Abstract
Authigenic carbonates are widely distributed in marine sediments, microbes, and anaerobic oxidation of methane (AOM) play a key role in their formation. The authigenic carbonates in marine sediments have been affected by weathering and diagenesis for a long time, it is difficult to understand their formation process by analyzing the samples collected in situ. A pore water environment with 10 °C, 6 MPa in the marine sediments was built in a bioreactor to study the stages and characteristics of authigenic carbonates formation induced by microbes. In experiments, FeCO3 is formed preferentially, and then FeCO3-MgCO3 complete isomorphous series and a small part of CaCO3 isomorphous mixture are formed. According to this, it is proposed that the formation of authigenic carbonates performed by AOM and related microbes needs to undergo three stages: the rise of alkalinity, the preferential formation of a carbonate mineral, and the formation of carbonate isomorphous series. This work provides experimental experience and reference basis for further understanding the formation mechanism of authigenic carbonates in marine sediments.
1. Introduction
The marine sediments are the largest methane reservoir on the earth. The methane in marine sediments was stored in the form of free methane, dissolved methane, and natural gas hydrates [1]. The annual methane generated from marine sediments reaches 85–300 Tg [2,3,4]. However, only 2% of methane generated was emitted into the atmosphere [5]. Previous work estimated that more than 80% of the methane migrating upward through marine sediments was consumed through anaerobic oxidation of methane coupled with sulphate reduction (AOM) mediated by the microbial consortium of anaerobic methane-oxidizing archaea and sulfate-reducing bacteria [6,7]:
HCO3− and HS− was produced by AOM, which increases the alkalinity of pore water, thus favoring the precipitation of authigenic carbonates and pyrite [8]. Authigenic carbonate was formed by the interaction between bicarbonate, calcium, iron, and magnesium within the pore water of the marine sediment [9]. Authigenic carbonates were very common in several methane-bearing cold seeps where the AOM was occurred. It was formed in different types such as calcite, siderite, aragonite, high-magnesium calcite, low-magnesium calcite [10], dolomite, and ankerite in methane-bearing cold seeps of different areas.
The study of authigenic carbonates in the cold seep area mainly focus on the determination of the origin, formation time of authigenic carbonates, and the source of seepage fluid by isotope method [11,12,13]. Some papers have discussed the different formation model of authigenic carbonates in the methane-bearing cold seep by studying sediment cores [14,15]. However, the particle size of carbonates in marine sediments is very small, and it is difficult to obtain single component minerals by conventional operation. Therefore, most studies on authigenic carbonates in the cold seep area are limited to determine the total amount of minerals [16], and few studies on the formation process of authigenic carbonate.
The laboratory experiment related to authigenic carbonates, some study the formation duration of carbonate minerals under cold seep conditions [17], and some study the relationship between biominerals and the microbes [18]. However, there are few researches on the formation mechanism of carbonate minerals in marine sediments. The previous research is limited to the exploration of the forming conditions of authigenic carbonates [19] and the simple description of the mineral morphology [20].
In this paper, artificial pore water, marine sediments collected from the South China Sea, and microbial solution incubated from sediments were used to study the formation stage and characteristics of authigenic carbonates induced by microbes in a bioreactor with low temperature and high pressure. The results reflect the stages and model of authigenic carbonates formation in marine sediments to some extent. This work improves our understanding of the formation of authigenic carbonates from a microscopic perspective.
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Vitro Incubation of Microbes Strain
The basal medium composition was shown in Appendix A. 10 g seabed sediment from the northern continental slope of the South China Sea mixed 250 mL sterile anaerobic medium was put into culture flask. After 5 min of N2 (99.99% purity, Dalian Special Gas Corporation) flushing and 2 min of CH4 (99.99% purity, Dalian Special Gas Corporation) flushing, culture flask was placed at 35 °C during incubation. To supplement the carbon substrate, excess methane was replenished every two days. Black crusts stuck on the inner wall of culture flask, some black suspension was generated after 10 days’ culture. Then, the supernatant of culture flask was obtained and placed it into fresh medium as new enrichment cultures. Repeating this process until the microbial concentration and purity met the requirements (OD value of microbial solution reach 2.1), an enriched microbial solution was obtained.
2.1.2. Artificial Pore Water
The pore water composition, 4.75 m below seafloor in site T1 of Qiongdongnan basin, the South China Sea, was referred to configure artificial pore water (Table 1). At site T1, the ratio of Mg2+/Ca2+ rose and the concentration of Mg2+,Ca2+, Sr2+, Mn2+, and SO42− reduced with depth [21]. These geochemical variations were consistent with the characteristics of pore water variations in the marine sediments [22].

Table 1.
The composition of artificial pore water.
2.1.3. Sediments and Granite Slices
Sediment samples taken from the seabed of the Northern continental slope of the South China Sea, were stored at 4 °C in the dark for 6 months until in the experiment started. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) were performed to analyze the sediments. The result shows that the sediments consist of (in wt.%) quartz 27, plagioclase 3, kaolinite 10, illite 45, and smectite 15 on average.
Limited by the laboratory experiment conditions, the amount of authigenic minerals formed during the experiment was small, which makes it difficult to collect and test. Granite slices, which were washed in adequate dilute hydrochloric acid (3.6 in wt.%) and then rinsed by distilled water and dried at 35 °C before the experiment, were used as intermediate carriers to collect authigenic minerals on it. Before the experiment, the results of XRD show that the granite slices consist of (in wt.%) 22 quartz, 33 plagioclase, 37 alkali feldspar, 5 biotite, and 3 kaolinite on average. Under the experiment conditions, the biotite can be regarded as nonreactive substance during short experiment duration.
2.2. Experimental Apparatus and Procedures
2.2.1. Experimental Apparatus
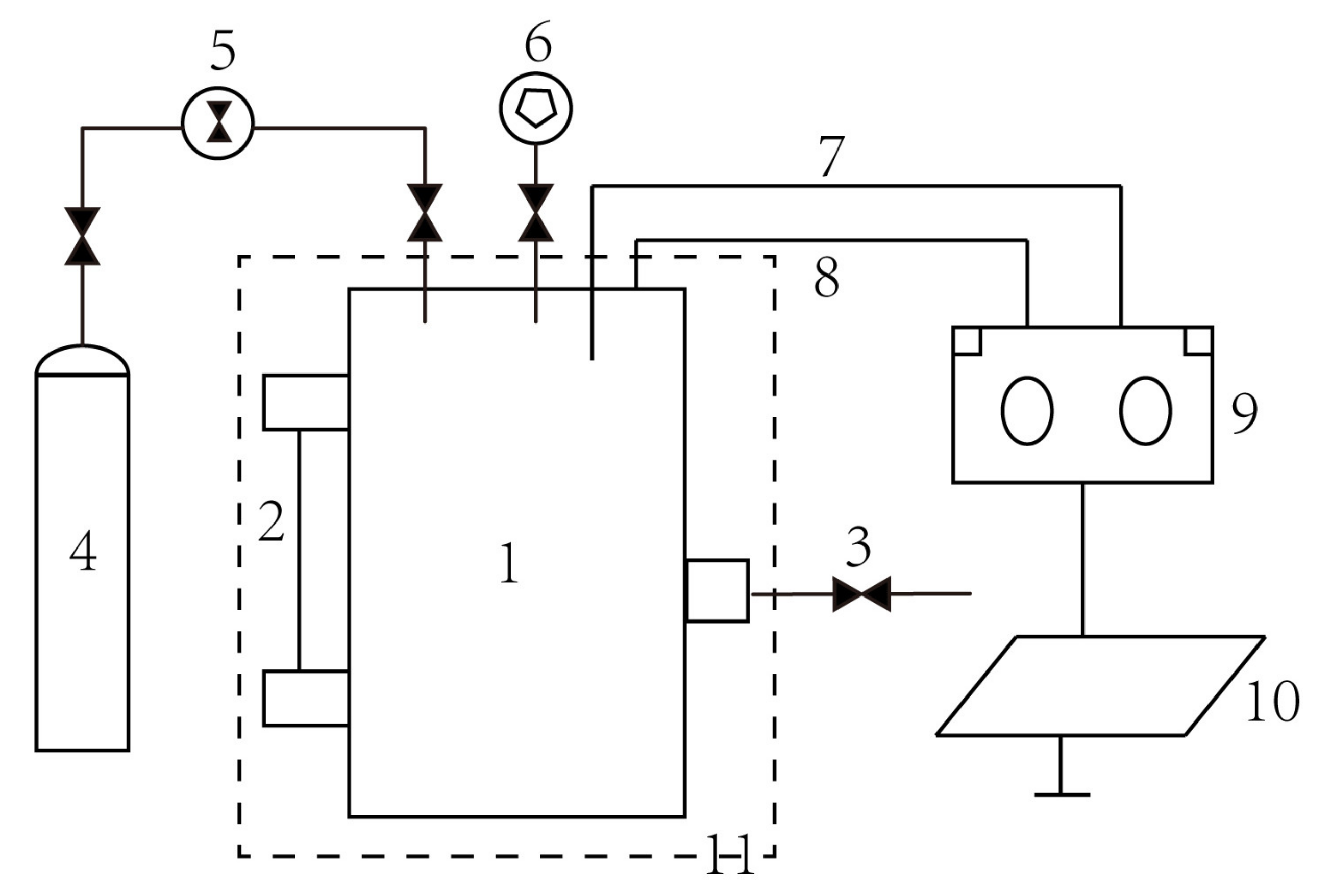
The experimental apparatus consisted of bioreactor, methane supply, temperature control system (air bath), and data acquisition system (Figure 1). The experiment was conducted in the low-temperature and high-pressure bioreactor, in which external control and monitoring of pressure and temperature were available with a volume of 1.0 L [23].
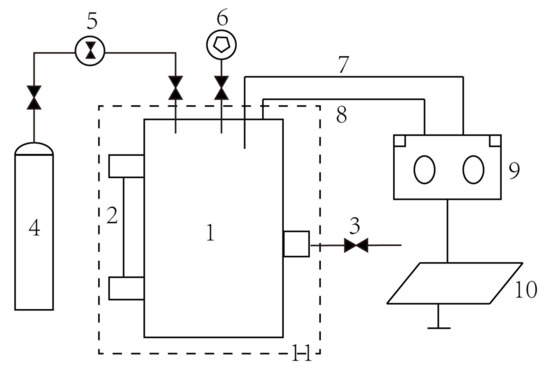
Figure 1.
The experimental system. 1. Bioreactor, 2. Sight glass, 3. Sample collection, 4. Methane cylinder, 5. Regulator, 6. Vacuum Pump, 7. Temperature sensor, 8. Pressure sensor, 9. Data acquisition, 10. Computer, 11. Air bath.
2.2.2. Experimental Procedures
The marine sediments [24,25,26] cover a wide range of water depth, from about 470 m to 3000 m corresponding to the pressure from 4.7 MPa to 30 MPa and the range of temperature from 2.2 to 10 °C [27]. Microbial metabolism was extremely slow if the temperature below 4 °C. Previous experiment studies showed that when the temperature was higher than 4 °C, the metabolism of anaerobic microbes accelerated, and authigenic minerals are easier to form [19]. Therefore, the experimental temperature should be 10 °C. The experimental pressure, selected 6 MPa, was slightly lower than the critical equilibrium pressure at 10 °C for avoiding gas hydrate formation [28].
The amounts of 150 cm3 sediments, 670 mL artificial pore water, 50 mL bacteria solution were put into the bioreactor in sequence. Sediment was at the bottom of the bioreactor, pore space of sediment was full of artificial pore water, and the surface of the water is 11 cm higher than that of sediment. Then, two pieces of granite slices were placed on the sediment surface and the bioreactor was quickly sealed. After evacuation, methane was injected into the bioreactor to rise and maintain pressure at the top of bioreactor. Temperature (10 °C) and pressure (6 MPa) in the bioreactor were kept constant during the experiment. The experiment lasted 17 days, fluid samples were taken for analysis at regular intervals (1–2 days at the initial stage, 2–3 days in the late stage). Scanning electron microscope (SEM) and energy-dispersive X-ray spectrometer (EDS) were performed to view and analyze granite slices surface. 16S rDNA sequencing was used to determine bacteria diversity. More details about experimental procedures and methodology can be found in [19,20].
2.2.3. Analytical Methods
The Eh, optical density (OD), and the concentration of HCO3−, Ca2+ of the fluid samples were measured. A batch of clean and evacuated sampling tubes were used to collect fluid samples through sample collection on the side of the bioreactor. These sampling tubes were sealed until measuring. HCO3− concentration was determined following the acid-base neutralization titration method. Phenolphthalein, Methyl orange, and diluted hydrochloric acid were used in this method. Ca2+ concentration was quantified by EDTA and an indicator of calconcarboxylic acid. OD was the absorbance of a material for a given wavelength (λ = 600) per unit distance and was measured with a UV-VIS spectrophotometer (T6 series, Purkinje General Corporation, Beijing, China). Eh was determined using HANNA multi-parameter water quality portable meter (HI9128, Woonsocket, RI, USA ). Instrument model of SEM, EDS, and XRD was JSM-6700F (JEOL Corporation, Tokyo, Japan), INCAX-SIGHT (Oxford, UK), and DX-2700 (Kemait NDT Co., Ltd., China), respectively. The accelerating voltage and working distance of the SEM were 15.0 kV and about 15 mm. All mineral morphologies were viewed using the backscattered electron (BSE) mode. The granite slices were detected using SEM with thin section form, and it was coated with gold dust before the test. The 16S rDNA sequencing was performed by Sangon Biotech (Shanghai, China).
3. Results
3.1. Fluid Chemistry and Microbes
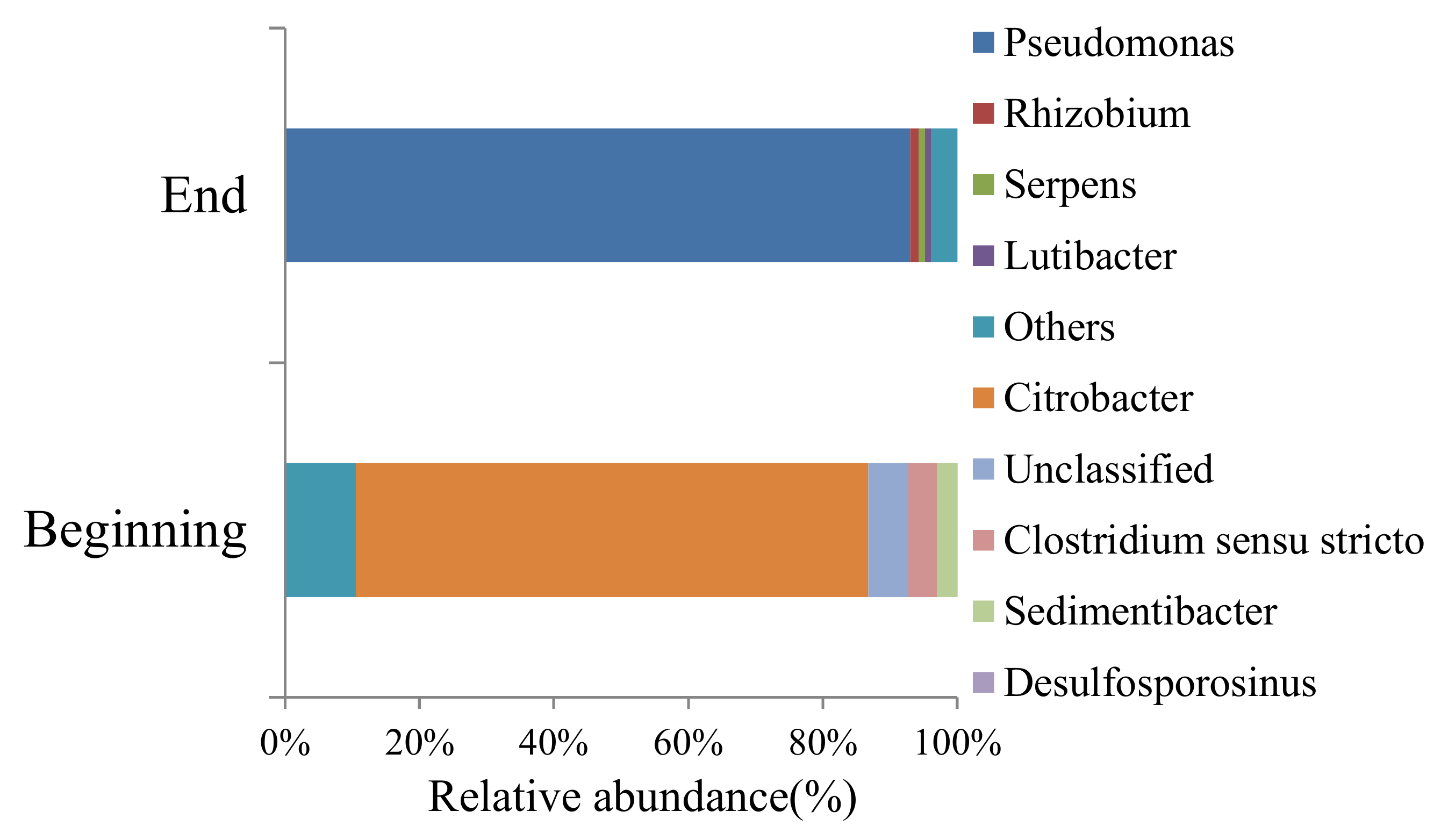
The relative abundance of bacteria strain at the beginning and at the end of the experiment are shown in Figure 2. At the beginning, Citrobacter and other bacteria account for 86% relative abundance in the whole microbial community. The type of microbial community changes a lot at the end of the experiment. Pseudomonas account for 93% relative abundance at the end of the experiment and many bacteria at the beginning disappeared. The environment condition during the experiment have a great influence on microbial community, and it is beneficial to the growth of Pseudomonas. Figure 3a reflect the change of microbes concentration. The fluctuation in Figure 3a caused by microbial community replacement and mineral dissolution, which will be discussed in Section 4.1.
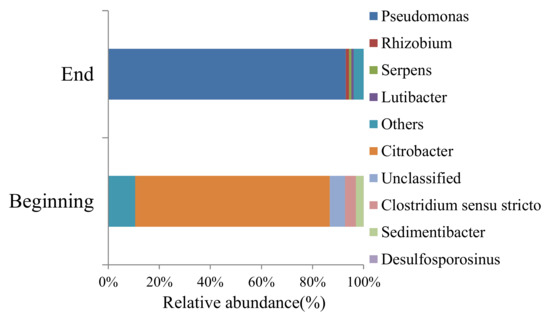
Figure 2.
The microbes strain at the beginning and at the end of the experiment.
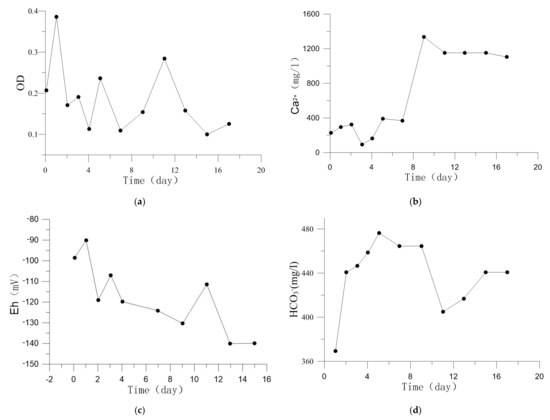
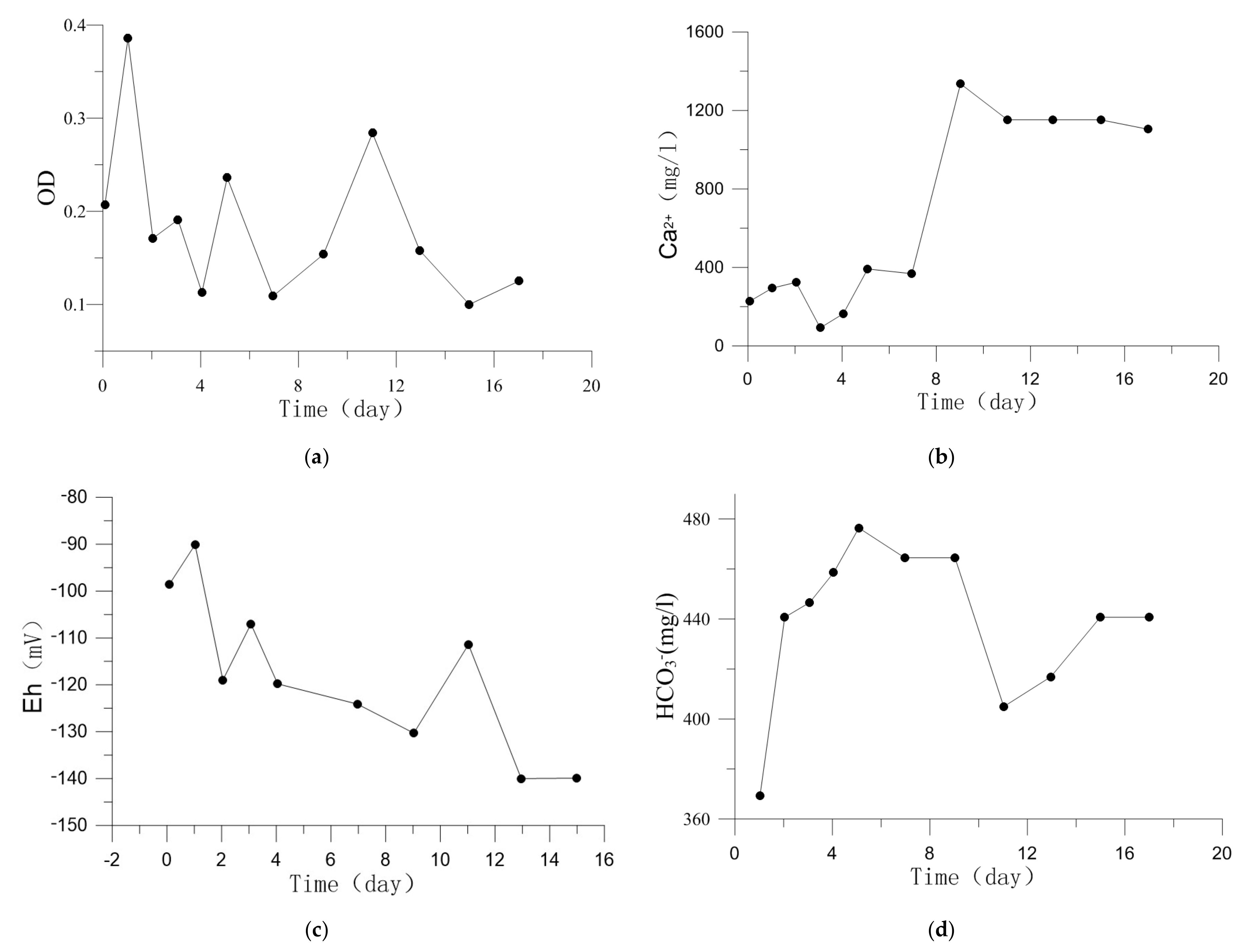
Figure 3.
Fluid chemistry change during the experiment. (a) OD. (b) Ca2+ concentrations. (c) Eh. (d) HCO3− concentrations.
The evolution of Ca2+ concentration is shown in Figure 3b. The dissolution of smectite-Ca contributed to its rise and the precipitation of carbonate make it less. More details were discussed in Section 4.1.
According to Equations (1)–(4), AOM produces HCO3− and carbonates precipitation consumes HCO3−, both of which affect HCO3− concentration in Figure 3d [29].
Low Eh value (<−100 mV) is favorable for AOM performance. The lower Eh value is, the more favorable for AOM performance [30]. Sulphur-containing compounds produced by microbial metabolism accumulate in pore water, which will cause the decrease of Eh [31,32,33,34,35]. Figure 3c shows Eh value dropped from −90 mV to −140 mV, it is a beneficial range for the growth and metabolism of microbes related to AOM [34]. Previous researches find that when Eh is less than 770 mV [36], the occurrence form of Fe is Fe2+, and Fe3+ disappears. Therefore, Fe2+ was inferred to be the main form in the experiments.
3.2. Authigenic Minerals
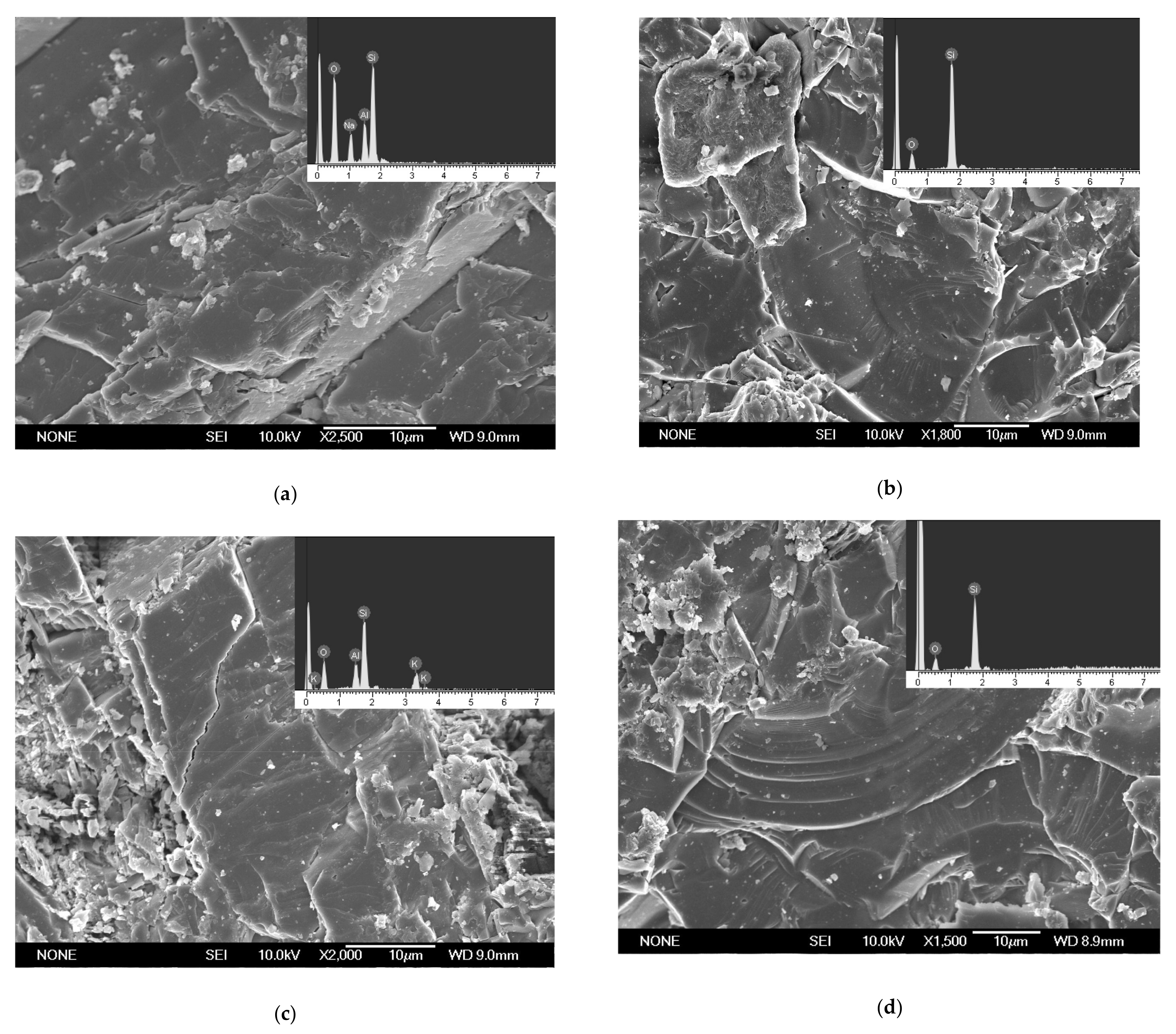
The microscopic morphology and atomic composition of granite slices surface before and after the experiment was determined by SEM and EDS.
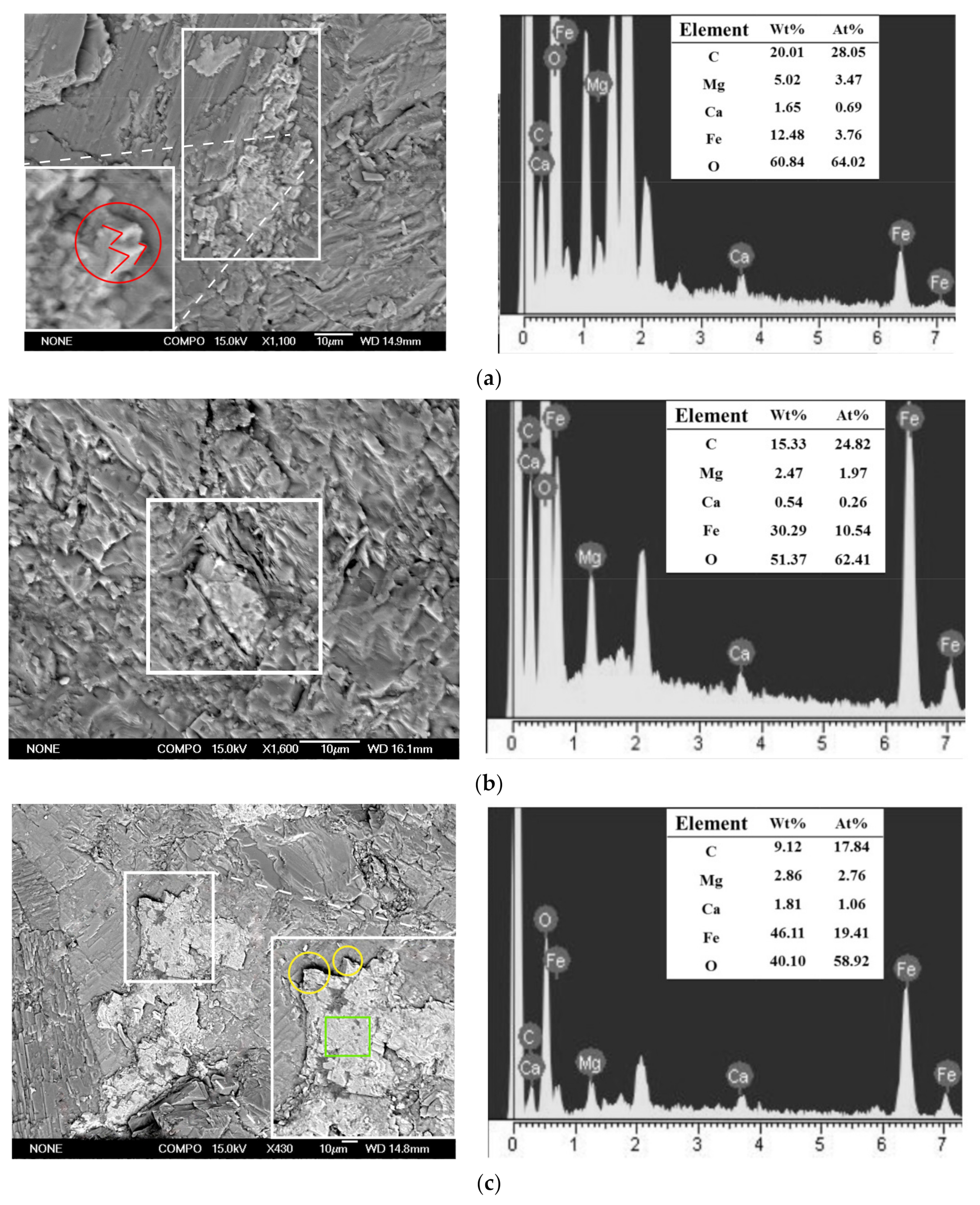
Before the experiment, low-albite (Figure 4a), quartz (Figure 4b,d), and k-feldspar (Figure 4c) were found on the slices. These minerals were the component of granite. Carbonates were found on the slices after the experiment (Figure 5). The faces and edges of the mineral crystals are complete (Figure 5c, green square and yellow circles), and the edges are sharp (Figure 5c, yellow circles), indicating that the mineral was formed in situ without weathering. In addition, these minerals contained C, Fe, Ca, and Mg elements that granite lacked. Therefore, the minerals in Figure 5 were the authigenic carbonates formed during the experiment. EDS analyses showed that these carbonates mainly contained the elements C, O, Fe, Ca, and Mg. The minerals in Figure 5 were the mixture of FeCO3, MgCO3, and CaCO3. Carbonates A was 15 μm × 8 μm in size with granular aggregate shape, and carbonates B was 20 μm × 16 μm in size with dense massive aggregate shape. The distribution range of carbonates C is large and uneven, showing amorphous morphology with an area of 5576 μm2. The formation of carbonates was the direct consequence of AOM and the original record of methane seepage and carbon migration in marine sediments [37,38]. The direct cause of carbonates formation is HCO3− reacting with Fe2+, Ca2+, and Mg2+ in pore water.
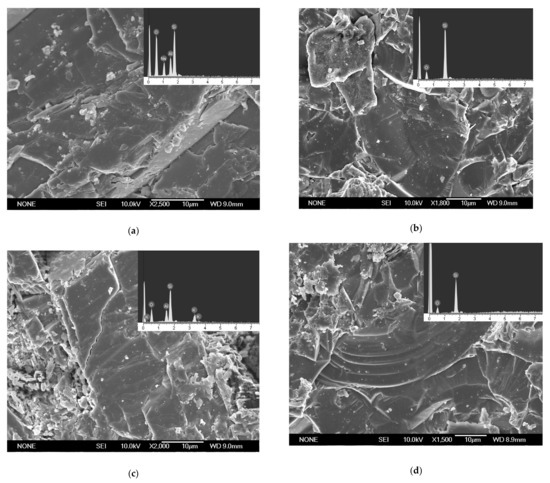
Figure 4.
Granite slices surface prior to the experiment. (a) Low-albite. (b) Quartz. (c) K-feldspar. (d) Quartz.
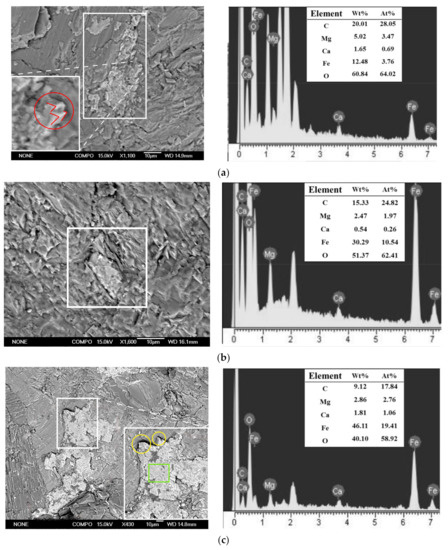
Figure 5.
Authigenic carbonates formed after the experiment. (a) Carbonates A. (b) Carbonates B. (c) Carbonates C. Authigenic carbonates (white square); crystal faces (green square); crystal edges (yellow circle); rhombohedral twin (red circle); acute angle of rhombohedron (red line).
4. Discussion
4.1. The Role of Microbes in Chemical Composition Changes of Pore Water and Carbonates Formation
Citrobacter and pseudomonas were the dominant strain at the beginning and end of the experiment, respectively. The function of formation of carbonates induced by Citrobacter was reported by previous literature [18]. Ma et al. [39] injected Citrobacter into a bottle containing liquid medium. After 55 days’ experiment, they found that a large amount of authigenic carbonates were formed in the bottle, which proved that Citrobacter has the biological function of promoting carbonates formation. The extracellular polymeric substances secreted by microbes contain polysaccharides and small molecular organic acids, which have the ability to adsorb and accumulate cations. Some work found that pseudomonas has the ability to secrete extracellular polymeric substances [40] and promote biological mineralization on carbonates [41,42,43].
The interaction between bacteria and minerals is an important process in terms of mineral formation. Clay minerals have the property of adsorbing bacteria, and some bacteria have the ability to weather minerals. The sediments used in the experiment contained smectite, kaolinite, and illite. Smectite, kaolinite, and illite have the properties of adsorbing bacteria on the solid surface or in the soil solution [44,45,46]. The higher the concentration of electrolyte in the solution, the higher the adsorption rate of clay minerals to bacteria [44]. The concentration of electrolyte of pore water in the experiment is at least 710 mM. Therefore, we infer that the bacteria were adsorbed by clay minerals after the bacteria solution were put into the bioreactor.
Comparing the bacteria strain at the beginning and end of the experiment, we know that the amount of pseudomonas in the pore water increased during the experiment. Pseudomonas were capable of destroying the crystal structure of smectite, initiating it to dissolve [47]. The sediments used in the experiment were collected from shallow sea, so smectite-Ca is the main form of smectite. Ca2+ and bacteria will be released into the pore water due to the dissolution of smectite-Ca [48]. The pseudomonas released destroyed the crystal structure of smectite-Ca, causing it to dissolve further. Therefore, Ca2+ concentration in Figure 3b showed tendency to rise during the experiment and a mutation in 7–9 day. Ca2+ concentration rose rapidly in 7–9 day increasing the ion product of carbonates and promotes the capture of HCO3− in 9–11 day in Figure 3d.
4.2. The Formation Sequence of Carbonates of the Experiment
The carbonates formed in the experiment was dominant by FeCO3, it was mixed with some MgCO3 and CaCO3. The isomorphism plays an important role in changing the composition of carbonates in the natural environment. Mixed components carbonates are usually formed from single component carbonate through isomorphism process [49]. From Table 2, we know the FeCO3-MgCO3 is complete isomorphous series. On the contrary, the CaCO3-FeCO3 and CaCO3-MgCO3 is incomplete isomorphous series or double salt. In the isomorphous series of FeCO3-MgCO3, Fe and Mg can be substituted with each other in any proportion. In the isomorphous series of CaCO3-FeCO3 and CaCO3-MgCO3, substitution between Fe, Ca, and Mg can only perform in a limited proportion range.

Table 2.
Isomorphism among different carbonates.
The solubility product constant of FeCO3 (3.13 × 10−11) is much smaller than that of MgCO3 (6.82 × 10−6) and CaCO3 (2.8 × 10−9) and FeCO3 is the main carbonates formed in the experiment. We propose an inference that FeCO3 is formed first in the experiment, and then the Fe in FeCO3 is constantly substituted by Mg to form FeCO3-MgCO3 isomorphous series. Due to the large difference in cation radius, only incomplete isomorphous series and double salt can be formed between CaCO3-FeCO3 and CaCO3-MgCO3, so Ca accounts for a small proportion in the isomorphous mixture of carbonates. We will explain this inference in terms of atomic composition and morphology of carbonates in the following paragraphs.
In terms of atomic composition, the earlier FeCO3 forms, the lower the ratio of Fe/Mg in FeCO3-MgCO3 isomorphous series. Within some siderite concretions in marine sediments of England, the Fe/Mg ratio decreases systematically from center to edge of concretions [50]. It is suggested that Fe can be continuously substituted by Mg in siderite in natural marine sediments. Therefore, we believe the carbonates C forms the latest (At% Fe/Mg = 7), followed by the carbonates B (At% Fe/Mg = 5), and the carbonates A formed the earliest (At% Fe/Mg = 1).
The morphology of mineral crystals and mineral aggregates reflects the formation time of minerals and external environmental conditions. The formation of mineral crystals needs a period of time. If there is enough time for minerals to precipitate, mineral crystals are easy to form characteristic morphology, otherwise, amorphous morphology is formed. Carbonates A shows granular aggregate morphology and rhombohedral twins (Figure 5a, red circle) with complete crystal structure. The rhombohedral twins are composed of two rhombohedral monocrystal with different orientations. The crystal of rhombohedral twins grows fully with complete crystal form and the formation time is earlier [49]. Rhombohedron or rhombohedral twins crystal is a typical crystal habit of carbonates. When the temperature decreases, the crystal form of carbonates transforms from obtuse rhombohedron to acute rhombohedron [51]. The low temperature set in the experiment has a good corresponding relationship with the formation of acute rhombohedron (Figure 5a, red circle and red line). The crystals of rhombohedron or rhombohedral twins and the granular aggregate or dense massive aggregate are typical characteristic morphology of FeCO3-MgCO3 isomorphous series [52]. Therefore, carbonates A is FeCO3-MgCO3 isomorphous series and it have been formed for a relatively long time. The morphology of carbonates B show dense massive aggregate. Carbonate B has formed a characteristic aggregate morphology, but the characteristic crystal form of FeCO3-MgCO3 isomorphous series has not been observed. This indicates that it formed later than carbonates A. Carbonates C shows irregular morphology, the crystal form of it is not developed. There is no characteristic morphology in crystal or in aggregate, indicating that the formation duration of carbonates C is very short and formed after carbonates A and carbonates B.
In conclusion, the sequence of carbonates formation in the experiment is carbonates A, carbonates B, carbonates C. We proves our inference in terms of atomic composition and morphology of carbonates.
4.3. Formation Stages of Authigenic Carbonates Induced by Microbes in the Marine Sediment
Authigenic minerals is the minerals formed in the ocean in situ, and authigenic carbonate is one of them. Authigenic carbonate is widely distributed in the modern ocean. Its composition and morphology are varied and was significantly affected by microbial action. However, the stage and model of authigenic carbonate formation induced by microbes in marine sediments have not been proposed. We will discuss this problem in this section. There are many ways for microbes to induce the formation of authigenic carbonates [53]. In this section, we only discuss the stage and model of authigenic carbonate formation performed by AOM and related microbes.
In order to distinguish the types of marine authigenic minerals, Zhu summarized the basis and contents of previous studies on the classification of the formation stages of marine authigenic minerals [49]. He proposed that the formation stage of authigenic minerals has the characteristically physical and chemical environment. Therefore, the formation stage of authigenic minerals should be regarded as the basis of the classification of authigenic minerals. On this basis, three stages of authigenic mineral formation are proposed in his theory: sedimentary stage (also known as hydrogenic stage), halmyrolysis stage, and syndiagenesis stage.
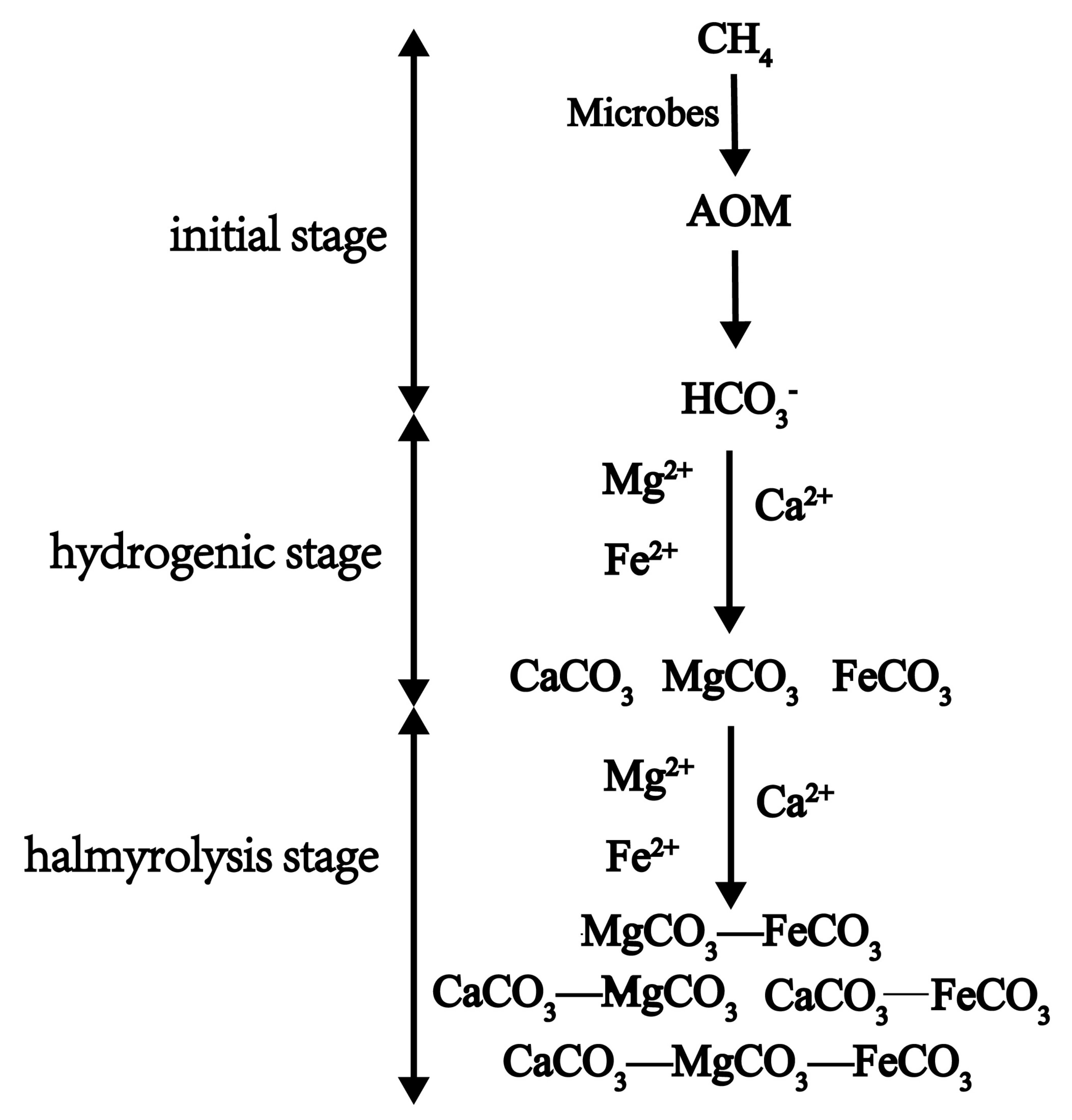
According to our work and previous studies, we propose a formation process of authigenic carbonates performed by AOM and related microbes in marine sediments as Figure 6. The initial stage, the concentration of HCO3− in the pore water increases continuously due to the performance of AOM and related microbes, which leads to the increase of alkalinity in pore water [54]. The hydrogenic stage, according to the relationship between the ion product and solubility product of various carbonate minerals, a kind of carbonate mineral is preferentially formed. The halmyrolysis stage, according to the isomorphous degree of carbonates of different isomorphous series, isomorphism occurs between the carbonate formed in hydrogenic stage and different kinds of metal cations which are abundant in pore water of marine sediments. The isomorphous series of carbonates with two members, three members, or more were formed.
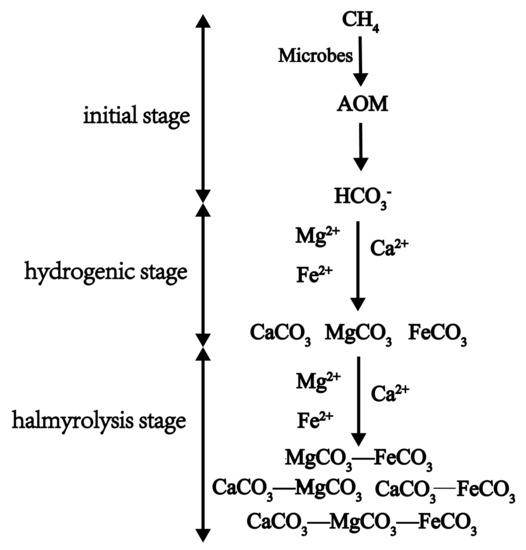
Figure 6.
Formation stages of authigenic carbonates induced by AOM and related microbes.
The increase of alkalinity is a direct result of the performance of AOM, which has been confirmed in previous experiments and field observations [9,55,56]. It is the same trend of alkalinity increase in Figure 3d. The formation mechanism of single component carbonate is very clear and will not be discuss. The isomorphous series of carbonates with two members or three members was common in the field. The isomorphism of two members such as the high magnesium calcite, calcium magnesite, monohydrocalcite, aragonite [11,38,57], and Mg-doped amorphous calcium carbonate [58]. The isomorphism of three members (CaCO3-MgCO3-FeCO3) such as siderite doped with Ca and Mg, ankerite in the field observation [38,59], mixture of CaCO3-MgCO3-FeCO3 in the laboratory experiment [20,34].
The formation and evolution of carbonates in field are actually on long time scales, and isomorphous substitution of carbonates is complex with numerous members, such as Mn, Ni, Si, Zn, Pb, Sr, Ba, Co, etc. In this paper, we only consider the isomorphous series member of Ca, Mg, and Fe. The amount of authigenic carbonates formed in the experiment cannot reach the minimum amount limited by FTIR and XRD detection, so the mineral crystal structure and isomorphous series cannot be identified accurately, which needs to be further studied with longer experimental duration and better experimental design.
5. Conclusions
- The microbial community changed during the experiment. Citrobacter and pseudomonas were the dominant strain at the beginning and end of the experiment, respectively. Both of them have the ability to promote the formation of carbonate minerals.
- In the experiment, FeCO3 is formed first, and then the complete isomorphous series of FeCO3-MgCO3 is formed. However, CaCO3 accounts for a small proportion in the isomorphous mixture of carbonates.
- We propose a formation process of authigenic carbonates performed by AOM and related microbes in marine sediments. It is divided into three stages. (1) The alkalinity of pore water increases during the initial stage. (2) A kind of carbonate mineral is preferentially formed during the hydrogenic stage. (3) The isomorphous series of carbonates were formed during the late halmyrolysis stage.
Author Contributions
Conceptualization, X.L.; investigation, J.W., Z.S., Z.W.; resources, S.S.; writing—original draft preparation, Z.W.; writing—review and editing, Y.C., H.T.; supervision, T.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 41877185.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Acknowledgments
Thanks for Yukun Bai, Keqi Bei, Mingcong Wei, Fugang Wang, Chenghao Zhong for contributions and help in investigation and draft preparation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
The medium composition was improved base on previous study [23] (g/L):KH2PO4 0.744, K2HPO4·2H2O 0.865, MgCl2·6H2O 11.896, (NH4)2SO4 0.2248, CaCl2·2H2O 1.3344, Na2SO4 2.932, NaCl 23.3, pH = 7, Na2MoO4·2H2O 0.02, CuSO4·5H2O 0.02, FeSO4·7H2O 0.01251, ZnSO4·7H2O 0.2, MnCl2·4H2O 0.00263, yeast extract 1, ascorbic acid 1.33, sodium lactate (wt. 50%) 10 mL/L, and demineralized water. Prior to the addition of the ascorbic acid and FeSO4·7H2O, the medium was boiled in 121 °C 20 min. H2S is the metabolic product of AOM. In many incubation investigations, it has been proved that sulfides have an inhibiting effect on AOM performance and consortium growth [60,61]. In order to avoid the effect of sulfide on incubation, continuous culture mode and membrane bioreactors were used to perform incubation [62,63]. Therefore, H2S is not used in our microbes incubation.
References
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2016, 55, 126–168. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef]
- Liu, L.H.; Wu, N.Y. Simulation of advective methane flux and AOM in Shenhu area, the northern South China Sea. Environ. Earth Sci. 2014, 71, 697–707. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed]
- Reeburgh, W.S. Global Methane Biogeochemistry. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 71–94. [Google Scholar]
- Boetius, A.; Ravenschlag, K. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef]
- Egger, M.; Riedinger, N. Global diffusive fluxes of methane in marine sediments. Nat. Geosci. 2018, 11, 421–425. [Google Scholar] [CrossRef]
- Wu, D.; Sun, T. Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence. Int. J. Environ. Res. Public Health 2019, 16, 2299. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Q. Formation of authigenic carbonates at a methane seep site in the middle Okinawa Trough, East China Sea. J. Asian Earth Sci. 2019, 185, 104028. [Google Scholar] [CrossRef]
- De Boever, E.; Swennen, R. Lower Eocene carbonate cemented chimneys (Varna, NE Bulgaria): Formation mechanisms and the (a)biological mediation of chimney growth? Sediment. Geol. 2006, 185, 159–173. [Google Scholar] [CrossRef]
- Tong, H.; Feng, D. Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology. Mar. Pet. Geol. 2013, 43, 260–271. [Google Scholar] [CrossRef]
- Ruban, A.; Rudmin, M. The Formation of Authigenic Carbonates at a Methane Seep Site in the Northern Part of the Laptev Sea. Minerals 2020, 10, 948. [Google Scholar] [CrossRef]
- Thiagarajan, N.; Cremerie, A.; Blattler, C.; Lepland, A.; Kirsimae, K.; Higgins, J.; Brunstad, H.; Eiler, J. Stable and clumped isotope characterization of authigenic carbonates in methane cold seep environments. Geochim. Cosmochim. Acta 2020, 279, 204–219. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Sauer, S.; Crémière, A.; Knies, J.; Lepland, A.; Sahy, D.; Martma, T.; Noble, S.R.; Shoneberger, J.; Klug, M.; Schubert, C.J. U-Th chronology and formation controls of methane-derived authigenic carbonates from the Hola trough seep area, northern Norway. Chem. Geol. 2017, 470, 164–179. [Google Scholar] [CrossRef]
- Song, J. Marine Biogeochemistry; China Science Publishing & Media Ltd.: Beijing, China, 2020. [Google Scholar]
- Ye, T.; Guangrong, G. Experimental and numerical simulation of the formation of cold seep carbonates in marine sediments. Int. J. Environ. Res. Public Health 2019, 16, 1433. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, H. Bio-precipitation of carbonate and phosphate minerals induced by the bacterium citrobacter freundii ZW123 in an anaerobic environment. Minerals 2020, 10, 65. [Google Scholar] [CrossRef]
- Wei, M. The Formation of Authigenic Minerals during Methane Seeping in Seafloor: Insight from Laboratory Test; Jilin University: Changchun, Jilin, China, 2016. [Google Scholar]
- Xu, T.F.; Bei, K.Q. Laboratory experiment and numerical simulation on authigenic mineral formation induced by seabed methane seeps. Mar. Pet. Geol. 2017, 88, 950–960. [Google Scholar] [CrossRef]
- Wu, D. Early Diagenesis Records and Geochemical Characteristics of Gas Hydrate in the South China Sea; Zhejiang University: Zhejiang, China, 2008. [Google Scholar]
- Daidai, W.; Nengyou, W. Geochemical Characteristics of Shallow Sediments in Gas Hydrate-bearing Area, Northeastern South China Sea. In Proceedings of the Fourth Chinese National Conference on Sedimentology, Qingdao, China, 16–20 October 2009; Volume 30, pp. 41–51. [Google Scholar]
- Liu, X.; Xu, T. Experiment on anaerobic oxidation of methane and precipitation of carbonate mediated by microbes. J. Cent. South Univ. (Sci. Technol.) 2016, 47, 1473–1479. [Google Scholar] [CrossRef]
- Hongxiang, G.; Yongge, S. Factors controlling the types of microbial consortia in cold-seep environments: A molecular and isotopic investigation of authigenic carbonates from the South China Sea. Chem. Geol. 2013, 354, 55–64. [Google Scholar] [CrossRef]
- Kleindienst, S.; Ramette, A. Distribution and in situ abundance of sulfate-reducing bacteria in diverse marine hydrocarbon seep sediments. Environ. Microbiol. 2012, 14, 2689–2710. [Google Scholar] [CrossRef]
- Bowles, M.W.; Samarkin, V.A. Weak coupling between sulfate reduction and the anaerobic oxidation of methane in methane-rich seafloor sediments during ex situ incubation. Geochim. Cosmochim. Acta 2011, 75, 500–519. [Google Scholar] [CrossRef]
- Chunshuang, J.; Jiyang, W. A Preliminary Study of the Gas Hydrate Stability Zone in the South China Sea. Acta Geol. Sin. Engl. Ed. 2002, 76, 423–428. [Google Scholar] [CrossRef]
- Liu, C.L.; Ye, Y.G. Experimental studies on the P-T stability conditions and influencing factors of gas hydrate in different systems. Sci. China Earth Sci. 2013, 56, 594–600. [Google Scholar] [CrossRef]
- Luff, R.; Wallmann, K. Fluid flow, methane fluxes, carbonate precipitation and biogeochemical turnover in gas hydrate-bearing sediments at Hydrate Ridge, Cascadia Margin: Numerical modeling and mass balances. Geochim. Cosmochim. Acta 2003, 67, 3403–3421. [Google Scholar] [CrossRef]
- Chapelle, F. Ground-Water Microbiology and Geochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Schulz, H.D. Redox Measurements in Marine Sediments; Springer: Berlin, Germany, 2000. [Google Scholar]
- Widdel, F.; Bak, F. Gram-Negative Mesophilic Sulfate-Reducing Bacteria; Springer: New York, NY, USA, 1992. [Google Scholar]
- Peck, H.D.; LeGall, J. Biochemistry of Dissimilatory Sulphate Reduction [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1982, 298, 443–466. [Google Scholar]
- Mingcong, W.; Tianfu, X. The Effection of the Temperature and Pressure Condition on the Formation of Authigene Minerals and Iron Sulfide in the Microorganisms System. Acta Sedimentol. Sin. 2018, 36, 664–673. [Google Scholar]
- Postgate, J.R. The Sulphate-Reducing Bacteria; Cambridge University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Shen, Z. Fundamentals of hydrogeochemistry; Geological Publishing House: Beijing, China, 1993. [Google Scholar]
- Feng, D.; Chen, D. Authigenic carbonates from an active cold seep of the northern South China Sea: New insights into fluid sources and past seepage activity. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 74–83. [Google Scholar] [CrossRef]
- Li, J.; Peng, X. Biogeochemical processes controlling authigenic carbonate formation within the sediment column from the Okinawa Trough. Geochim. Cosmochim. Acta 2018, 222, 363–382. [Google Scholar] [CrossRef]
- Heng, M.; Fuchun, L. Morphological evolution during the formation of carbonate spherulite in Citrobacter freundii culture. Geol. J. China Univ. 2009, 15, 429–436. [Google Scholar]
- Greenfield, L.J. Metabolism and concentration of calcium and magnesium and precipitation of calcium carbonate by a marine bacterium. Ann. N. Y. Acad. Sci. 1963, 109, 23–45. [Google Scholar] [CrossRef]
- Jones, B.; Peng, X. Hot spring deposits on a cliff face: A case study from Jifei, Yunnan Province, China. Sediment. Geol. 2014, 302, 1–28. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbe–mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2010, 51, 745–765. [Google Scholar] [CrossRef]
- Decho, A.W. Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 2010, 36, 137–144. [Google Scholar] [CrossRef]
- Xingmin, R. Thermodynamic Investigations on the Interactions of Bacteria with Soil Clay Minerals; Huazhong Agricultural University: Hongshan, Hubei, China, 2008. [Google Scholar]
- Xiurong, H.; Guanglie, L. Study on the mechanism of the interaction between montmorillonite and bacterium. Acta Pharm. Sin. 2002, 37, 718–720. [Google Scholar]
- Zhiyu, T. Study on the Determination Method of Bacterial Number Adsorbed on the Surface of Clay Minerals and on the Enrichment Method of Teace Composition in Assembly of Clay Minerals; Nanjing Agricultural University: Nanjing, China, 2015. [Google Scholar]
- Zhu, T.T.; Fan, J. Experimental study on the interaction between Pseudomonas mendocina and montmorillonite. Bull. Miner. Petrol. Geochem. 2011, 30, 304–310. [Google Scholar]
- Ebinger, M.H. Water-Rock Interactions and the pH Stability of Groundwater from Yucca Mountain, Nevada; Office of Scientific & Technical Information: Oak Ridge, TN, USA, 1992. [Google Scholar]
- Erqing, Z. Marine Authigenic Mineral; Ocean Press: Lancing, UK, 1988. [Google Scholar]
- Curtis, C.D.; Coleman, M.L. Pore water evolution during sediment burial from isotopic and mineral chemistry of calcite, dolomite and siderite concretions. Geochim. Cosmochim. Acta 1986, 50, 2321–2334. [Google Scholar] [CrossRef]
- Zhaolu, P. Crystallography and Mineralogy; Geological Publishing House: Beijing, China, 1993. [Google Scholar]
- Shanrong, Z. Crystallography and Mineralogy; Higher Education Press: Beijing, China, 2011. [Google Scholar]
- Yarong, H. Study of Mineralization Mechanism of Carbonates and Sulfates Mediated by Marine Microbes; University of Science and Technology of China: Hefei, Anhui, China, 2019. [Google Scholar]
- Bazzaro, M.; Ogrinc, N. Geochemical signatures of intense episodic anaerobic oxidation of methane in near-surface sediments of a recently discovered cold seep (Kveithola trough, NW Barents Sea). Mar. Geol. 2020, 425, 106189. [Google Scholar] [CrossRef]
- Orcutt, B.; Boetius, A. Molecular biogeochemistry of sulfate reduction, methanogenesis and the anaerobic oxidation of methane at Gulf of Mexico cold seeps. Geochim. Cosmochim. Acta 2005, 69, 4267–4281. [Google Scholar] [CrossRef]
- Milucka, J.; Ferdelman, T.G. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 2012, 491, 541–546. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X. Geochemical evidence of methane seepage in the sediments of the Qiongdongnan Basin, South China Sea. Chem. Geol. 2020, 543, 119588. [Google Scholar] [CrossRef]
- Opitz, P.; Besch, L. Insights into the In Vitro Formation of Apatite from Mg-Stabilized Amorphous Calcium Carbonate. Adv. Funct. Mater. 2021, 31, 2007830. [Google Scholar] [CrossRef]
- Isaack, A.; Gischler, E. Facies variations in response to Holocene sea-level and climate change on Bora Bora, French Polynesia: Unravelling the role of synsedimentary siderite in a tropical marine, mixed carbonate-siliciclastic lagoon. Mar. Geol. 2017, 390, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Henriet, J.P. Stimulation of in vitro anaerobic oxidation of methane rate in a continuous high-pressure bioreactor. Bioresour. Technol. 2010, 101, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Clech, P.L. Membrane fouling in membrane bioreactors for wastewater treatment. J. Environ. Eng. 2002, 128, 1018–1029. [Google Scholar] [CrossRef]
- Timmers, P.H.A.; Gieteling, J. Growth of anaerobic methane-oxidizing archaea and sulfate-reducing bacteria in a high-pressure membrane capsule bioreactor. Appl. Environ. Microbiol. 2015, 81, 1286–1296. [Google Scholar] [CrossRef]
- Girguis, P.R.; Orphan, V.J. Growth and methane oxidation rates of anaerobic methanotrophic archaea in a continuous-flow bioreactor. Appl. Environ. Microbiol. 2003, 69, 5472–5482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).