The Dual Role of Microplastics in Marine Environment: Sink and Vectors of Pollutants
Abstract
1. Introduction
1.1. The Overall Problem of Co-Contaminant Vehiculation
1.2. The Protagonists
1.2.1. MP Nature
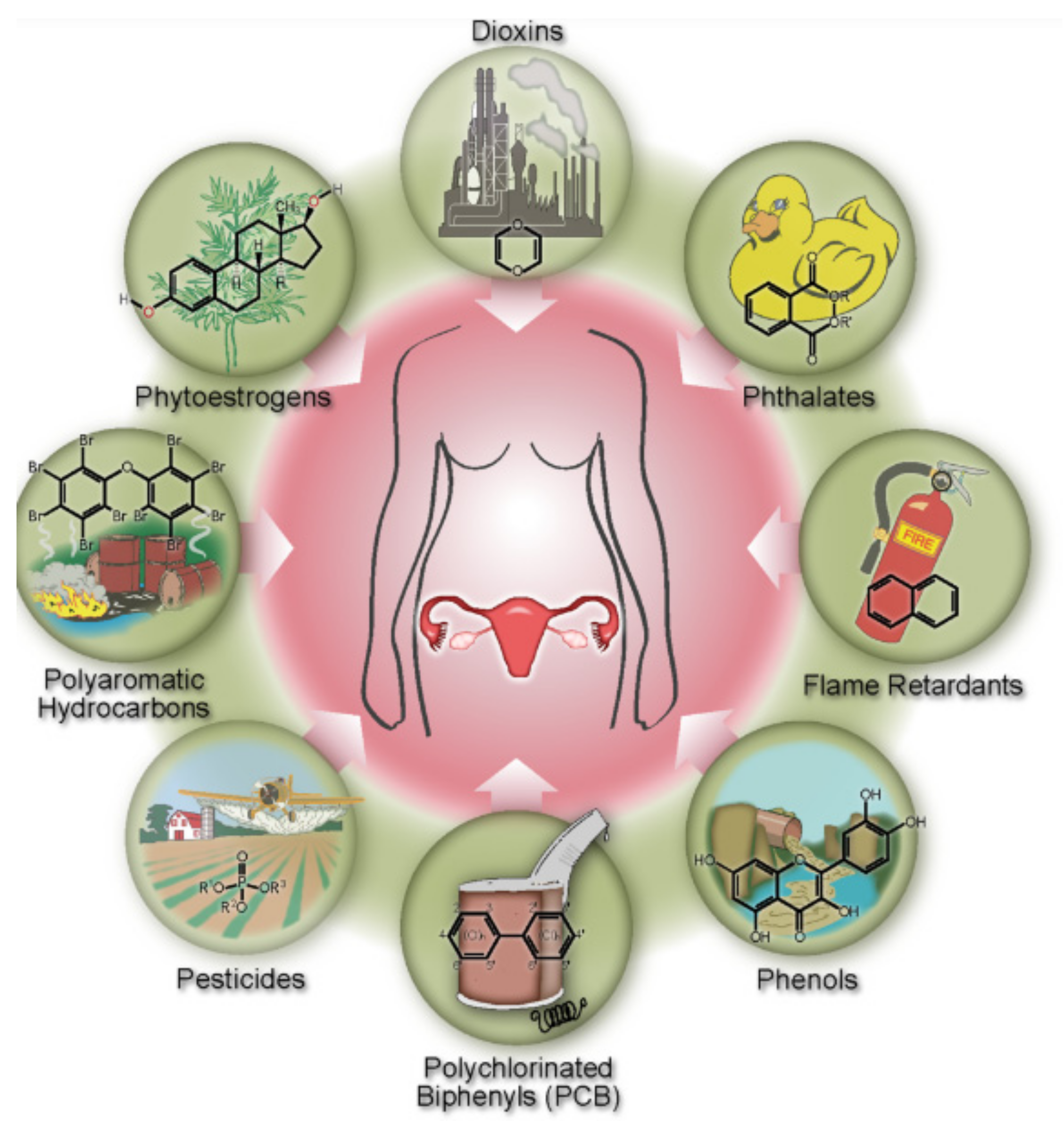
1.2.2. The Potential Sorbing Xenobiotics
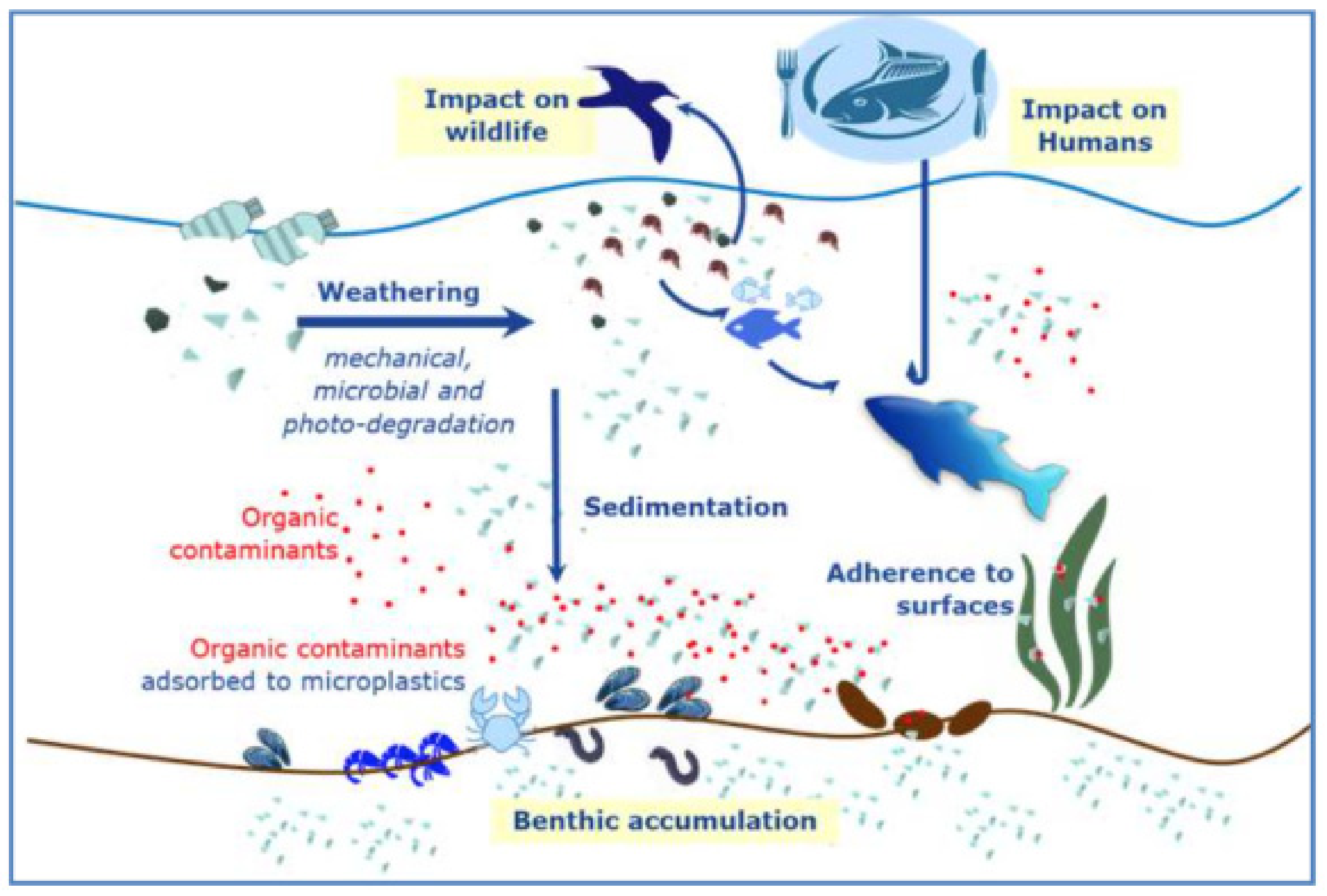
2. Sorption of Pollutants
2.1. The Two Sorption Routes
2.2. The Polymer Factor of Sorption
A Deeper Look at the Polymer Structure
2.3. The Weathering Factor
2.4. The Environmental Factors
3. The Pollutant Bioavailability
4. Interactive Toxicity
5. The Two Conceptual Schools
6. Conclusions
7. Final Considerations and Suggestions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 6689, 30571–30574. [Google Scholar] [CrossRef]
- The Ellen MacArthur Foundation: Rethinking the Future of Plastics & Catalysing Action; The Ellen MacArthur Foundation: Cowes, UK, 2017; pp. 1–67.
- Rai, P.K.; Lee, J.; Brown, R.J.C.; Kim, K.H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mat. 2021, 403, 123910. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M. Research Progress in Transfer, Accumulation and Effects of Microplastics in the Oceans. J. Mar. Sci. Eng. 2021, 9, 433. [Google Scholar] [CrossRef]
- Prichard, P.; Granek, E.F. Effects of pharmaceuticals and personal care products on marine organisms: From single-species studies to an ecosystem-based approach. Environ. Sci. Pollut. Res. 2016, 23, 22365–22384. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Choi, K.C. Treatment with bisphenol A and phthalate stimulated the growth of human LNCaP prostate cancer cells and induced a cell cycle by upregulating transcriptional levels of c-myc and c-fos. Korea Exp. Anim. Soc. Winter Symp. 2012, 2, 107. [Google Scholar]
- Amereh, F.; Babaeic, M.; Eslamia, A.; Fazelipourd, F.; Rafieea, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Poll. 2020, 261, 114158. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 325–340. [Google Scholar]
- Nakashima, E.; Isobe, A.; Kako, S.; Itai, T.; Takahashi, S. Quantification of toxic metals derived from macroplastic litter on Ookushi Beach. Jpn. Environ. Sci. Technol. 2012, 46, 10099–10105. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the Lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
- Rani, M.; Shim, J.W.; Han, M.G.; Jang, M.; Song, Y.K.; Hong, H.S. Benzotriazoletype ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef]
- Jasna, M.; Jelena, L.; Pero, T.; Dubravka, B.V.; Jasna, S.; Josko, P. Levels of trace metals on microplastic particles in beach sediments of the island of Vis, Adriatic Sea, Croatia. Mar. Pollut. Bull. 2018, 137, 231–236. [Google Scholar]
- Jeong, C.; Kang, H.; Lee, Y.H.; Kim, M.; Lee, J.; Seo, J.S.; Wang, M.; Lee, J. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont Rotifer Brachionus koreanus via multi-xenobiotic resistance (MXR) disruption. Environ. Sci. Technol. 2018, 52, 11411–11418. [Google Scholar] [CrossRef]
- Leon, V.M.; Garcia, I.; Gonzalez, E.; Sampler, R.; Fernandez-Gonzalez, V.; Muniategui- Lorenzo, S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ. Pollut. 2018, 236, 442–453. [Google Scholar] [CrossRef]
- Turner, A. Black plastics: Linear and circular economies, hazardous additives and marine pollution. Environ. Int. 2018, 117, 308–318. [Google Scholar] [CrossRef]
- Kaminuma, T.; Ohtake, C.; Kabuyama, N. Distribution andorigin of plastic resin pellets as environmental pollutants at theEast China Sea area. Bull. Nat. Health Sci. 2000, 118, 90–99. [Google Scholar]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Bowmer, T.; Kershaw, P. Particles as a Vector in Transporting Persistent, Bio-Accumulating and Toxic Substances in the Oceans, Proceedings of the GESAMP International Workshop on Micro-Plastic, Paris, France, 28–30 June 2010; Bowmer, T., Kershaw, P., Eds.; UNESCO-IOC: Paris, France, 2010. [Google Scholar]
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Antunes, J.C.; Frias, J.G.L.; Micaelo, A.C.; Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuar. Coast Shelf Sci. 2013, 130, 62–69. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef]
- Gewert, B.; Ogonowski, M.; Barth, A.; MacLeod, M. Abundance, and composition of near surface microplastics and plastic debris in the Stockholm Archipelago, Baltic Sea. Mar. Pollut. Bull. 2017, 120, 292–302. [Google Scholar] [CrossRef]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xiong, X.; Hu, H.J.; Wu, C.X.; Bi, Y.H.; Wu, Y.H.; Zhou, B.S.; Lam, P.K.S.; Liu, J.T. Occurrence and characteristics of microplastic pollution in Xiangxi bay of three Gorges Reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef]
- Herzke, D.; Anker-Nilssen, T.; Nøst, T.H.; Götsch, A.; Christensen-Dalsgaard, S.; Langset, M.; Fangel, K.; Koelmans, A.A. Negligible impact of ingested microplastics on tissue concentrations of persistent organic pollutants in northern fulmars off coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. [Google Scholar] [CrossRef]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—But should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Hurley, R.; Iversen, K.; Olsen, M. Testing of Methodology for Measuring Microplastics in Blue Mussels (Mytilus spp.) and Sediments, and Recommendations for Future Monitoring of Microplastics (R & D Project), 2017; p. 87. Available online: https://www.researchgate.net/publication/321724113 (accessed on 8 June 2021).
- Yu, Q.; Hu, X.; Yang, B.; Zhang, G.; Wang, J.; Ling, W. Distribution, abundance, and risks of microplastics in the environment. Chemosphere 2020, 249, 126059. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Zhu, Z.L.; Yang, Y.X.; Sun, Y.R.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Zhu, Y.; Song, B.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Poll. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1. [Google Scholar] [CrossRef]
- Ma, J.; Sheng, G.D.; Chen, Q.L.; O’Connor, P. Do combined nanoscale polystyrene and tetracycline impact on the incidence of resistance genes and microbial community disturbance in Enchytraeus crypticus? J. Hazard. Mater. 2020, 387, 122012. [Google Scholar] [CrossRef] [PubMed]
- Velzeboer, I.; Kwadijk, C.J.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants bymicroplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Analyt. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplasticdan emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in ani-mals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Hu, J.; Wanzi, S.; Yuzhu, K.; Xiaoyu, G.; Weiling, S. Occurrence and removal of antibiotics and antibiotic resistance genes in natural and constructed riverine wetlands in Beijing, China. Sci. Total Environ. 2019, 664, 546–553. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Lee, H.; Chang, S.; Kim, S.K.; Kwon, J.H. Fugacity Analysis of polycyclic aromatic hydrocarbons between microplastics and seawater. Ocean Sci. J. 2017, 52, 43–55. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Q.; Lu, T.; Ke, M.; Zhu, Y.; Zhang, M.; Zhang, Z.; Du, B.; Pan, X.; Sun, L.; et al. The combined toxicity effect of Nanoplastics and glyphosate on Microcystis Aeruginosa growth. Environ. Pollut. 2018, 243, 1106–1112. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastic debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorbtion of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Cacador, I.; Canning-Clode, J. Microplastics as vectors for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 1–7. [Google Scholar] [CrossRef]
- Massos, A.; Turner, A. Cadmium, lead and bromine in beached microplastics. Environ. Pollut. 2017, 227, 139–145. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Tot. Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Davison, J. Genetic exchange between bacteria in the environment. Plasmid 1999, 42, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Campo, J.; Masiá, A.; Picó, Y.; Farré, M.; Barceló, D. Distribution, and fate of perfluoroalkyl substances in Mediterranean Spanish sewage treatment plants. Sci. Total Environ. 2014, 472, 912–922. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A.; Siegrist, H. Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Mayer, P.; Vaes, W.H.J.; Hermens, J.L.M. Absorption of hydrophobic compounds into the poly(dimethylsiloxane) coating of solid phase microextraction fibers: High partition coefficients and fluorescence microscopy images. Anal. Chem. 2000, 72, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Vakros, J.; Karapanagioti, H.K. Surface properties of marine microplastics that affect their interaction with pollutants and microbes. CIESM Workshop Monogr. 2014, 46, 55–60. [Google Scholar]
- Luthy, R.G.; Aiken, G.R.; Brusseau, M.L.; Cunningham, S.D.; Gschwend, P.M.; Pignatello, J.J.; Reinhard, M.; Traina, S.J.; Weber, W.J.; Westall, J.C. Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 1997, 31, 3341–3347. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsson, O.; Bucheli, T.D.; Jonker, M.T.O.; Koelmans, A.A.; Van Noort, P.C.M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Echeandía, J.S. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. Trends Analyt. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- O’Connor, I.A.; Golsteijn, L.; Hendriks, A.J. Review of the partitioning of chemicals into different plastics: Consequences for the risk assessment of marine plastic debris. Mar. Pollut. Bull. 2016, 113, 17–24. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by microsized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Syberg, K.; Khan, F.R.; Selck, H.; Palmqvist, A.; Banta, G.T.; Daley, J.; Sano, L.; Duhaimeet, M.B. Microplastics: Addressing Ecological Risk Through Lessons Learned. Environ. Toxicol. Chem. 2015, 34, 945–953. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- de Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, G.W.; Theriault, R.P. Polymeric Materials: Structure, Properties, Applications; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2011; pp. 67–78. ISBN 978-1-56990-310-0. [Google Scholar]
- Carraher, C.E.; Seymour, R.B. Seymour/Carraher’s Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2003; pp. 43–45. ISBN 978-0-8247-0806-1. [Google Scholar]
- Weissbach, W. Materials Science and Materials Testing; Vieweg + Teubner Verlag: Berlin, Germany, 2007; ISBN 3-8348-0295-6. [Google Scholar]
- Becker, G.W.; Bottenbruch, L.; Binsack, R.; Braun, D. Engineering Thermoplastics: Polyamides; Hanser Verlag: Munich, Germany, 1998; ISBN 3-446-16486-3. (In German) [Google Scholar]
- Haopeng, W.; Keum, J.K.; Hiltner, A.; Baer, E.; Freeman, B.; Rozanski, A.; Galeski, A. Confined Crystallization of Polyethylene Oxide in Nanolayer Assemblies. Science 2009, 323, 757–760. [Google Scholar]
- Müller, A.; Becker, R.; Dorgerloh, U.; Simon, F.G.; Braun, U. The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics. Environ. Pollut. 2018, 240, 639–646. [Google Scholar] [CrossRef]
- Pascall, M.A.; Zabik, M.E.; Zabik, M.J.; Hernandez, R.J. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J. Agric. Food Chem. 2005, 53, 164–169. [Google Scholar] [CrossRef]
- Smedes, F.; Geertsma, R.W.; Van Der Zande, T.; Booij, K. Polymer-water partition coefficients of hydrophobic compounds for passive sampling: Application of cosolvent models for validation. Environ. Sci. Technol. 2009, 43, 7047–7054. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Turnbull, A. Weathering of polymers: Mechanisms of degradation and stabilization, testing strategies and modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Material Weathering, 3rd ed.; Society of Plastics Engineer’s Inc.: Toronto, ON, Canada, 2003; p. 59. [Google Scholar]
- Arnold, J.C. Environmental stress cracking initiation in glassy polymers. Trends Polym. Sci. 1996, 4, 403–408. [Google Scholar]
- Christensen, M.L.; Klausen, M.M.; Christensen, P.V. Test of precoat filtration technology for treatment of swimming pool water. Water Sci. Technol. 2018, 77, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Fayolle, B.; Richaud, E.; Colin, X.; Verdu, J. Review: Degradation-induced embrittlement in semi-crystalline polymers having their amorphous phase in rubbery state. J. Mater. Sci. 2008, 43, 6999–7012. [Google Scholar] [CrossRef]
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stabil. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Jahnke, A.; Arp, H.P.H.; Escher, B.I.; Gewert, B.; Gorokhova, E.; Kühnel, D.; Ogonowski, M.; Potthoff, A.; Rummel, C.; Schmitt-Jansen, M.; et al. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 2017, 4, 85–90. [Google Scholar] [CrossRef]
- Fisner, M.; Taniguchi, S.; Moreira, F.; Bicego, M.C.; Turra, A. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, C.; Mülow-Stollin, U.; Hering, S.; Richter, J.; Piechotta, C.; Paul, A.; Braun, U. A first pilot study on the sorption of environmental pollutants on various microplastic materials. J. Environ. Anal. Chem. 2017, 4, 191. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, A.; Wan, Y.; Liu, H.; Wang, K.; Peng, H.; Dong, Z.; Hu, J. Occurrences of three classes of antibiotics in a natural river basin: Association with antibiotic resistant Escherichia coli. Environ. Sci. Technol. 2014, 48, 14317–14325. [Google Scholar] [CrossRef]
- Li, Z.; Hong, H.; Liao, L.; Ackley, C.J.; Schulz, L.A.; MacDonald, R.A.; Miheliche, A.L.; Emard, S.M. A mechanistic study of ciprofloxacin removal by kaolinite. Coll. Surf. B Bioint. 2011, 88, 339–344. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Munoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 1–9. [Google Scholar] [CrossRef]
- Ahrens, M.J.; Hertz, J.; Lamoureux, E.M.; Lopez, G.R.; McElroy, A.E.; Brownawell, B.J. The role of digestive surfactants in determining bioavailability of sediment-bound hydrophobic organic contaminants to 2 deposit feeding polychaetes. Mar. Ecol. Prog. Ser. 2001, 212, 145–157. [Google Scholar] [CrossRef]
- Käarrman, A.; Schönlau, C.; Engwall, M. Exposure and Effects of Microplastics on Wildlife. A Review of Existing Data; DiVA: Bielefeld, Germany, 2016; pp. 1–39. [Google Scholar]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of Plastic Additives to Marine Organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo a pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Au, S.Y.; Lee, C.M.; Weinstein, J.E.; van den Hurk, P.; Klainek, S.J. Trophic Transfer of Microplastics in Aquatic Ecosystems: Identifying Critical Research Needs. Integ. Environ. Assess. Manag. 2017, 13, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Lacroix, C.; Fernández, G.C.; Hégaret, H.; Lambert, C.; Le Goïc, N.L.; Frére, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. To polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Sørensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Fabbri, E.; Franzellitti, S. Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environ. Toxicol. Chem. 2015, 35, 799–815. [Google Scholar] [CrossRef]
- Moreno-González, R.; Rodríguez-Mozaz, S.; Huerta, B.; Barceló, D.; León, V.M. Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon? Environ. Res. 2016, 146, 282–298. [Google Scholar] [CrossRef]
- Nagtegaal, M.; Ternes, T.A.; Baumann, W.; Nagel, R. Detection of UV sunscreen agents in water and fish of the Meerfelder Maar the Eifel, Germany. Umweltwiss. Schadst. Forsch. 1997, 9, 79–86. [Google Scholar] [CrossRef]
- Tang, G.; Liu, M.; Zhou, Q.; He, H.; Chen, K.; Zhang, H.; Hu, J.; Huang, Q.; Luo, Y.; Ke, H.; et al. Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Rochman, C.M.; Lewison, R.L.; Eriksen, M.; Allen, H.; Cook, A.M.; Teh, S.J. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014, 476–477, 622–633. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Provencher, J.F.; Bond, A.L.; Hedd, A.; Montevecchi, W.A.; Muzaffar, S.B.; Courchesne, S.J.; Gilchrist, H.G.; Jamieson, S.E.; Merkel, F.R.; Falk, K.; et al. Prevalence of marine debris in marine birds from the North Atlantic. Mar. Pollut. Bull. 2014, 84, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chae, Y.; An, Y. Mixture toxicity of nickel and microplastics with different functional groups on Daphnia magna. Environ. Sci. Technol. 2017, 51, 12852–12858. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.G.; Lahive, E.; Spurgeon, D.J.; Svendsen, C.; Heutink, R.; Van Bodegom, P.M.; Baas, J. Acute toxicity of organic pesticides to Daphnia magna is unchanged by co-exposure to polystyrene microplastics. Ecotoxicol. Environ. Saf. 2018, 166, 26–34. [Google Scholar]
- Zhang, R.; Tang, J.; Li, J.; Zheng, Q.; Liu, D.; Chen, Y.; Zou, Y.; Chen, X.; Luo, C.; Zhang, G. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: Occurrence, distribution, and ecological risks. Environ. Pollut. 2013, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Koelmans, A.A. Accumulation of plastic debris and associated contaminants in aquatic food webs. Environ. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; De Writte, B.; Vethaak, D.; Hostens, K.; Leslie, H.A. Leslie, Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef]
- Magara, G.; Elia, C.A.; Syberg, K.; Khan, F.R. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. J. Toxicol. Environ. Health Part A 2018, 81, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Grigorakis, S.; Drouillard, K.G. Effect of microplastic amendment to food on diet assimilation efficiencies of PCBs by fish. Environ. Sci. Technol. 2018, 52, 10796–10802. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Leslie, H.A. Plastic debris is a human health issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Farré, M.; Karapanagioti, H.K.; Barceló, D. Levels and fate of perfluoroalkyl substances in beached plastic pellets and sediments collected from Greece. Mar. Pollut. Bull. 2014, 87, 286–291. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic Moves Pollutants and Additives to Worms, Reducing Functions Linked to Health and Biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Kooi, M.; Reisser, J.; Slat, B.; Ferrari, F.F.; Schmid, M.S.; Cunsolo, S.; Brambini, R.; Noble, K.; Sirks, L.A.; Linders, T.E.W.; et al. The effect of particle properties on the depth profile of buoyant plastics in the ocean. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Chaudhry, Q.; Stone, V.; Fernandes, T.F. A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ. Toxicol. Chem. 2009, 28, 2142–2149. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arienzo, M.; Ferrara, L.; Trifuoggi, M. The Dual Role of Microplastics in Marine Environment: Sink and Vectors of Pollutants. J. Mar. Sci. Eng. 2021, 9, 642. https://doi.org/10.3390/jmse9060642
Arienzo M, Ferrara L, Trifuoggi M. The Dual Role of Microplastics in Marine Environment: Sink and Vectors of Pollutants. Journal of Marine Science and Engineering. 2021; 9(6):642. https://doi.org/10.3390/jmse9060642
Chicago/Turabian StyleArienzo, Michele, Luciano Ferrara, and Marco Trifuoggi. 2021. "The Dual Role of Microplastics in Marine Environment: Sink and Vectors of Pollutants" Journal of Marine Science and Engineering 9, no. 6: 642. https://doi.org/10.3390/jmse9060642
APA StyleArienzo, M., Ferrara, L., & Trifuoggi, M. (2021). The Dual Role of Microplastics in Marine Environment: Sink and Vectors of Pollutants. Journal of Marine Science and Engineering, 9(6), 642. https://doi.org/10.3390/jmse9060642







