1. Introduction
Primary production in seasonally ice-covered polar oceans is driven by ice-associated and planktonic algae. While the proportional contributions of these two communities to primary production is variable [
1], there is widespread support for the view that ice algae represent a significant source of carbon, not least because they are available in early spring before ice break-up allows planktonic algae to develop [
2,
3]. Although sea ice productivity often decreases greatly after spring, the remaining biomass persists throughout the season and continues to provide carbon to food webs well into the autumn [
3,
4]. The timing and flux of carbon flow during this process is an important coupling in the Arctic marine ecosystem [
5]. In recent years, climatic warming has reduced the thickness and area of Arctic sea ice, which has led to concerns over the impacts of shifting patterns of primary productivity on Arctic ecosystems [
6,
7]. Sea ice algal biomass is generally dominated by diatoms concentrated at the ice–water interface [
8,
9], and these cells become available to seed phytoplankton [
10] and to support higher trophic levels either by grazing within the ice matrix or when they are released to the water column [
1]. The timing of such release of ice algae to the water column is potentially significant, as it may change how and when other organisms can exploit the ice algae food resource [
11] and when seeding of spring phytoplankton growth occurs [
10].
Snow cover is a key variable affecting sea ice algae biomass and photosynthetic parameters but can have complex effects. Small reductions in thick snow cover can enhance ice algal accumulation, but rapid reduction in snow cover often results in equally rapid loss of ice algae [
12]. Loss of algal biomass following sudden snow loss can be both physical, due to increased basal ablation increasing loss rates, and biological, through photoinhibition reducing growth [
13,
14]. Biomass can also fall after sudden increases in irradiance due to emigration of motile taxa, including flagellates and many pennate diatoms [
14]. The amount of light transmitted through the snow–ice matrix and the ability of algal photobiology to acclimate to sudden changes in light intensity over different time scales are therefore critical to understanding increases, decreases, and losses of algal biomass in response to a changing Arctic snow cover.
Climate modelling has predicted both increased and decreased thicknesses of snow cover in the Arctic Ocean [
15]; warmer air temperatures may make the coverage period much shorter, and the snow cover more dynamic with more frequent, sudden snow loss events. Most recent modelling suggests increased precipitation but less snow accumulation and more rainfall as the atmosphere warms [
16]. There is considerable evidence of general declines in the duration of the snow-covered period in the Eurasian Arctic [
17,
18,
19]; thus understanding the responses of ice algae to rapid shifts in snow cover has therefore become more important to understanding possible trajectories of change in the Arctic. At the local scale, however, snow trajectories are highly variable spatially and there is considerable inter-annual variability. For any given site, it is therefore important to compare responses across a range of snow thicknesses and hence irradiance scenarios to develop a complete insight into whether algal biomass is likely to change in response to broader climatic changes.
There are generally two approaches for studying effects of snowmelt on the ice algae community: first, manipulative field experiments involving complete or partial artificial snow removal, following effects from a subsequent time series [
12,
20], and second, measurement of selected parameters such as biomass or primary production as a function of natural variation in snow depth over space or time [
21,
22,
23]. The limitations of these two approaches for understanding large-scale, rapid increases in irradiance in response to sudden snowmelt are evident. For logistical reasons, most snow-clearing experiments can only treat relatively small areas (a few square metres) and are not accompanied by realistic changes in air and sea ice temperatures during weather events, and therefore they may not accurately mimic whole-ecosystem algal responses during snowmelt events. Natural gradients, on the other hand, allow algae to acclimate to the prevailing irradiance and do not provide the sudden photoinhibitory stress driven by rapid snowmelt. Regrettably, researchers have few opportunities to study photobiological responses to snowmelt during the large-scale snowmelt events that sea ice algae often experience in nature.
In this paper, we are able to present a fortuitous account of a sudden weather-driven warming and large-scale snow loss event impacting an ice algal community on an entire fjord during the spring growth season at Kangerlussuaq, a Greenland fjord. This is, to the authors’ knowledge, the first study of its kind, addressing the photobiological consequences of a rapid, wholesale natural loss of the snow cover over an entire fjord system. This type of event—sudden warming, with rainfall precipitation reducing snow cover—is very specifically a prediction of the most recent climate modelling [
16]. In addition, there are still relatively limited data in the literature on in situ photosynthesis–irradiance parameters describing acclimation to under-ice irradiance, even though these are important in most current models relating ice algal biomass to snow cover and sea ice properties [
24,
25]. The specific objectives were therefore to study the effects on the photophysiology of ice algae following sudden snow loss, as well as the role of a ca. 17 °C air temperature increase within 36 h.
2. Materials and Methods
2.1. Site Description and Sampling Design
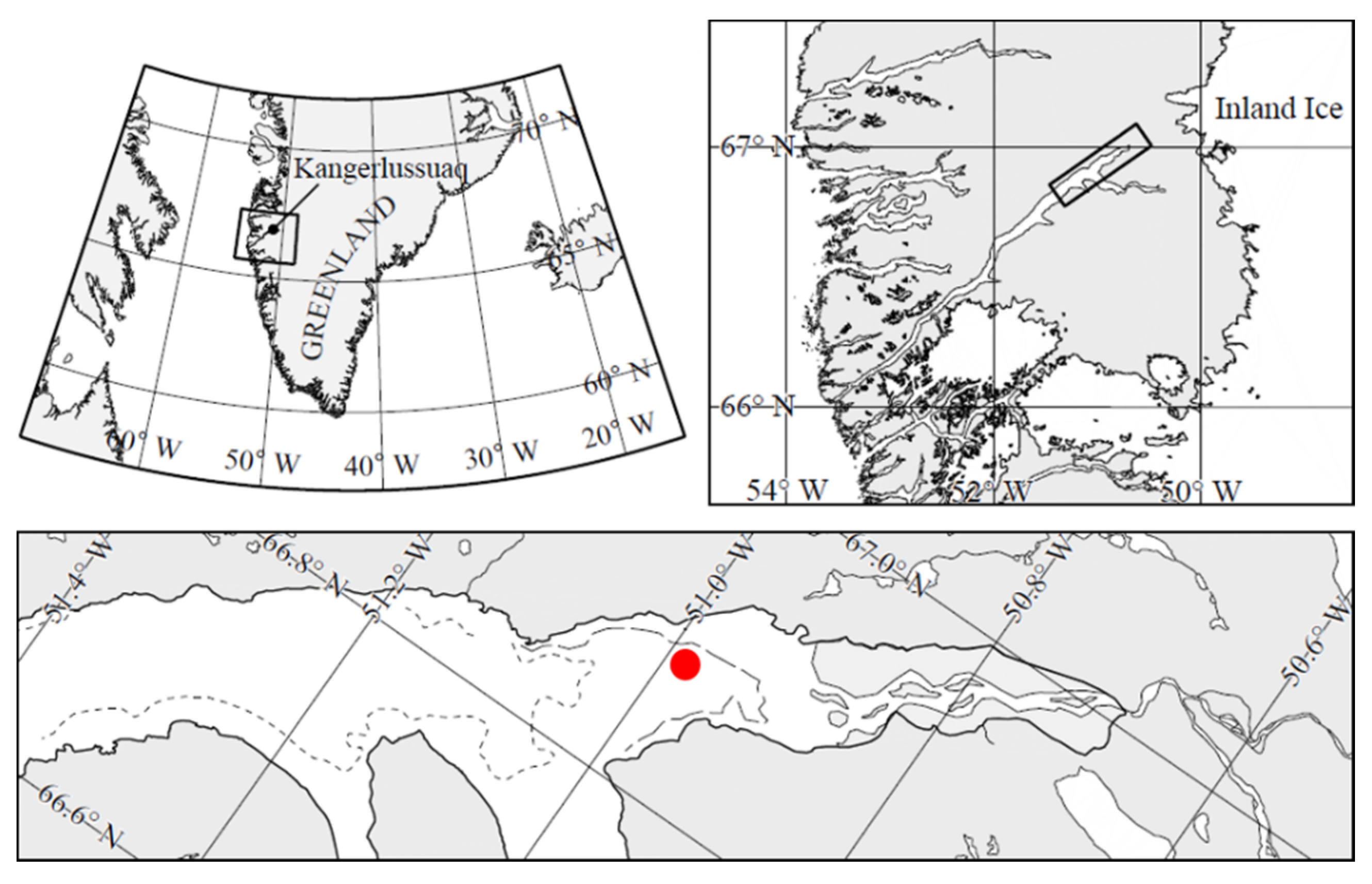
Our observations were made from 12 to 19 March 2013 at 66°56.780 N; 50°52.853 W, in Kangerlussuaq (
Figure 1), a 180 km long, narrow (1–5 km) Arctic fjord located on the Arctic Circle on the west coast of Greenland, about 25 km from the Greenland ice sheet. It consists of a relatively shallow (30 to 40 m depth) outer part that connects with the open ocean and a deep-water inner part with depths up to 280 m [
26]. Water depth at the sampling site was ca. 130 m. The tide is diurnal with a maximum tidal range of about 2–4 m [
27]. The area has a continental climate with low average winter temperatures of −15 to −25 °C and low precipitation of ca. 5 mm per month during December–July (
www.dmi.dk (accessed on 1 July 2021)). The fjord is ice-covered between October and early June to a thickness of about 0.5–1.0 m.
The sea ice station shown in
Figure 1 was established on 12 March 2013 (Julian Day 71), at which time a near-uniform ca. 10 mm thick snowpack covered the entire fjord (
Figure 2A). High air temperatures and rainfall over Days 74 and 75 caused the entire snow cover of the fjord to melt by Day 75, with the meltwater on the ice preventing access on Days 75 and 76. By Day 77, the warm event had removed all snow from the entire fjord including the study area (
Figure 2B).
Sea ice was sampled twice during the cold pre-snowmelt period (Days 71 and 73), which was followed by the melting event (Days 74 to 76), during which the ice station was inaccessible (
Figure 3A). After the snowmelt, ice cores were again collected twice (Days 77 and 78). In situ fluorescence imaging was performed on Days 73 (cold, pre-snowmelt) and 78 (warm, post-snowmelt). Sampling was carried out on a 5 m-wide × 10 m-long sampling area, marked out during the initial establishment of the experiment on Day 71.
2.2. Under-Ice Irradiance
Throughout the experiment, air temperature (sun-screened Campbell 107 temperature probe), downwelling and upwelling photosynthetic photon flux density (PAR) (Id and Iu) at 0.5 m above the surface (Li-COR Li-191 PAR sensor), and under-ice PAR (Ii) (Li-COR –Li-192 submersible PAR sensor) were recorded at the sea ice station at five-minute intervals using a Campbell CR10X data logger. The under-ice sensor was mounted on a black pole and lowered through a hole in the ice to a measuring position just below the ice and allowed to freeze in place. Transmittance (τ) was calculated as the ratio between under-ice PAR (Ii) and downwelling PAR at the surface (Id), and albedo was calculated as [Iu/Id].
To quantify how snow cover affected the spectral distribution of light available to the algae, spectral irradiances were obtained below the ice both with and without snow cover, using a TriOS Ramses ACC VIS cosine-corrected hyperspectral radiometer, measuring from 320 to 950 nm with a 3.3 nm resolution (
www.trios.de (accessed on 1 July 2021)). The sensor was mounted on an articulating arm that was lowered through a 90 mm hole in the ice, and once there, the arm unfolded to allow measurements to be obtained just below the ice at a horizontal distance of about 70 cm from the hole. Measurements were taken around local noon with a clear sky.
2.3. Collection, Processing, and Analysis of Ice Cores
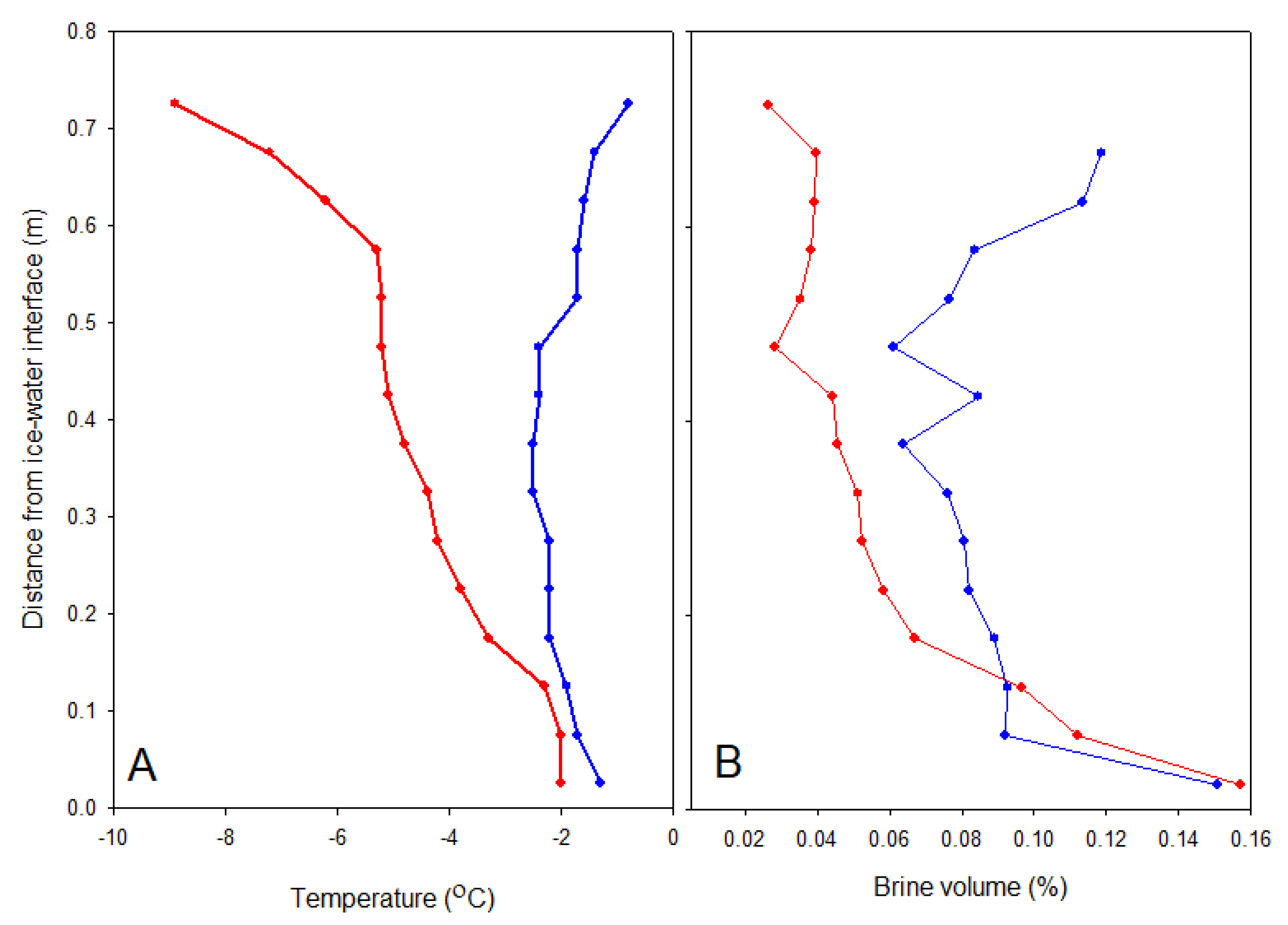
Eight ice cores were obtained on each sampling occasion using a 90 mm diameter Kovacs ice corer driven by a battery-powered drill. Their lengths were measured to the nearest 1 mm. Two of the cores were immediately placed in a horizontal cradle and temperature measured to 0.1 °C precision in 3 mm diameter holes drilled every 2.5 cm to the centre of the core. These cores were then cut into 50 mm sections, and each section was sealed in a polyethylene bag and placed in insulated boxes for transportation back to the laboratory within 4 h. The sections were then thawed in individual pots at 4 °C in the dark, and salinity was measured on the thawed sample with a YSI handheld CTD (PRO30) to nearest 0.1 precision. Brine salinity and volume were estimated according to the formulae of Cox and Weeks [
28].
Three of the cores from each sampling were used for biological analysis. The lower 30 mm of these cores was removed and immediately used in the field for fluorescence imaging (see next section). The remainder of the core was cut into 50 mm sections. All sections were then sealed in an opaque plastic container to minimise exposure to ambient irradiance and low temperatures and transported to the laboratory. At the laboratory, these biological core sections were placed in individual pots to thaw at 4 °C for ca. 12 h in the dark and to ensure dark adaptation of photosystems before Phyto-PAM analysis (
Section 2.5), with an additional two volumes of pre-filtered seawater to prevent osmotic shock [
20]. Each sample was immediately subsampled for the Phyto-PAM and filtered as soon as the last visible ice had disappeared, thus ensuring minimum time for any biological degradation or grazing of the algae in the thawed sample. An exact volume of thawed ice (0.25 to 0.5 L) was filtered for estimation of chlorophyll a (Chl
a) through glass fibre filters (GF75 Advantec, nominal pore size 0.7 µm), using a vacuum of <30 kPa, and the filters packed in aluminium foil and frozen at −20 °C.
Chl a was extracted in 5 mL of 96% ethanol at 5 °C for at least 6 h. Samples were centrifuged and the fluorescence of the supernatant measured with a Turner Design fluorometer (TD-700) using excitation of 430 and emission of 665 nm and converted into Chl a concentrations using a calibration series of pure pigment.
Three of the non-biological cores were analysed for dissolved organic carbon (DOC), on the lower 30 mm. The lower 30 mm was returned to the laboratory in acid-washed containers for thawing at 4 °C. Subsamples (50 mL) were filtered with a syringe filtration system through pre-combusted (450–500 °C for 2 h) GF/F filters into acid-washed 60 mL HDPE-bottles and preserved by addition of ultrapure 2 N HCl to a final concentration of 0.1%. DOC was later measured using a TOC-VCPH Total Organic Carbon Analyzer (Shimadzu). Deep-water reference Batch 9 Lot # 09/09 (Hansell Laboratory, University of Miami), a four-component freshwater standard series with exactly known C concentration, and freshly produced Milli-Q water as blank were used as reference material. Chl a and DOC concentrations were corrected for brine volume.
2.4. In Situ Variable Chlorophyll Fluorescence of Photosystem II—Imaging-PAM
Stress on the photosystems of the sea ice algae from the rapid increase in irradiance after snowmelt was examined through the variable fluorescence of photosystem II (PSII), determined by pulse amplitude modulated (PAM) fluorometry. Variable chlorophyll fluorescence was measured in the field on the intact lower 30 mm section of ice cores using a Walz Imaging-PAM fluorometer. Imaging PAM fluorometry allows resolution of the distribution of pigment systems and the activity of PSII in two dimensions, and full details of methods applied here are given in Hawes et al. [
9]. In brief, the instrument was fitted with a lens imaging an area of 30 mm × 24 mm, and the saturation pulse method was used to determine ambient (
F) and maximum (
Fm’) fluorescence yields of samples adapted to under-ice light. From these, we derived the effective quantum yield of PSII (Φ
PSII), which is a unitless ratio that is a proxy for the quantum efficiency of photosystem II in electron transfer at the prevailing irradiance; further details are given by Schreiber et al. [
29]. We calculated average values of Φ
PSII for the entire area of each image obtained as described previously [
14].
Our approach was designed to determine ΦPSII in freshly collected ice cores to provide insights into the degree of high-light-induced down-regulation of photosynthesis under the prevailing irradiance regime and to compare this with maximum quantum yield of PSII after overnight dark acclimation (ΦPSII_max) to discriminate down-regulation from persistent photodamage. Fluorescence imaging was carried out in a darkened tent on the sea ice immediately after sampling in the field. Two types of measurements were made: a single value from triplicate cores collected at local noon on each of Days 73 and 78 and a time course from 09:00 to 17:00 on Day 78. For the time course, each ice core was retained after imaging and thawed overnight (12 h) at 4 °C in the dark as described above for determination of subsequent dark-adapted maximum yield (ΦPSII_max) using the Phyto-PAM, as described below.
2.5. Variable Chlorophyll Fluorescence of Photosystem II—Phyto-PAM
Triplicate subsamples of the dark-adapted thawed samples as described in
Section 2.3 were taken for variable chlorophyll fluorescence estimates in a Phyto-PAM System II Emitter-Detector (Phyto-ED) unit with Phyto-WIN software (
www.walz.com.de (accessed on 1 July 2021)), taking care to maintain samples in darkness or very dim light. Measurements of Φ
PSII_max were performed as described previously [
20] on all core sections from the three biological cores. There was no detectable chlorophyll fluorescence in ice sections further up in the core, nor was there any measurable Chl
a or DOC beyond the lower 30 mm.
In addition, rapid light curves (RLCs) were made for each subsample. RLCs are primarily designed to determine the photophysiological status of cells under a given light regime by exposing cells to short periods of gradually increasing actinic irradiance and measuring ΦPSII by providing a saturation pulse at the end of each irradiance. The product of ΦPSII and actinic irradiance is taken as a measure of electron flow through PSII at that irradiance and referred to as relative electron transfer rate (rETR). From the curves of rETR vs. irradiance, we derived rETR_max, the maximum rate of electron flow at light saturation, α, the slope of the light-limited portion of the RLC, and Ek, the irradiance at which rETR was light-saturated, using the algorithms within the Phyto-PAM software. Values of all photobiological parameters for each replicate were the mean of the three subsamples.
2.6. Taxonomic Analysis and Relative Abundance
Samples (100 mL) of thawed bottom ice collected pre-snowmelt on Day 73 and after the snowmelt on Day 77 were preserved with acidic Lugol’s solution and stored in the dark for species identification. The ice algae were enumerated according to the Utermöhl method [
30]. The samples were allowed to settle in 50 mL settling chambers for 48 h before counting using a Zeiss Axiovert 135M (40×) inverted microscope. A total of four diagonals were counted in each sample. Identification of species and morphological groups were primarily based on the work by Tomas [
31], the relative abundance of algae was estimated as percent of total count, and the algal biomass was calculated according to the ALGESYS protocol.
2.7. Statistical Analysis
All statistical analysis was performed using JASP Version 0.11.1. Differences between sampling dates were compared with repeated-measures ANOVA. Post hoc multiple comparisons were conducted with a Bonferroni procedure. The assumption of sphericity was tested with Mauchly’s test for all repeated-measures ANOVAs. If violated, the degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity. For the diel experiment, comparisons were between in situ and dark-acclimated fluorescence yields with time of day as a fixed factor. Data were tested for normality and for heterogeneity of variances with Levene’s test and log10-transformed where necessary.
4. Discussion
Snow-clearing experiments are commonly used for investigating effects of snowmelt on polar sea ice algae [
12,
14,
32]. The fortuitous weather event documented in this paper has allowed us to explore how the results of such experiments compare against a natural snow loss event featuring a 17 °C atmospheric warming. In contrast to snow-clearing experiments in which air temperature remains unchanged, the main effect apart from increased irradiance is removal of the insulating effect of snow. Exposure to warmer air temperatures may lead to increased brine volume and potentially brine drainage and ultimately heat loss and melting of the sea ice. The general findings of snow-clearing experiments are that sudden increases in irradiance when snow is cleared cause rapid loss of shade-adapted taxa, leading to immediately lower sea ice algal biomass, and subsequent increases in taxa tolerant of higher irradiances [
12,
14,
20]. A similar response was seen here in the overall response of Kangerlussuaq Fjord to wholesale snow loss, suggesting that these large weather events produce similar effects to smaller-scale snow-clearing experiments.
Snow loss can lead to reduced sea ice algal biomass through both physical mechanisms and photobiological effects. Snow is a very effective thermal insulator, protecting sea ice from atmospheric temperature fluctuations. Loss of snow cover increases thermal exchange between ice and atmosphere; in this case, the sudden atmospheric warming increased heat transfer into the ice, leading to melting and loss of the millimetre-thin crystalline skeletal layer on the underside of the ice. A large fraction of the ice algal biomass is often associated with this crystalline layer [
8] as it provides a large surface area for colonisation, especially when actively growing [
33]. Documenting developments in the structure of the underside of the ice and its relation to biomass and photobiology is one of the benefits of the Imaging-PAM method. With this method, we were able in this study to observe the loss of the crystalline structure and replacement of its well-organised ice algal aggregates with a poorly defined ice surface and much smaller clumps of algae. Comparing the F and Φ
PSII_max images before and after snow loss illustrates the change in distribution and activity caused by the cold–warm event and confirms physical ablation of the skeletal layer as an important mechanism in the algal loss observed here. The corresponding decrease in Chl
a content and DOC confirms a substantial change in the community after snow loss. Brine drainage is an additional physical mechanism that can lead to loss of ice algae when air temperatures warm the ice and increase brine permeability. However, this mechanism was unlikely to be important for the measured photophysiological variables in the current study as all of the algae before the snowmelt were associated with the crystals of the skeletal brine layer in the lowest 10–15 mm, as seen in the fluorescence images (
Figure 5).
PAM fluorometric techniques are also valuable in establishing the extent of photoinhibition stress suffered by the ice algal community and its role in ice algal loss. Rapid changes from low to high irradiance cause photoinhibition in sea ice algae because they are characteristically extremely shade-adapted organisms [
1] and photoacclimate to the very low irradiances under snow-covered sea ice [
32,
34]. Φ
PSII_max is now well-established as an extremely sensitive indicator of photoinhibition that often responds rapidly and before any observable loss of biomass [
20,
35]. Here, as also seen in snow-clearing experiments [
20], its decrease immediately when the snow disappeared confirms the rapid onset of photoinhibition in the community. It is important, when interpreting these data, to note that the photobiological data from Day 77 after the snowmelt represents those algae that persisted or colonised in spite of increased irradiance. The flagellates and diatoms lost between Days 73 and 77 were either physically lost, killed by excess photostress, or migrated out of the ice. The remaining community—dominated especially by
N. frigida—is able to remain active and grow despite the photoinhibition evident from the lower Φ
PSII_max. In particular, rETR
max was not significantly lower in the post-snowmelt community, confirming that high-light-tolerant taxa remained active and may even have grown in the increased irradiance. The fact that the algal cell numbers and carbon content of the lower 30 mm did not change after the snowmelt even though Chl
a decreased suggests that the post-snowmelt community consisted of cells with a lower Chl
a content, due either to the change in taxonomic composition or acclimation by the pre-snowmelt taxa that persisted after snow loss. The contribution of
N. frigida is particularly interesting here.
N. frigida is known as a sea-ice specialist [
12,
36] and well-established as highly shade-tolerant species [
37]. Our study suggests that its adaptation to the sea ice habitat comes not only from being shade-adapted but also from an ability to tolerate higher light intensities and be robust in response to the rapid light fluctuations that are a feature of sea ice.
In this study, we applied two PAM fluorescence methods that provided complementary information on the nature of photoinhibition in the community after snowmelt. Imaging-PAM data measured in the field immediately after collection of cores illustrated the operational fluorescence yield Φ
PSII under the prevailing PAR at the time of collection, whereas Phyto-PAM data measured after thawing of core slices provided Φ
PSII_max after all reaction centres had had more than 12 h to relax in the dark in optimal conditions. The Imaging-PAM Φ
PSII data show a typical diurnal cycle of yield associated with photosynthetic activity and non-photosynthetic quenching with the diatinoxanthin–diadinoxanthin cycle. The post-snowmelt community was not showing any signs of photodamage, given that Φ
PSII before sunrise at 06:00 h in the fresh cores was not significantly lower than in the same cores after thawing overnight for the Phyto-PAM. Some non-photosynthetic quenching associated with energy dissipation is implied by the diurnal cycle in Φ
PSII_max observed in the Phyto-PAM, but there is apparently not any severe photodamage, and it is likely that the community would continue to acclimate to the higher irradiance as the higher irradiance persisted. There was also no indication of any decline in rETR at higher irradiances that would indicate photodamage. We have previously observed recovery of Φ
PSII_max and other photobiological parameters at this site after an initial decrease, in conjunction with colonisation of larger, higher-light taxa [
14,
20]. This is also a typical feature of the seasonal development of the ice algal community during a spring bloom in landfast seasonal ice, where early-season flagellates and small taxa are gradually replaced by the pennate diatom community [
22], climaxing in the larger taxa that are most high-light-tolerant [
38], before summer snowmelt.
This study, along with the snow-clearing experiments it can be compared and contrasted with, confirms the prime importance of snow cover as the predominant factor controlling the composition, timing, and productivity of the ice algal community [
39]. Comparing against two other studies from the same site and the same time of year (March, spring bloom) in 2011 [
14] and 2016 [
20], both the pre-snowmelt/snow-clearing and post-snow-removal communities were different. In 2011, under ca. 8 cm snow, the pre-bloom community was dominated by
Fragillariopsis oceanica, which was the main species lost after snow removal [
14]. F. oceanica was rare or absent at all times in the current study, whereas
N. frigida was rare in 2011 both before and after snow loss/removal. In 2016, flagellates were rare to begin with, but
N. frigida and other
Nitzschia spp. increased after snow removal. Light history can certainly explain these taxonomic differences: in both of those years, the under-ice irradiance was considerably lower than the ca. 60 µmol photons m
−2 s
−1 in 2013. Early-season communities dominated by small diatoms and flagellates can photosynthesise and grow at light intensities <10 µmol photons m
−2 s
−1 [
32,
40]. The inter-annual variability at this site, despite having a very uniform landfast ice structure and similar water temperature and unlimited nutrient availability at the time of the spring bloom, is testimony to how small differences in snow cover can have dramatic effects on the ice algal community. Although snow thickness is important, the high albedo of snow is its main property giving a high light attenuation, and thus presence or absence of snow cover is particularly important. In the present study, we can see a dramatic change when even a very thin snow cover is removed. Predicting the future of sea ice communities is therefore challenging, given the increasing frequency of wind and warming events with climate change, but one robust conclusion is that disruption of the normal progress of the spring bloom is likely to become more common. On the other hand, the results of this study and others suggest that some ice algal taxa, although briefly stressed when snow disappears, are capable of acclimating and recovering and continuing production even under greatly increased irradiances.
In conclusion, by documenting algal biomass, species composition, and photobiological responses to this whole-fjord snow loss event, we observed similar responses to artificial snow clearance. Photobiological effects of changes in irradiance appear to remain central to these effects, irrespective of the scale or cause of snow loss. Finally, we provided additional information here on the photobiological properties of first-year sea ice algae, which are highly dynamic features of the community. Many models exist that relate Chl
a to snow depth and ice thickness [
23,
39], but only recently have models begun to include photobiological parameters [
24]. Snow-clearing experiments, particularly longer-term experiments that allow both photostress but also include time for recovery, will remain an important tool for exploring the future of sea ice ecosystems.