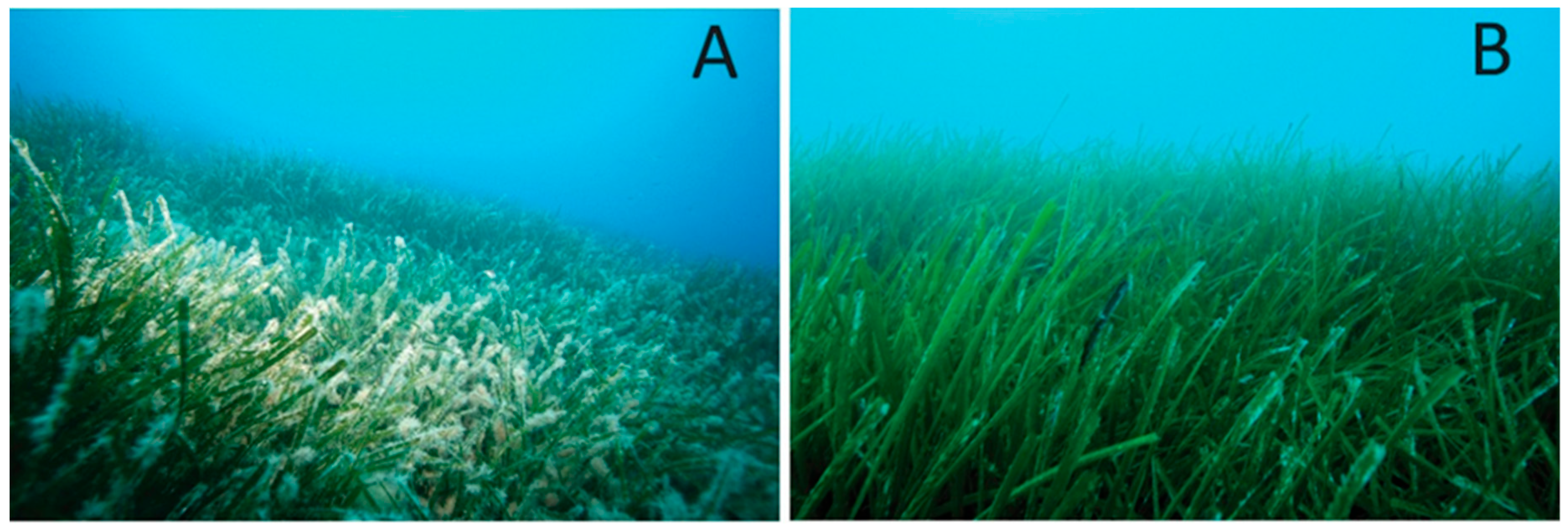
3.1. Mapping and Visual Observations
3.2. Posidonia oceanica Structural Descriptors
3.3. Main Drivers Affecting P. oceanica Descriptors
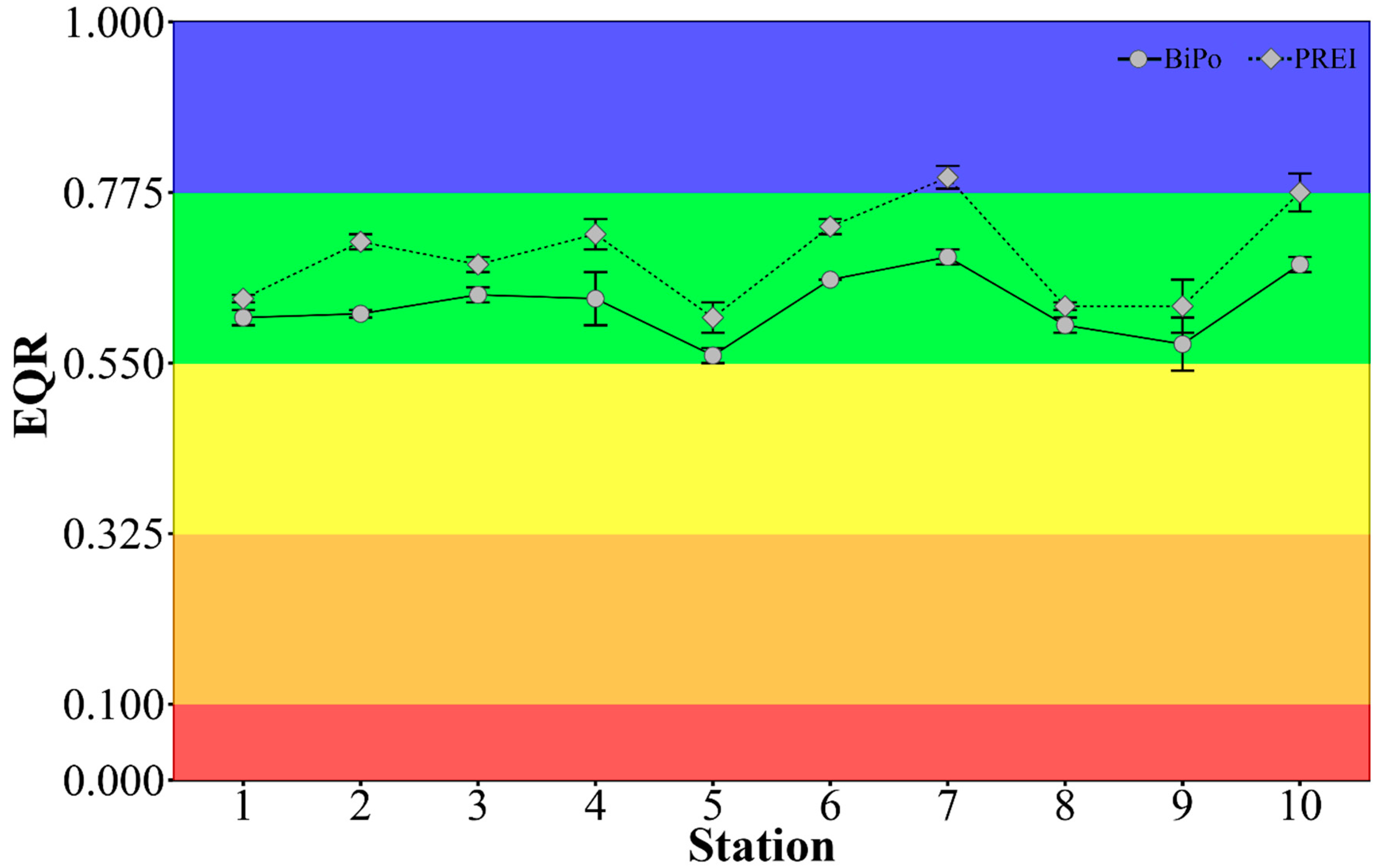
3.4. Ecological Status Class
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsworth, R.K.F.; Van Keulen, M.; Coles, R. Seagrass meadows in a globally changing environment. Mar. Pollut. Bull. 2014, 83, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Costanza, R.; De Groot, R.; Sutton, P.C.; Van Der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S.; et al. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998-13. [Google Scholar] [CrossRef] [Green Version]
- Lavery, P.S.; Mateo, M.A.; Serrano, O.; Rozaimi, M. Variability in the Carbon Storage of Seagrass Habitats and Its Implications for Global Estimates of Blue Carbon Ecosystem Service. PLoS ONE 2013, 8, e73748. [Google Scholar] [CrossRef] [Green Version]
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.A.; Montefalcone, M.; et al. Climate change and Mediterranean seagrass meadows: A synopsis for environmental managers. Mediterr. Mar. Sci. 2014, 15, 462. [Google Scholar] [CrossRef] [Green Version]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [Green Version]
- Topouzelis, K.; Makri, D.; Stoupas, N.; Papakonstantinou, A.; Katsanevakis, S. Seagrass mapping in Greek territorial waters using Landsat-8 satellite images. Int. J. Appl. Earth Obs. Geoinf. 2018, 67, 98–113. [Google Scholar] [CrossRef]
- Campagne, C.S.; Salles, J.-M.; Boissery, P.; Deter, J. The seagrass Posidonia oceanica: Ecosystem services identification and economic evaluation of goods and benefits. Mar. Pollut. Bull. 2015, 97, 391–400. [Google Scholar] [CrossRef]
- Jackson, E.; Rees, S.E.; Wilding, C.; Attrill, M.J. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 2015, 29, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Piazzi, L.; Balata, D.; Ceccherelli, G. Epiphyte assemblages of the Mediterranean seagrass Posidonia oceanica: An overview. Mar. Ecol. 2015, 37, 3–41. [Google Scholar] [CrossRef]
- Díaz-Almela, E.; Duarte, C.M. Management of Natura 2000 Habitats. 1120 *Posidonia Beds (Posidonion Oceanicae); European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Çinar, M. Polychaetes (Annelida: Polychaeta) Associated with Posidonia Oceanica (L.) Delile along the Coasts of Turkey and Northern Cyprus. In Proceedings of the First National Workshop on Posidonia oceanica (L.) Delile on the Coasts of Turkey, Gokceada, Turkey, 19–20 September 2013. [Google Scholar]
- Urra, J.; Mateo-Ramírez, Ángel; Marina, P.; Salas, C.; Gofas, S.; Rueda, J.L. Highly diverse molluscan assemblages of Posidonia oceanica meadows in northwestern Alboran Sea (W Mediterranean): Seasonal dynamics and environmental drivers. Estuarine Coast. Shelf Sci. 2013, 117, 136–147. [Google Scholar] [CrossRef]
- Zubak, I.; Schultz, S.; Kruschel, C.; Blindow, I. What drives fish community structure on Posidonia oceanica meadows? A review of available data across the Mediterranean basin. In Proceedings of the 4th Mediterranean Seagrass Workshop, Oristano, Italy, 18–22 May 2015. [Google Scholar]
- Marbá, N.; Krause-Jensen, D.; Alcoverro, T.; Birk, S.; Pedersen, A.; Neto, J.M.; Orfanidis, S.; Garmendia, J.M.; Muxika, I.; Borja, Á.; et al. Diversity of European seagrass indicators: Patterns within and across regions. Hydrobiologia 2012, 704, 265–278. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Boudouresque, C.-F.; Pasqualini, V.; Pergent, G. Impact of fish farming facilities on Posidonia oceanica meadows: A review. Mar. Ecol. 2006, 27, 310–319. [Google Scholar] [CrossRef]
- Apostolaki, E.T.; Marbá, N.; Holmer, M.; Karakassis, I. Fish farming enhances biomass and nutrient loss in Posidonia oceanica (L.) Delile. Estuarine Coast. Shelf Sci. 2009, 81, 390–400. [Google Scholar] [CrossRef]
- Bonacorsi, M.; Pergent-Martini, C.; Breand, N.; Pergent, G. Is Posidonia oceanica regression a general feature in the Mediterranean Sea? Mediterr. Mar. Sci. 2013, 14, 193. [Google Scholar] [CrossRef]
- Guillén, J.E.; Sánchez-Lizaso, J.L.; Jimenez, S.; Martínez, J.; Codina, A.; Montero, M.; Triviño, A.; Soler, G.; Zubcoff, J. Evolution of Posidonia oceanica seagrass meadows and its implications for management. J. Sea Res. 2013, 83, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Clabaut, P.; Harmelin, M.; Mateo, M.A.; Montefalcone, M.; Morri, C.; et al. Mediterranean Seagrass Meadows: Resilience and Contribution to Climate Change Mitigation. A Short Summary; IUCN: Málaga, Spain, 2012; p. 40. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, G.; Duarte, C.M.; Marbá, N. Clonality in seagrasses, emergent properties and seagrass landscapes. Mar. Ecol. Prog. Ser. 2005, 290, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Celebi, B.; Gucu, A.C.; Ok, M.; Sakinan, S.; Akoglu, E. Hydrographic indications to understand the absence of Posidonia oceanica in the Levant Sea (Eastern Mediterranean). Biol. Mar. Mediterr. 2006, 13, 34–38. [Google Scholar]
- Arnaud-Haond, S.; Marbá, N.; Diaz-Almela, E.; Serrão, E.A.; Duarte, C.M. Comparative Analysis of Stability—Genetic Diversity in Seagrass (Posidonia oceanica) Meadows Yields Unexpected Results. Chesap. Sci. 2009, 33, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Serra, I.A.; Innocenti, A.M.; Di Maida, G.; Calvo, S.; Migliaccio, M.; Zambianchi, E.; Pizzigalli, C.; Arnaud-Haond, S.; Duarte, C.M.; Serrão, E.A.; et al. Genetic structure in the Mediterranean seagrass Posidonia oceanica: Disentangling past vicariance events from contemporary patterns of gene flow. Mol. Ecol. 2010, 19, 557–568. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Palaeoclimatic conditions in the Mediterranean explain genetic diversity of Posidonia oceanica seagrass meadows. Sci. Rep. 2017, 7, 2732. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.L.Y.; Casazza, G.; Pergent-Martini, C.; Pergent, G. A biotic index using the seagrass Posidonia oceanica (BiPo), to evaluate ecological status of coastal waters. Ecol. Indic. 2010, 10, 380–389. [Google Scholar] [CrossRef]
- Montefalcone, M. Ecosystem health assessment using the Mediterranean seagrass Posidonia oceanica: A review. Ecol. Indic. 2009, 9, 595–604. [Google Scholar] [CrossRef]
- Giraud, G. Contribution à la Description et à la Phénologie Quantitative des Herbiers à Posidonia oceanica (L.) Delile. Ph.D. Thesis, Université Aix-Marseille II, Marseille, France, 1997; pp. 1–150. [Google Scholar]
- Gobert, S.; Lejeune, P.; Chéry, A.; Boissery, P.; Sartoretto, S.; Andral, B.; Lepoint, G.; Richir, J. Assessment of the ecological status of P. oceanica meadow with a no destructive shoot method. In Proceedings of the Mediterranean Seagrass Workshop, Essaouira, Morocco, 28 May–1 June 2012. [Google Scholar]
- Gobert, S.; Sartoretto, S.; Rico-Raimondino, V.; Andral, B.; Chery, A.; Lejeune, P.; Boissery, P. Assessment of the ecological status of Mediterranean French coastal waters as required by the Water Framework Directive using the Posidonia oceanica Rapid Easy Index: PREI. Mar. Pollut. Bull. 2009, 58, 1727–1733. [Google Scholar] [CrossRef] [Green Version]
- Gerakaris, V.; Panayotidis, P.; Vizzini, S.; Nicolaidou, A.; Economou-Amilli, A. Effectiveness of Posidonia oceanica biotic indices for assessing the ecological status of coastal waters in Saronikos Gulf (Aegean Sea, Eastern Mediterranean). Mediterr. Mar. Sci. 2017, 18, 161. [Google Scholar] [CrossRef] [Green Version]
- Dinno, A. Dunn’s Test of Multiple Comparisons Using Rank Sums R Package Version 1.3.5; Stata Software Package: College Station, TX, USA, 2017. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics R Package Version 2 (1); Stata Software Package: College Station, TX, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package R Package Version 2.4-6; Stata Software Package: College Station, TX, USA, 2018. [Google Scholar]
- Montefalcone, M.; Vacchi, M.; Carbone, C.; Cabella, R.; Schiaffino, C.F.; Elter, F.M.; Morri, C.; Bianchi, C.N.; Ferrari, M. Seagrass on the rocks: Posidonia oceanica settled on shallow-water hard substrata withstands wave stress beyond predictions. Estuarine Coast. Shelf Sci. 2016, 180, 114–122. [Google Scholar] [CrossRef]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth Surf. Process. Landforms 2016, 42, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Via, J.D.; Sturmbauer, C.; Schönweger, G.; Sötz, E.; Mathekowitsch, S.; Stifter, M.; Rieger, R. Light gradients and meadow structure in Posidonia oceanica:ecomorphological and functional correlates. Mar. Ecol. Prog. Ser. 1998, 163, 267–278. [Google Scholar] [CrossRef]
- Olesen, B.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 2002, 236, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Nicolai, M., Okem, A., Petzold, J., et al., Eds.; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Iacono, C.L.; Mateo, M.A.; Gràcia, E.; Guasch, L.; Carbonell, R.; Serrano, L.; Serrano, O.; Dañobeitia, J. Very high-resolution seismo-acoustic imaging of seagrass meadows (Mediterranean Sea): Implications for carbon sink estimates. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Serrano, O.; Mateo, M.A.; Dueñas-Bohórquez, A.; Renom, P.; López-Sáez, J.; Cortizas, A.M. The Posidonia oceanica marine sedimentary record: A Holocene archive of heavy metal pollution. Sci. Total Environ. 2011, 409, 4831–4840. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Duarte, C.M.; Diaz-Almela, E.; Marbá, N.; Sintes, T.; Serrão, E.A. Implications of Extreme Life Span in Clonal Organisms: Millenary Clones in Meadows of the Threatened Seagrass Posidonia oceanica. PLoS ONE 2012, 7, e30454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Almela, E.; Marbá, N.; Alvarez, E.; Santiago, R.; Holmer, M.; Grau, A.; Mirto, S.; Danovaro, R.; Petrou, A.; Argyrou, M.; et al. Benthic input rates predict seagrass (Posidonia oceanica) fish farm-induced decline. Mar. Pollut. Bull. 2008, 56, 1332–1342. [Google Scholar] [CrossRef] [Green Version]
- Balata, D.; Bertocci, I.; Piazzi, L.; Nesti, U. Comparison between epiphyte assemblages of leaves and rhizomes of the seagrass Posidonia oceanica subjected to different levels of anthropogenic eutrophication. Estuarine Coast. Shelf Sci. 2008, 79, 533–540. [Google Scholar] [CrossRef]
- Ruiz, J.; Marco-Méndez, C.; Sánchez-Lizaso, J.L. Remote influence of off-shore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadows. Mar. Environ. Res. 2010, 69, 118–126. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Collier, C.J.; Waycott, M.; McKenzie, L.J.; Cullen-Unsworth, L.C.; McKenzie, L.J. A framework for the resilience of seagrass ecosystems. Mar. Pollut. Bull. 2015, 100, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Marbá, N.; Santiago, R.; Diaz-Almela, E.; Alvarez, E.; Duarte, C.M. Seagrass (Posidonia oceanica) vertical growth as an early indicator of fish farm-derived stress. Estuarine Coast. Shelf Sci. 2006, 67, 475–483. [Google Scholar] [CrossRef]
- Holmer, M.; Argyrou, M.; Dalsgaard, T.; Danovaro, R.; Diaz-Almela, E.; Duarte, C.M.; Frederiksen, M.; Grau, A.; Karakassis, I.; Marbá, N.; et al. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Pollut. Bull. 2008, 56, 1618–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Torquemada, Y.; Díaz-Valdés, M.; Colilla, F.; Luna, B.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Descriptors from Posidonia oceanica (L.) Delile meadows in coastal waters of Valencia, Spain, in the context of the EU Water Framework Directive. ICES J. Mar. Sci. 2008, 65, 1492–1497. [Google Scholar] [CrossRef] [Green Version]
- Kletou, D.; Kleitou, P.; Savva, I.; Attrill, M.J.; Antoniou, C.; Hall-Spencer, J.M. Seagrass recovery after fish farm relocation in the eastern Mediterranean. Mar. Environ. Res. 2018, 140, 221–233. [Google Scholar] [CrossRef]
- Romero, J.; Alcoverro, T.; Roca, G.; Pérez, M. Bioindicators, Monitoring, and Management Using Mediterranean Seagrasses: What Have We Learned from the Implementation of the EU Water Framework Directive. In The Handbook of Environmental Chemistry; Springer: Heidelberg/Berlin, Germany, 2015; Volume 43, pp. 161–182. [Google Scholar]
- Personnic, S.; Boudouresque, C.F.; Astruch, P.; Ballesteros, E.; Blouet, S.; Bellan-Santini, D.; Bonhomme, P.; Thibault-Botha, D.; Feunteun, É.; Harmelin-Vivien, M.; et al. An Ecosystem-Based Approach to Assess the Status of a Mediterranean Ecosystem, the Posidonia oceanica Seagrass Meadow. PLoS ONE 2014, 9, e98994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudouresque, C.F.; Personnic, S.; Astruch, P.; Ballesteros, E.; Bellan-Santini, D.; Bonhomme, P.; Botha, D.; Feunteun, E.; Harmelin-Vivien, M.; Pergent, G.; et al. Ecosystem-Based Versus Species-Based Approach for Assessment of the Human Impact on the Mediterranean Seagrass Posidonia oceanica. In Marine Productivity: Perturbations and Resilience of Socio-Ecosystems; Springer: Heidelberg/Berlin, Germany, 2015; Volume 43, pp. 235–241. [Google Scholar]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Indic. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- Mayot, N.; Boudouresque, C.; Charbonnel, E. Changes over time of shoot density of the Mediterranean seagrass Posidonia oceanica at its depth limit. Biol. Mar. Mediterr. 2006, 13, 250–254. [Google Scholar]
- Vázquez-Luis, M.; Borg, J.A.; Morell, C.; Banach-Esteve, G.; Deudero, S. Influence of boat anchoring on Pinna nobilis: A field experiment using mimic units. Mar. Freshw. Res. 2015, 66, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, I.E.; Tenan, S.; Tavecchia, G.; Marbá, N.; Jordà, G.; Deudero, S.; Alvarez, E.; Duarte, C.M. Boat anchoring impacts coastal populations of the pen shell, the largest bivalve in the Mediterranean. Biol. Conserv. 2013, 160, 105–113. [Google Scholar] [CrossRef]
- Mabrouk, L.; Hamza, A.; Ben Brahim, M.; Bradai, M.-N. Temporal and depth distribution of microepiphytes on Posidonia oceanica (L.) Delile leaves in a meadow off Tunisia. Mar. Ecol. 2011, 32, 148–161. [Google Scholar] [CrossRef]
- Romero, J.; Pérez Vallmitjana, M.; Renom, P.; Invers i Brunet, O.; Mateo Mínguez, M.Á.; Tomás, F.; Manzanera, M.; Pedro Puente, X.d. Seguimiento de la Pradera de Posidonia oceanica de las Islas Medas. Ejercicio de 1999. Seguiment temporal de la reserva marina de les Illes Medes. Informe anual. Any 1999, 9–18. [Google Scholar]
- Borg, J.A.; Schembri, P.J. Bathymetric distribution of decapods associated with a Posidonia oceanica meadow in Malta. In The Biodiversity Crisis and Crustacea-Proceedings of the Fourth International Crustacean Congress; CRC Press: Boca Raton, FL, USA, 2000; p. 119. [Google Scholar]
- Panayotidis, P.; Simboura, N. Distribution and phenology of Posidonia oceanica in Saronikos gulf (Aegean sea, Greece). In International Workshop on Posidonia oceanica Beds; Boudouresque, C.F., Meinesz, A., Fresi, E., Gravez, V., Eds.; GIS Posidonie Publications: Pairs, France, 1989; Volume 2, pp. 43–48. [Google Scholar]
- Mabrouk, L.; Asma, H.; Bradai, M.N. Temporal and bathymetric variation of epiphyte cover and leaf biomass in a southern Posidonia oceanica (L.) meadow: The case of Mahdia coast, Tunisia. Mar. Ecol. 2016, 38, e12394. [Google Scholar] [CrossRef]
- Mauro, L.; Paola, G.; Margherita, V.; Rugiada, R.; Francesca, B.; Primo, M.; Duccio, S.; Enrica, F. Human impact on a small barrier reef meadow of Posidonia oceanica (L.) Delile on the north Tyrrhenian coast (Italy). Mar. Pollut. Bull. 2013, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Alcoverro, T.; Duarte, C.M.; Romero, J. Annual growth dynamics of Posidonia oceanica: Contribution of large-scale versus local factors to seasonality. Mar. Ecol. Prog. Ser. 1995, 120, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Gerakaris, V.; Panayotidis, P. Use of Posidonia oceanica meadows as Biological Quality Element, according to the European Water Framework Directive (WFD, 2000/60/EC): A case study in south Aegean Sea. In Proceedings of the 9th Symposium on Oceanography & Fisheries, Patras, Greece, 13–16 May 2009. [Google Scholar]
- Pergent-Martini, C.; Rico-Raimondino, V.; Pergent, G. Primary production of Posidonia oceanica in the Mediterranean Basin. Mar. Biol. 1994, 120, 9–15. [Google Scholar] [CrossRef]
- Scardi, M.; Chessa, L.A.; Fresi, E.; Pais, A.; Serra, S. Optimizing interpolation of shoot density data from a Posidonia oceanica seagrass bed. Mar. Ecol. 2006, 27, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Sghaier, Y.; Zakhama-Sraieb, R.; Charfi-Cheikhrouha, F.; Societa Italiana di Biologia Marina, L. Status of Posidonia oceanica meadows along the eastern coast of Tunisia. Biol. Mar. Mediterr. 2006, 13, 85–91. [Google Scholar]
- Sghaier, Y.R.; Zakhama-Sraieb, R.; Charfi Cheikhrouha, F. Posidonia oceanica meadows along the eastern coast of Tunisia: Feature and health status. Bull. Inst. Natn. Scien. Tech. Mer Salammbô 2011, 38, 89–99. [Google Scholar]
- Costantino, G.; Mastrototaro, F.; Tursi, A.; Torchia, G.; Pititto, F.; Salerno, G.; Lembo, G.; Sion, L.; D’Onghia, G.; Carlucci, R.; et al. Distribution and bio-ecological features of Posidonia oceanica meadows along the coasts of the southern Adriatic and northern Ionian Seas. Chem. Ecol. 2010, 26, 91–104. [Google Scholar] [CrossRef]
- Marbá, N.; Duarte, C.M.; Diaz-Almela, E.; Terrados, J.; Alvarez, E.; Martínez, R.; Santiago, R.; Gacia, E.; Grau, A.M. Direct evidence of imbalanced seagrass (Posidonia oceanica) shoot population dynamics in the Spanish Mediterranean. Estuaries Coasts 2005, 28, 53–62. [Google Scholar] [CrossRef]
- Mabrouk, L.; Hamza, A.; Sahraoui, H.; Bradai, M. Données sur les caractéristiques et la phénologie de l’herbier de” Posidonia oceanica” (L.) delile sur les côtes de Mahdia (region est de la Tunisie). Bulletin de l’Institut National des Sciences et Technologies de la Mer 2009, 36, 139–147. [Google Scholar]
- Costantino, G.; Mastrototaro, F.; Carlucci, R.; Matarrese, A.; Panza, M. First data on meadow structure of a large Posidonia oceanica bed along the Southern Tyrrhenian coast (Mediterranean Sea). Biol. Mar. Mediterr. 2006, 13, 199–205. [Google Scholar]
- Peirano, A.; Cocito, S.; Banfi, V.; Cupido, R.; Damasso, V.; Farina, G.; Lombardi, C.; Mauro, R.; Morri, C.; Roncarolo, I.; et al. Phenology of the Mediterranean seagrass Posidonia oceanica (L.) Delile: Medium and long-term cycles and climate inferences. Aquat. Bot. 2011, 94, 77–92. [Google Scholar] [CrossRef]
- Buia, M.C.; Zupo, V.; Mazzella, L. Primary Production and Growth Dynamics in Posidonia oceanica. Mar. Ecol. 1992, 13, 2–16. [Google Scholar] [CrossRef]
- Department of Fisheries and Marine Research (DFMR). Creation of Monitoring Stations and Data Collection for the Implementation of the Monitoring Program (2000/60/EC) of Habitat 1120 ’Marine Benthic Vegetation with Posidonia oceanica’, in the Marine area of Kavo Kiti/Pervolia, Implemented by Marine & Environmental Research (MER) Lab; DFMR: Nicosia, Cyprus, 2016.
- Pergent, G.; Pergent-Martini, C.; Bein, A.; Dedeken, M.; Oberti, P.; Orsini, A.; Santucci, J.-F.; Short, F. Dynamic of Posidonia oceanica seagrass meadows in the northwestern Mediterranean: Could climate change be to blame? Comptes Rendus Biol. 2015, 338, 484–493. [Google Scholar] [CrossRef]
- Vasapollo, C. Spatio-Temporal Variability of Plant Features and Motile Invertebrates in Posidonia oceanica Seagrass Meadows. Ph.D. Thesis, Open University, Milton Keynes, UK, 2009. [Google Scholar]
- Royo, C.L.Y.; Pergent, G.; Alcoverro, T.; Buia, M.C.; Casazza, G.; Martínez-Crego, B.; Perez, M.; Silvestre, F.; Romero, J. The seagrass Posidonia oceanica as indicator of coastal water quality: Experimental intercalibration of classification systems. Ecol. Indic. 2011, 11, 557–563. [Google Scholar] [CrossRef]
- Zupo, V.; Buia, M.C.; Gambi, M.C.; Lorenti, M.; Procaccini, G. Temporal variations in the spatial distribution of shoot density in a Posidonia oceanica meadow and patterns of genetic diversity. Mar. Ecol. 2006, 27, 328–338. [Google Scholar] [CrossRef]
- Guala, I.; Iveša, L.; Krstinić, P.; Jakl, Z.; Šijan, M.; Prvan, M.; Sertic, M.; Špika, M.; Nikolić, V.; Žuljević, A.; et al. Assessment of Posidonia oceanica status along the north Croatian coast (Adriatic Sea). PeerJ PrePrints 2015, e1418. [Google Scholar] [CrossRef]
- Rotini, A.; Belmonte, A.; Barrote, I.; Micheli, C.; Peirano, A.; Santos, R.; Silva, J.; Migliore, L. Effectiveness and consistency of a suite of descriptors for assessing the ecological status of seagrass meadows (Posidonia oceanica L. Delile). Estuarine Coast. Shelf Sci. 2013, 130, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Costantino, G.; Ungaro, N.; Mastrototaro, F.; Battista, D.; Blonda, M.; Pastorelli, A.; Carlucci, R.; Tursi, A. Recent data from the monitoring of Posidonia oceanica in two Marine Protected Areas of the Apulian coasts (central-eastern Mediterranean Sea). Biol. Mar. Mediterr. 2010, 17, 164–166. [Google Scholar]
- Hadjimichael, M.; Bruggeman, A.; Lange, M.A. Tragedy of the few? A political ecology perspective of the right to the sea: The Cyprus marine aquaculture sector. Mar. Policy 2014, 49, 12–19. [Google Scholar] [CrossRef]
- Sheaves, M.; Coles, R.; Dale, P.; Grech, A.; Pressey, R.L.; Waltham, N.J. Enhancing the Value and Validity of EIA: Serious Science to Protect Australia’s Great Barrier Reef. Conserv. Lett. 2016, 9, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Unsworth, R.K.F.; Cullen-Unsworth, L.C. Strategies to enhance the resilience of the world’s seagrass meadows. J. Appl. Ecol. 2016, 53, 967–972. [Google Scholar] [CrossRef] [Green Version]





| Depth (m) | Country | Location | Mean Shoot Densities (Shoot m−2) | Source |
|---|---|---|---|---|
| 5 ± 1 | Malta and Spain | Comino, Majorca | 1185–1243 | [59] |
| Spain | Majorca | 384–1013 | [60] | |
| Cyprus | Vasiliko Bay | 617–954 | Present study | |
| Tunisia | Mahdia | 807 | [61] | |
| Spain | Catalonia | 481–789 | [62] | |
| Malta | Northern Malta | 787 | [63] | |
| Greece | Saronikos Gulf | 500–750 | [64] | |
| Tunisia | Mahdia | 413–748 | [65] | |
| Italy | Tuscany | 593–697 | [66] | |
| Spain | Catalonia | 111–627 | [67] | |
| Greece | Southern Aegean Sea | 610 | [68] | |
| Italy | Sardinia | 525 | [69] | |
| Italy | Sardinia | 69–525 | [70] | |
| Tunisia | Eastern Tunisia | 362–496 | [71,72] | |
| Italy | Gulf of Taranto | 494 | [73] | |
| Spain | Balearic Islands | 367–484 | [74] | |
| Italy | Gulf of Naples | 473 | [69] | |
| Tunisia | Mahdia | 456 | [75] | |
| Turkey | Central Aegean Sea | 450 | [69] | |
| Italy | Gulf of Naples | 431 | [76] | |
| Italy | Monterosso | 349 | [77] | |
| Italy | Gulf of Naples | 341 | [78] | |
| Spain | Campomanes | 292 | [74] | |
| 15 ± 1 | Cyprus | Cape Kiti | 509–568 | [79] |
| Greece | Southern Aegean | 518 | [68] | |
| Malta | Northern Malta | 486 | [63] | |
| Cyprus | Vasiliko Bay | 395–478 | Present study | |
| France | Corsica | 176–472 | [29] | |
| France | Corsica | 211–448 | [80] | |
| Spain | Balearic Islands | 433 | [74] | |
| Italy | Gulf of Naples | 244–413 | [81] | |
| Greece | Saronikos Gulf | 231–406 | [34] | |
| Spain | Catalonia | 398 | [74] | |
| Italy | Sardinia | 395 | [69] | |
| Italy | Gulf of Naples | 220–388 | [82] | |
| Italy | Gulf of Naples | 301–345 | [76] | |
| Italy | Gulf of Naples | 137–343 | [83] | |
| Spain | Catalonia | 173–340 | [62] | |
| France | Corsica | 285–334 | [82] | |
| Croatia | Northern Adriatic Sea | 141–324 | [84] | |
| Spain | Catalonia | 159–288 | [82] | |
| Italy | Ligurian Sea | 260 | [85] | |
| Italy | North-Eastern Ionian Sea | 225–234 | [73] | |
| Italy | Southern Adriatic | 189 | [86] | |
| Italy | Southern Adriatic | 135–163 | [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kletou, D.; Kleitou, P.; Savva, I.; Attrill, M.J.; Charalambous, S.; Loucaides, A.; Hall-Spencer, J.M. Seagrass of Vasiliko Bay, Eastern Mediterranean: Lost Cause or Priority Conservation Habitat? J. Mar. Sci. Eng. 2020, 8, 717. https://doi.org/10.3390/jmse8090717
Kletou D, Kleitou P, Savva I, Attrill MJ, Charalambous S, Loucaides A, Hall-Spencer JM. Seagrass of Vasiliko Bay, Eastern Mediterranean: Lost Cause or Priority Conservation Habitat? Journal of Marine Science and Engineering. 2020; 8(9):717. https://doi.org/10.3390/jmse8090717
Chicago/Turabian StyleKletou, Demetris, Periklis Kleitou, Ioannis Savva, Martin J. Attrill, Stephanos Charalambous, Alexis Loucaides, and Jason M. Hall-Spencer. 2020. "Seagrass of Vasiliko Bay, Eastern Mediterranean: Lost Cause or Priority Conservation Habitat?" Journal of Marine Science and Engineering 8, no. 9: 717. https://doi.org/10.3390/jmse8090717
APA StyleKletou, D., Kleitou, P., Savva, I., Attrill, M. J., Charalambous, S., Loucaides, A., & Hall-Spencer, J. M. (2020). Seagrass of Vasiliko Bay, Eastern Mediterranean: Lost Cause or Priority Conservation Habitat? Journal of Marine Science and Engineering, 8(9), 717. https://doi.org/10.3390/jmse8090717






