The Use of Living Mussels as a Substratum for Growing Seedlings of Two Sargassum Species from the Perspective of Coastal Seaweed Bed Restoration in the East China Sea
Abstract
1. Introduction
2. Material and Methods
2.1. Selection of Mussel Specifications
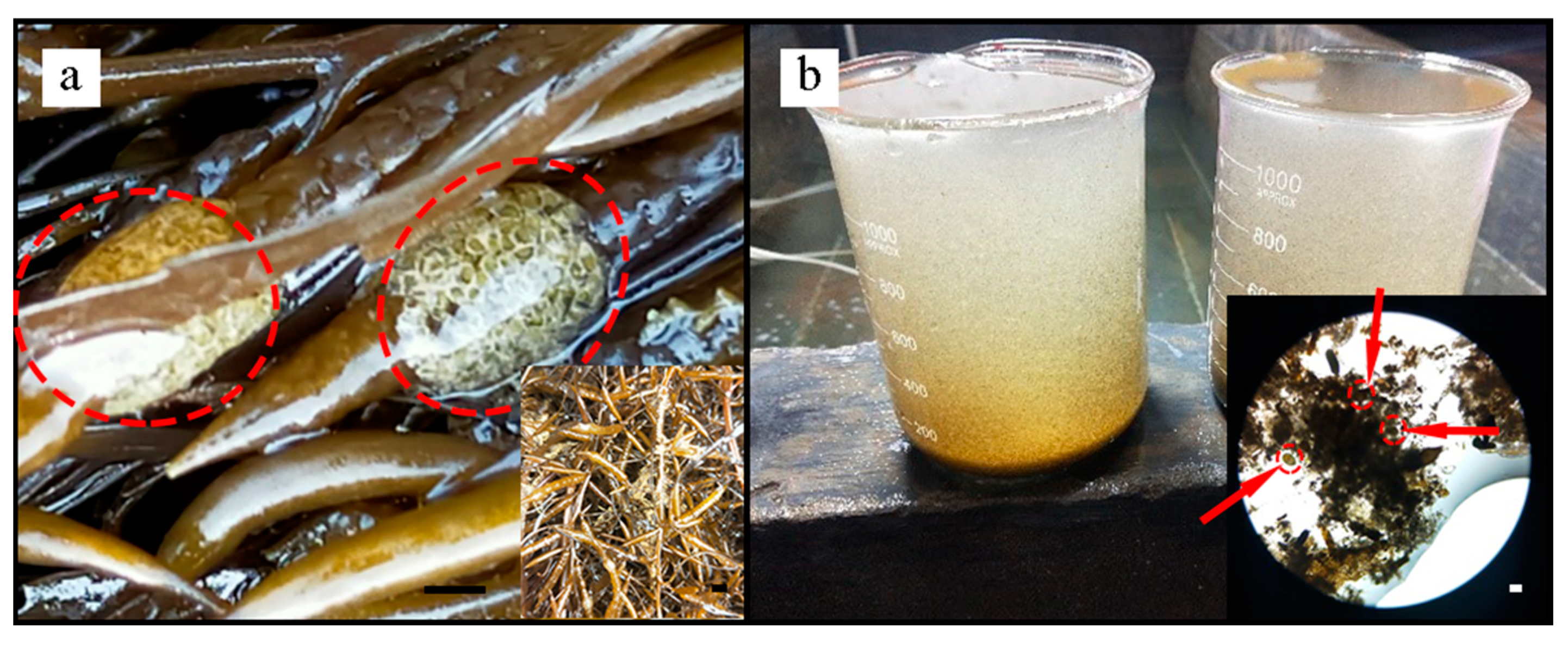
2.2. Collection of Zygotes from Seaweed
2.3. Experimental Design
2.4. Data Analysis
3. Results
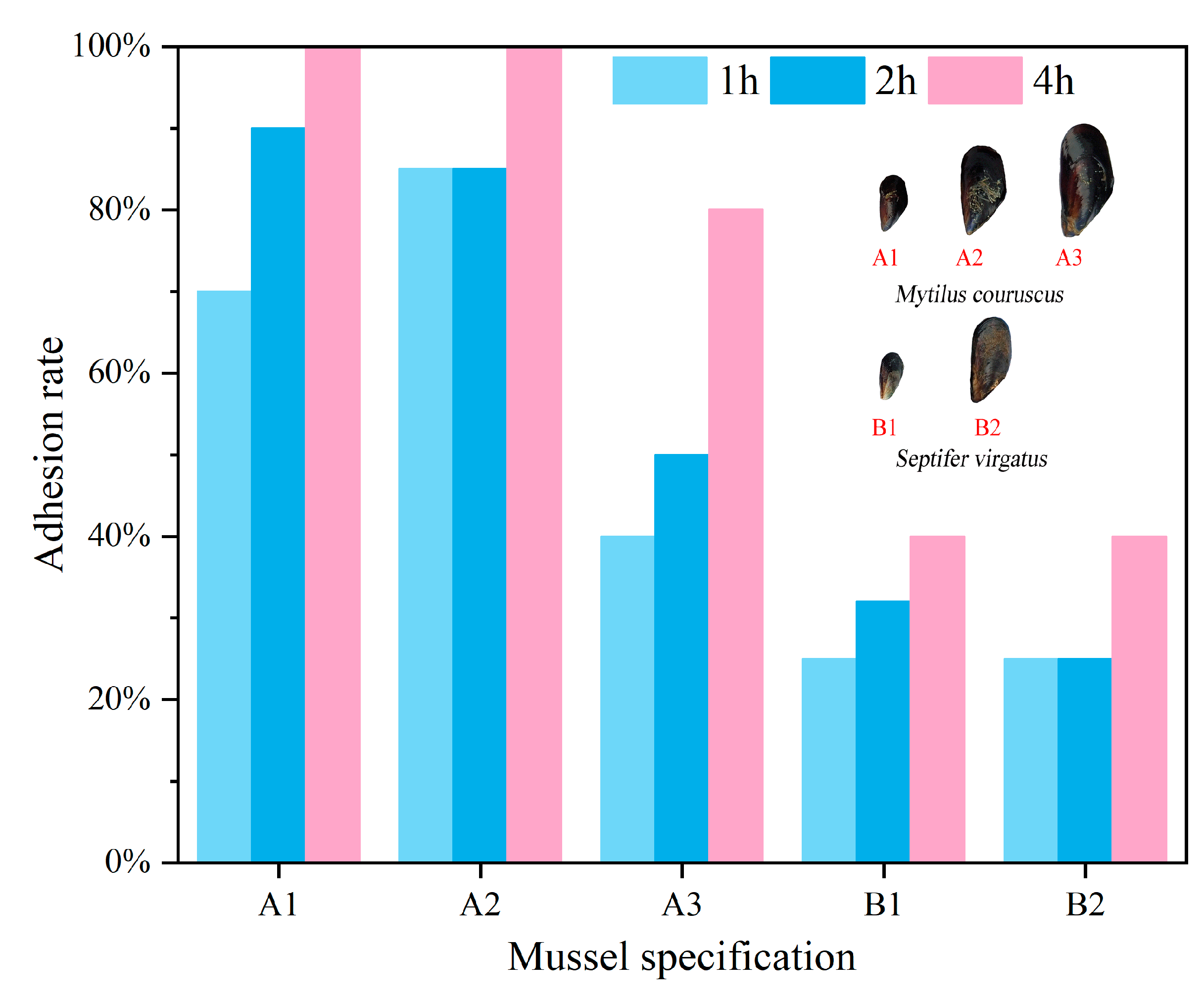
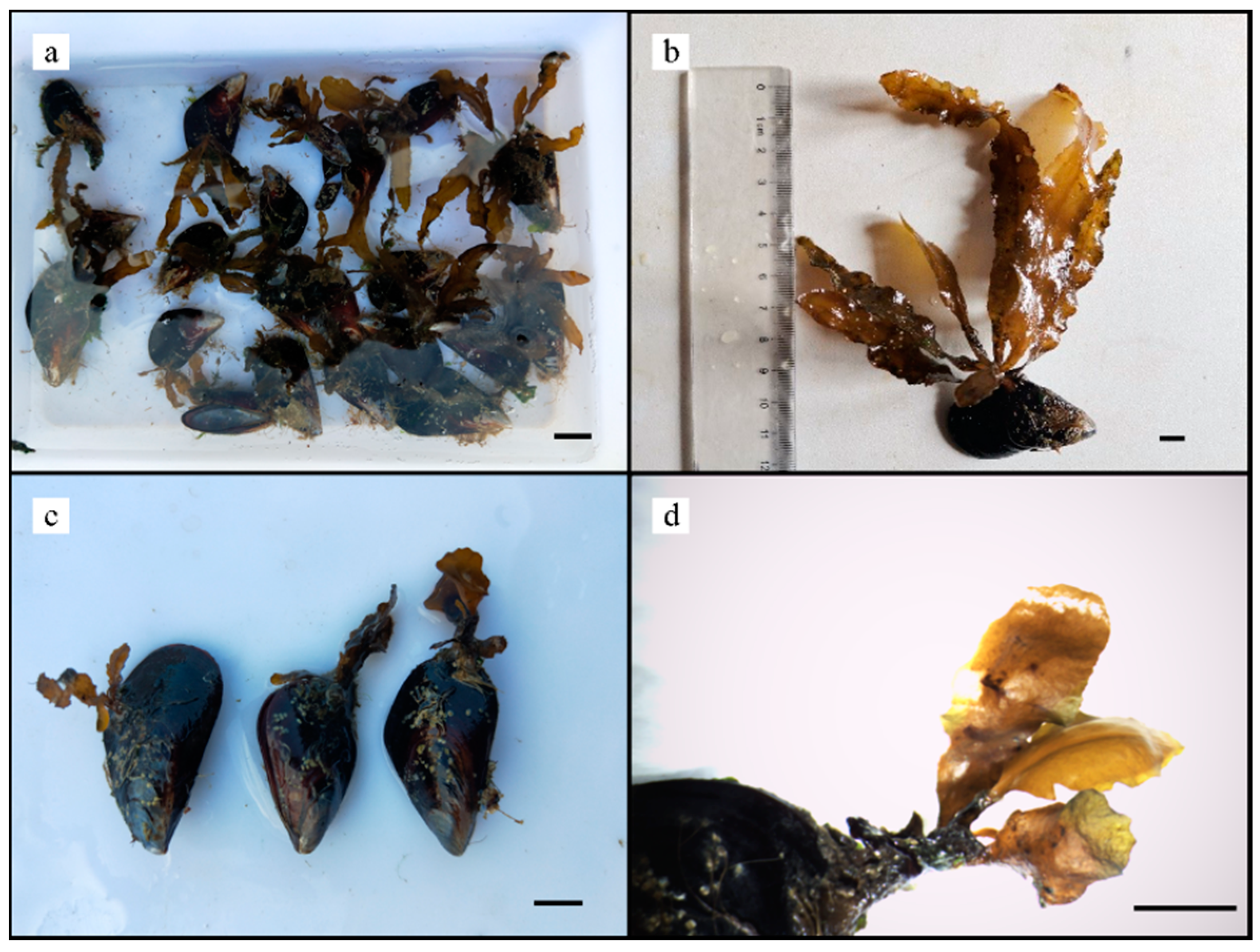
3.1. Adhesion Force and Rate of Mussels
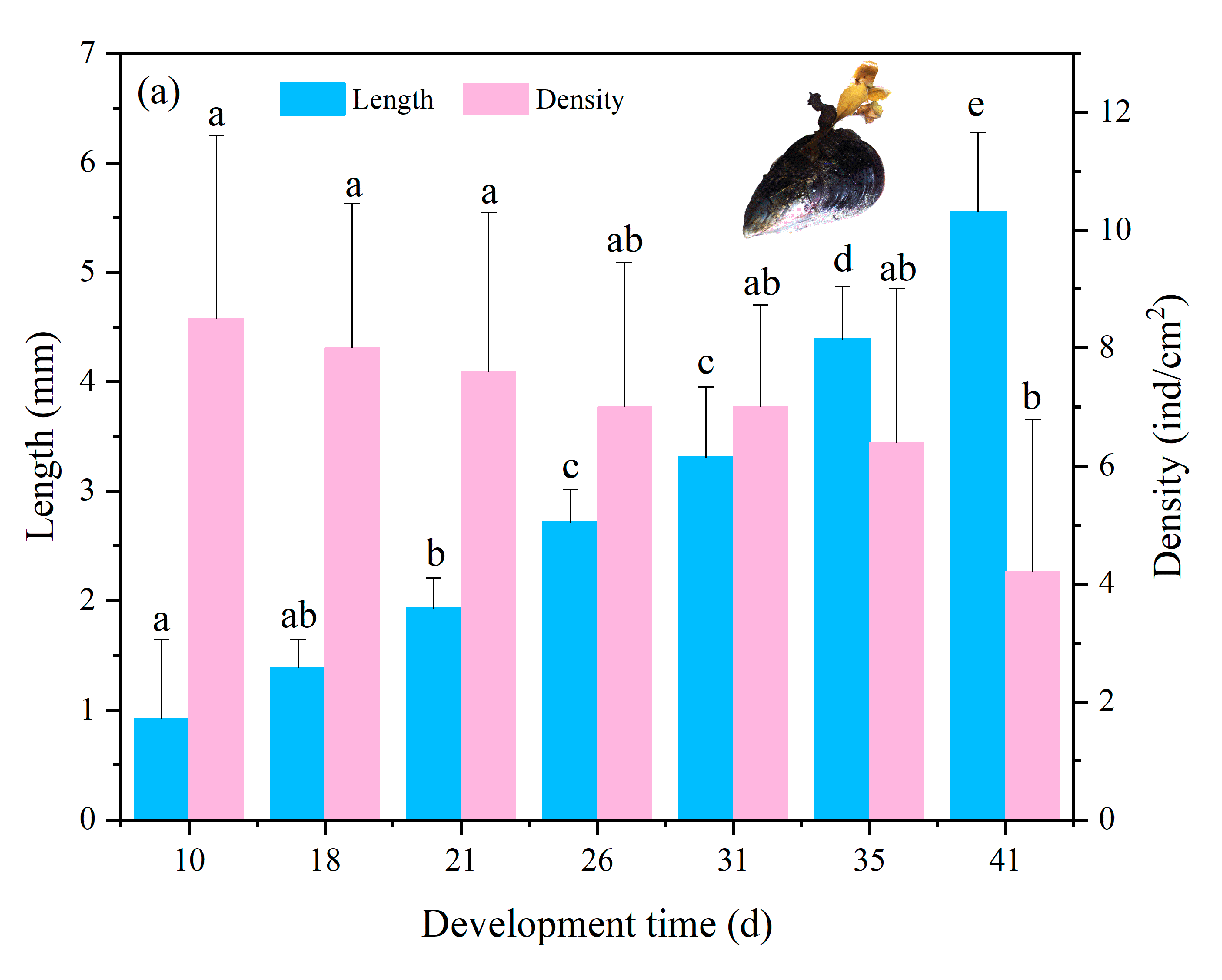
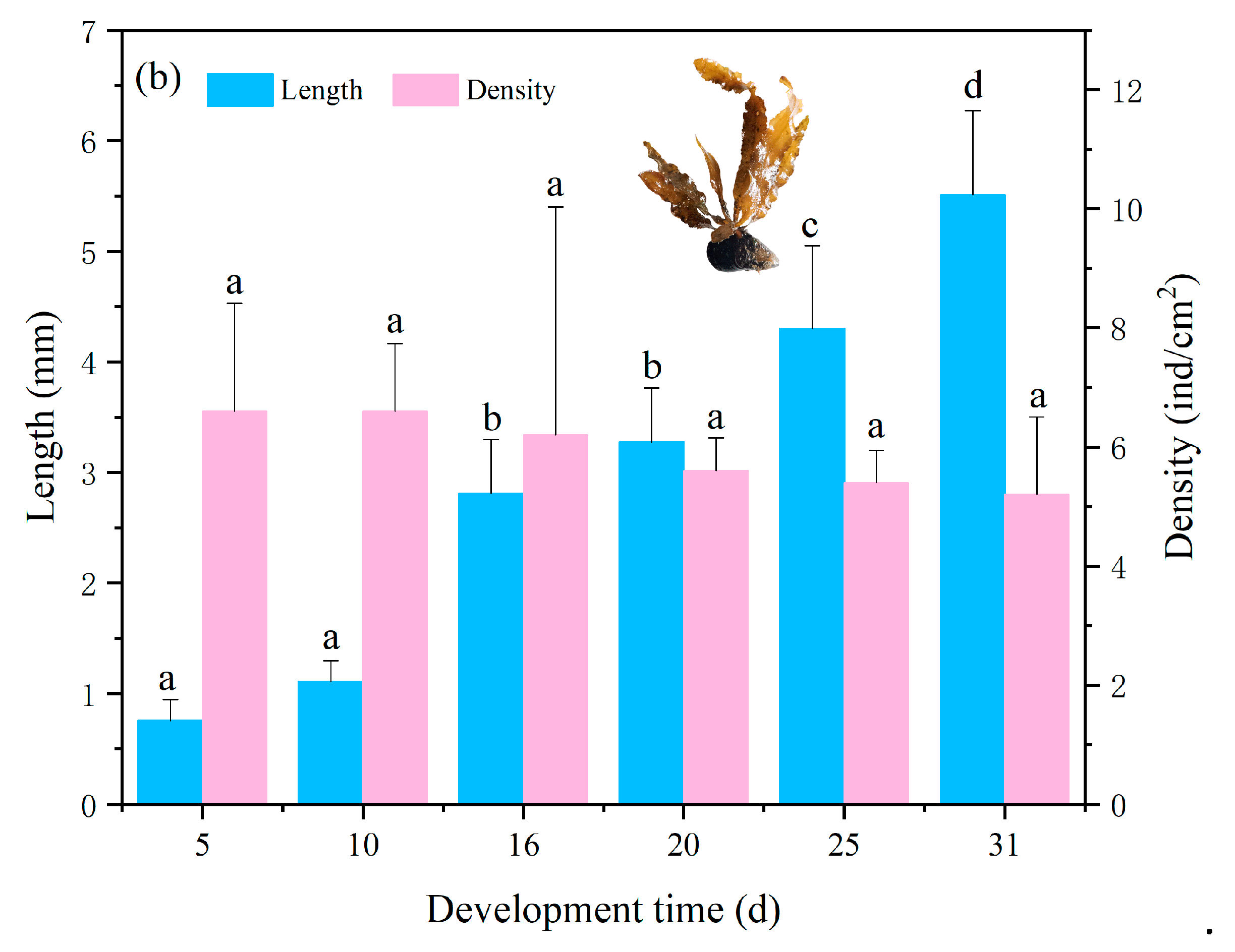
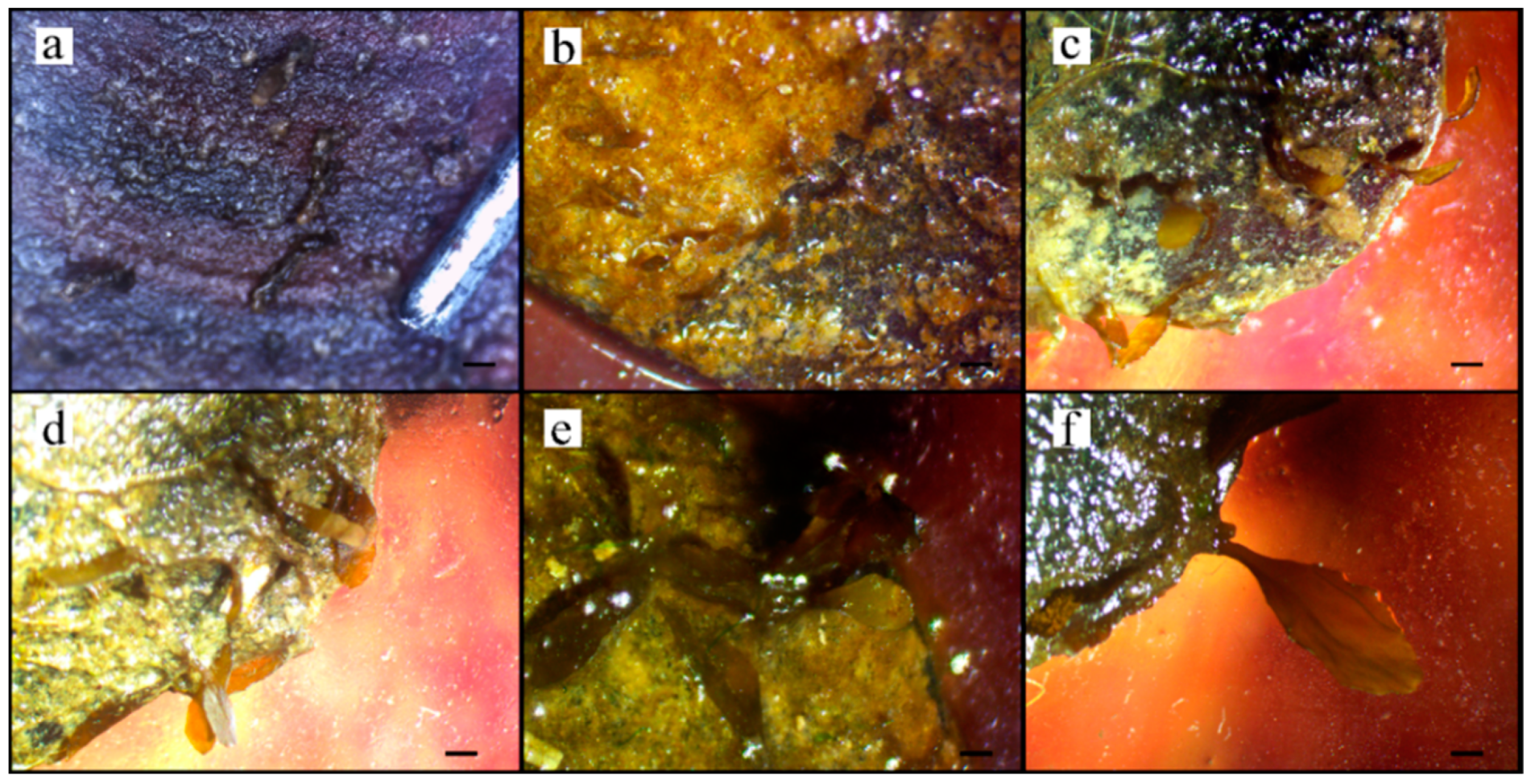
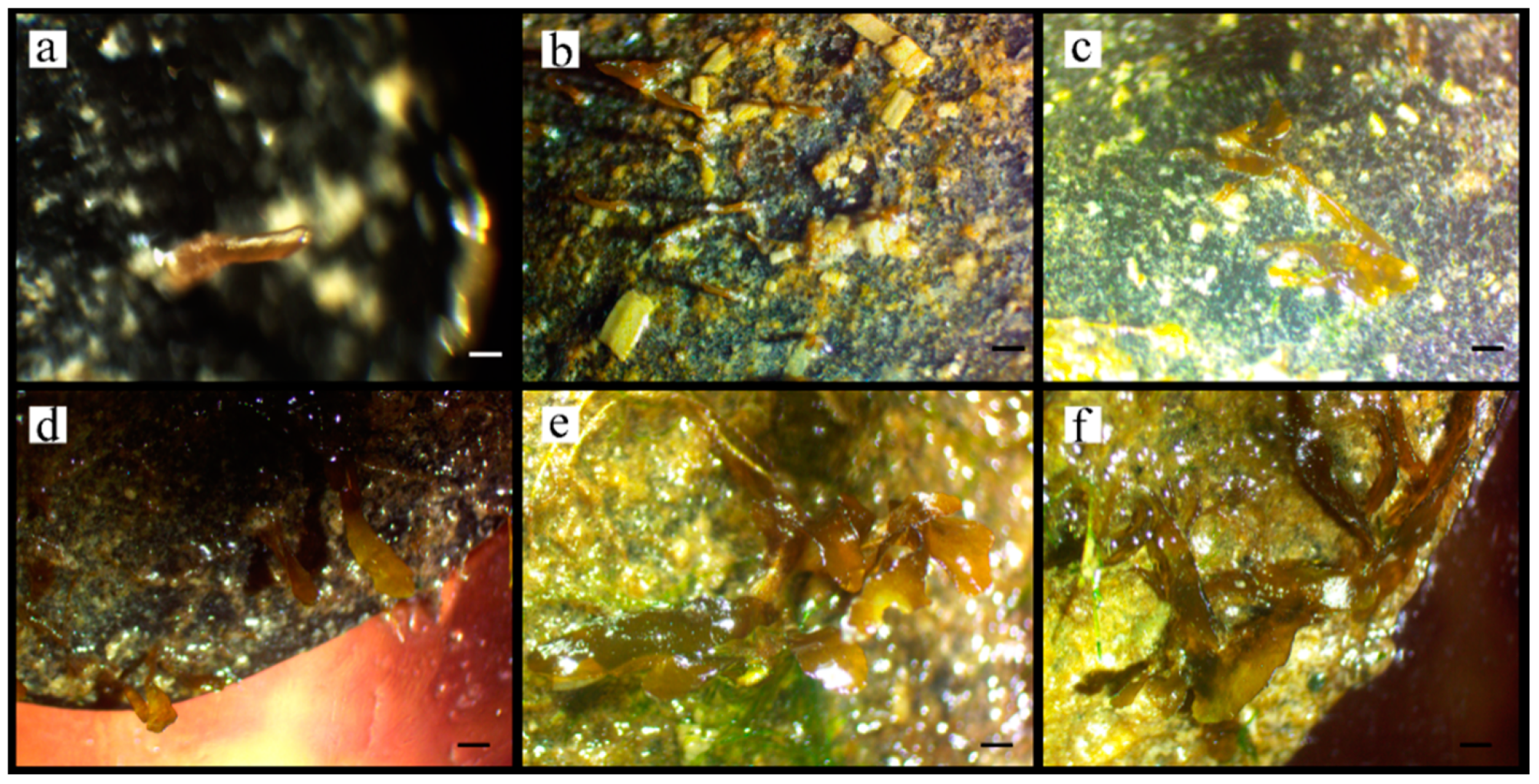
3.2. Adhesion and Growth of Zygotes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, T.A.; Volesky, B.; Vieira, R.H.S.F. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef]
- Wood, G.; Marzinelli, E.M.; Coleman, M.A.; Campbell, A.H.; Santini, N.S.; Kajlich, L.; Verdura, J.; Wodak, J.; Steinberg, P.D.; Vergés, A. Restoring subtidal marine macrophytes in the Anthropocene: Trajectories and future-proofing. Mar. Freshw. Res. 2019, 70, 7. [Google Scholar] [CrossRef]
- Huang, B.X.; Ding, L.P.; Tan, H.Q.; Sun, G.D. Species diversity and distribution of genus sargassum in China seas. Oceanol. Limnol. Sinica 2013, 44, 69–76. [Google Scholar]
- Abdullah, M.I.; Fredriksen, S. Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J. Mar. Biol. Assoc U. K. 2004, 84, 887–894. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W. Loss, Status and Trends for Coastal Marine Habitats of Europe. Oceanogr. Mar. Biol. 2007, 45, 345–405. [Google Scholar]
- Connell, S.D.; Russell, B.D.; Turner, D.J.; Shepherd, S.A.; Kildea, T.; Miller, D.; Airoldi, L.; Cheshire, A. Recovering a lost baseline: Missing kelp forests from a metropolitan coast. Mar. Ecol. Prog. Ser. 2008, 360, 63–72. [Google Scholar] [CrossRef]
- Gorman, D.; Russell, B.D.; Connell, S.D. Land-to-sea connectivity: Linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecol. Appl. 2009, 19, 1114–1126. [Google Scholar] [CrossRef]
- Karen, F.D.; Thomas, W. Rise of Turfs: A New Battlefront for Globally Declining Kelp Forests. BioScience 2018, 68, 64–76. [Google Scholar]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Wernberg, T.; Filbee-Dexter, K. Missing the marine forest for the trees. Mar. Ecol. Prog. Ser. 2019, 612, 209–215. [Google Scholar] [CrossRef]
- Ling, S.D. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: A new and impoverished reef state. Oecologia 2008, 156, 883–894. [Google Scholar] [CrossRef]
- Ambrose, R.F. Mitigating the Effects of a Coastal Power Plant on a Kelp Forest Community_ Rationale and Requirements for an Artificial Reef. Bull. Mar. Sci. 1994, 55, 694–708. [Google Scholar]
- Carter, J.M.; Carpenter, A.L.; Foster, M.S.; Jessee, W.N. Benthic succession on an artificial reef designed to support a kelp-reef community. Bull. Mar. Sci. 1985, 37, 86–113. [Google Scholar]
- Layton, C.; Coleman, M.A.; Marzinelli, E.M.; Steinberg, P.D.; Swearer, S.E.; Vergés, A.; Wernberg, T.; Johnson, C.R. Kelp Forest Restoration in Australia. Front. Mar. Sci. 2020, 7, 74. [Google Scholar] [CrossRef]
- Terawaki, T.; Hasegawa, H.; Arai, S.; Ohno, M. Management-free techniques for restoration of Eisenia and Ecklonia beds along the central Pacific coast of Japan. J. Appl. Phycol. 2001, 13, 13–17. [Google Scholar] [CrossRef]
- Umezaki, I. Ecological Studies of Sargassum horneri (TURNER) C. AGARDH in Obama Bay, Japan Sea. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1193–1200. [Google Scholar] [CrossRef]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefali, M.E.; Cebrian, E. Restoration of a Canopy-Forming Alga Based on Recruitment Enhancement: Methods and Long-Term Success Assessment. Front. Plant. Sci. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Lee, J.H.; Han, S.H.; Jeon, W.B.; Kim, G.Y.; Kim, S.; Kim, S.; Lee, H.R.; Hwang, D.S.; Jung, S.; et al. A new approach to the restoration of seaweed beds using Sargassum fulvellum. J. Appl. Phycol. 2020, 32, 2575–2581. [Google Scholar] [CrossRef]
- Fredriksen, S.; Filbee-Dexter, K.; Norderhaug, K.M.; Steen, H.; Bodvin, T.; Coleman, M.A.; Moy, F.; Wernberg, T. Green gravel: A novel restoration tool to combat kelp forest decline. Sci. Rep. UK 2020, 10, 3983. [Google Scholar] [CrossRef] [PubMed]
- Fuente, G.D.L.; Chiantore, M.; Asnaghi, V.; Kaleb, S.; Falace, A. First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 2019, 7, e7290. [Google Scholar] [CrossRef]
- Largo, D.B.; Diola, A.G.; Rance, G.M.S. Culture of the brown seaweed Sargassum siliquosum J. Agardh (Phaeophyceae, Ochrophyta): From hatchery to out-planting. J. Appl. Phycol. 2020, 32, 1–18. [Google Scholar] [CrossRef]
- Smale, D.A.; Epstein, G.; Hughes, E.; Mogg, A.O.M.; Moore, P.J. Patterns and drivers of understory macroalgal assemblage structure within subtidal kelp forests. Biodivers. Conserv. 2020, 29, 4173–4192. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.G.; Zhong, C.H.; Yan, X.H.; Zhang, L.N.; Liu, Y. Study on seed breeding of Sargassum horneri. Acta Hydrobiol. Sin. 2018, 42, 428–438. [Google Scholar]
- Zhang, J. Indoor Cultivation of the Brown Alga Sargassum Vachellianum and Sargassum Horneri: Morphological Observation and Techniques of Artificial Seeding. Master’s Thesis, Shagnhai Ocean University, Shanghai, China, 2012. [Google Scholar]
- Platell, M.E.; Ang, H.P.; Hesp, S.A.; Potter, I.C. Comparisons between the influences of habitat, body size and season on the dietary composition of the sparid Acanthopagrus latus in a large marine embayment. Estuar. Coast. Shelf Sci. 2007, 72, 626–634. [Google Scholar] [CrossRef]
- Sun, P.; Ling, J.Z.; Zhang, H.; Tang, B.J.; Jiang, Y.Z. Diet composition and feeding habits of black sea bream (Acanthopagrus schlegelii) in Xiangshan Bay based on high-throughput sequencing. Acta Ecol. Sin. 2021, 41, 1221–1228. [Google Scholar]
- Liu, S.P.; Zhao, Y.C.; Yang, N.; Wang, C.D. Amethod for Seedling Seedling Seeding of Fixed Living Algae on the Sea Surface. Chinese Patent ZL201110097012.7, 3 August 2011. [Google Scholar]
- Tang, L.Q.; Wang, Q.X.; Liu, H.J.; Zhang, Z.P.; Liu, C.Y.; Zhou, J. Habitat suitability of Stichopus japonicas, Scapharca broughtonii and Mytilus edulis in the shallow waters of Xiaoheishan Isalnd. Acta Ecol. Sin. 2017, 37, 668–682. [Google Scholar]
- Lv, J.F.; Du, X.X.; Qu, X.W.; Liu, S.P. Investigation of the transport technology of Mytilus edulis and Saccharina japonica. Mar. Sci. 2019, 43, 88–94. [Google Scholar]





| Specifications | Species | W (g) | L (mm) | SW (mm) |
|---|---|---|---|---|
| A1 | Mytilus coruscus | 0.822 ± 0.154 c | 20.708 ± 1.306 c | 11.992 ± 1.117 b |
| A2 | Mytilus coruscus | 2.203 ± 0.335 b | 30.485 ± 1.828 b | 17.028 ± 0.486 a |
| A3 | Mytilus coruscus | 5.907 ± 0.793 a | 40.148 ± 2.356 a | 22.876 ± 0.372 a |
| B1 | Septifer virgatus | 1.113 ± 0.300 b | 19.916 ± 1.374 b | 11.290 ± 0.614 b |
| B2 | Septifer virgatus | 3.790 ± 1.051 a | 30.740 ± 1.291 a | 15.980 ± 1.991 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, K.; Feng, M.; Chen, J.; Zhang, S.; Bi, Y. The Use of Living Mussels as a Substratum for Growing Seedlings of Two Sargassum Species from the Perspective of Coastal Seaweed Bed Restoration in the East China Sea. J. Mar. Sci. Eng. 2021, 9, 558. https://doi.org/10.3390/jmse9060558
Li X, Wang K, Feng M, Chen J, Zhang S, Bi Y. The Use of Living Mussels as a Substratum for Growing Seedlings of Two Sargassum Species from the Perspective of Coastal Seaweed Bed Restoration in the East China Sea. Journal of Marine Science and Engineering. 2021; 9(6):558. https://doi.org/10.3390/jmse9060558
Chicago/Turabian StyleLi, Xunmeng, Kai Wang, Meiping Feng, Jianqu Chen, Shouyu Zhang, and Yuanxin Bi. 2021. "The Use of Living Mussels as a Substratum for Growing Seedlings of Two Sargassum Species from the Perspective of Coastal Seaweed Bed Restoration in the East China Sea" Journal of Marine Science and Engineering 9, no. 6: 558. https://doi.org/10.3390/jmse9060558
APA StyleLi, X., Wang, K., Feng, M., Chen, J., Zhang, S., & Bi, Y. (2021). The Use of Living Mussels as a Substratum for Growing Seedlings of Two Sargassum Species from the Perspective of Coastal Seaweed Bed Restoration in the East China Sea. Journal of Marine Science and Engineering, 9(6), 558. https://doi.org/10.3390/jmse9060558






